Abstract
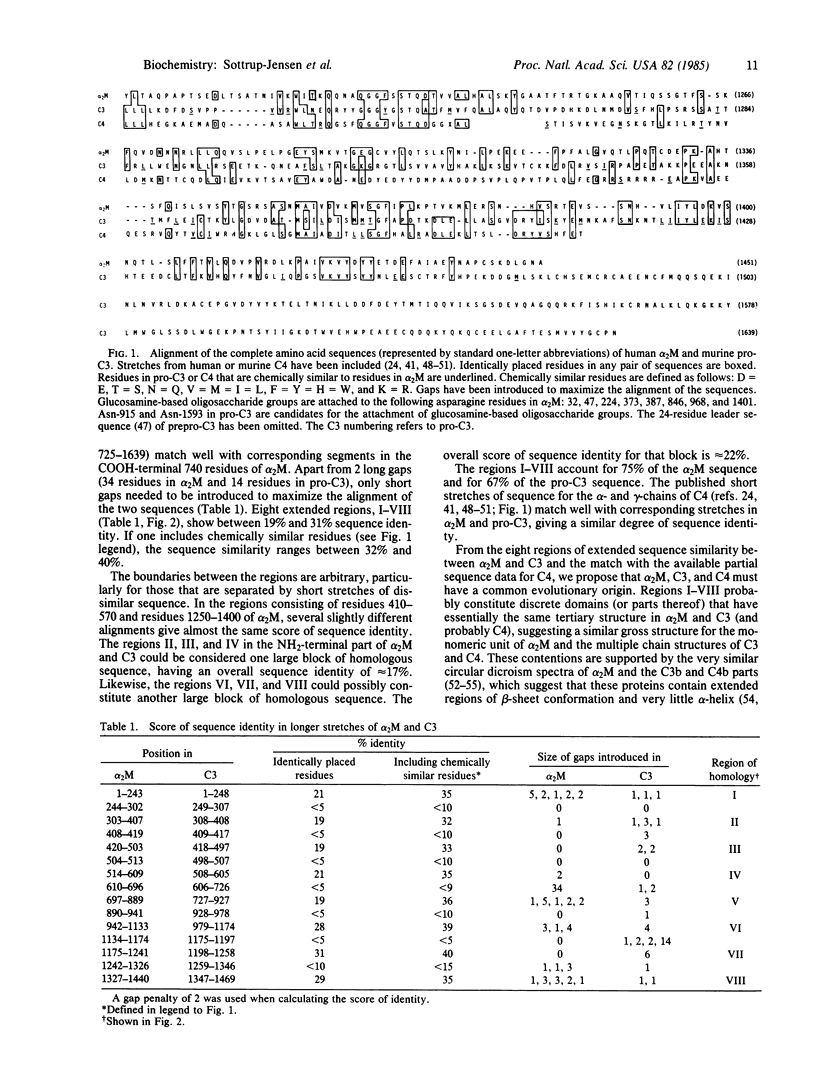
A comparison of the sequence of the subunit of human alpha 2-macroglobulin (alpha 2M; 1451 amino acid residues) with that of murine complement component pro-C3 (1639 amino acid residues) reveals eight extended regions of sequence similarity. These regions contain between 19% and 31% identically placed residues and account for 75% and 67%, respectively, of the polypeptide chains of alpha 2M and pro-C3. Published sequence data for complement component C4 show that segments of this protein match well with corresponding stretches in alpha 2M and pro-C3. It is proposed that alpha 2M, C3 and C4, which all contain a unique activatable beta-cysteinyl-gamma-glutamyl thiol ester, have a common evolutionary origin and are homologous proteins. Several larger regions of low sequence similarity indicate the presence of structural domains in each of these proteins that specifically modify an underlying common gross structure. The quartets of basic residues in pro-C3 and pro-C4, at which cleavage takes place to produce the mature subunits of these proteins, and most of the residues forming the anaphylatoxin peptides of C3 and C4 (C3a and C4a) are absent in alpha 2M. In addition, C3 and C4 contain large portions, which extend beyond the COOH terminus of alpha 2M.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Björk I., Fish W. W. Evidence for similar conformational changes in alpha 2-macroglobulin on reaction with primary amines or proteolytic enzymes. Biochem J. 1982 Nov 1;207(2):347–356. doi: 10.1042/bj2070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the thiol and reactive acyl groups of human complement component C4. Biochem J. 1981 Nov 1;199(2):359–370. doi: 10.1042/bj1990359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti D. N., Campbell R. D., Gagnon J. Amino acid sequence of a polymorphic segment from fragment C4d of human complement component C4. FEBS Lett. 1983 Apr 18;154(2):387–390. doi: 10.1016/0014-5793(83)80188-4. [DOI] [PubMed] [Google Scholar]
- Domdey H., Wiebauer K., Kazmaier M., Müller V., Odink K., Fey G. Characterization of the mRNA and cloned cDNA specifying the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7619–7623. doi: 10.1073/pnas.79.24.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir S., Acad B. A., Sonn J., Furman E., Kedem J. Preservation of myocardial oxygen balance and functional reserve by coronary vasodilators. Arch Int Physiol Biochim. 1985 Sep;93(3):231–239. doi: 10.3109/13813458509069925. [DOI] [PubMed] [Google Scholar]
- Fey G. H., Lundwall A., Wetsel R. A., Tack B. F., de Bruijn M. H., Domdey H. Nucleotide sequence of complementary DNA and derived amino acid sequence of murine complement protein C3. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):333–344. doi: 10.1098/rstb.1984.0094. [DOI] [PubMed] [Google Scholar]
- Gadd K. J., Reid K. B. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981 May 1;195(2):471–480. doi: 10.1042/bj1950471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G., Abraham G. N., Williams J., Colten H. R. NH2-terminal sequence analysis of pro-C4, the precursor of the fourth component of guinea pig complement. J Biol Chem. 1980 Aug 10;255(15):7071–7074. [PubMed] [Google Scholar]
- Goldberger G., Thomas M. L., Tack B. F., Williams J., Colten H. R., Abraham G. N. NH2-terminal structure and cleavage of guinea pig pro-C3, the precursor of the third complement component. J Biol Chem. 1981 Dec 25;256(24):12617–12619. [PubMed] [Google Scholar]
- Gorski J. P., Howard J. B. Effect of methylamine on the structure and function of the fourth component of human complement, C4. J Biol Chem. 1980 Nov 10;255(21):10025–10028. [PubMed] [Google Scholar]
- Gorski J. P., Hugli T. E., Müller-Eberhard H. J. Characterization of human C4a anaphylatoxin. J Biol Chem. 1981 Mar 25;256(6):2707–2711. [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Thomas M. L., Tack B. F. Sequence determination of the thiolester site of the fourth component of human complement. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7388–7392. doi: 10.1073/pnas.78.12.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter M. K., Thomas M. L., Rosen F. S., Tack B. F. Binding of C3b proceeds by a transesterification reaction at the thiolester site. Nature. 1982 Jul 1;298(5869):72–75. doi: 10.1038/298072b0. [DOI] [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., Vermeulen M., Swenson R. P. The temperature-sensitive bond in human alpha 2-macroglobulin is the alkylamine-reactive site. J Biol Chem. 1980 May 10;255(9):3820–3823. [PubMed] [Google Scholar]
- Huber R., Scholze H., Pâques E. P., Deisenhofer J. Crystal structure analysis and molecular model of human C3a anaphylatoxin. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1389–1399. doi: 10.1515/bchm2.1980.361.2.1389. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Human anaphylatoxin (C3a) from the third component of complement. Primary structure. J Biol Chem. 1975 Nov 10;250(21):8293–8301. [PubMed] [Google Scholar]
- Hugli T. E., Morgan W. T., Müller-Eberhard H. J. Circular dichroism of C3a anaphylatoxin. Effects of pH, heat, guanidinium chloride, and mercaptoethanol on conformation and function. J Biol Chem. 1975 Feb 25;250(4):1479–1483. [PubMed] [Google Scholar]
- Isenman D. E., Cooper N. R. The structure and function of the third component of human complement--I. The nature and extent of conformational changes accompanying C3 activation. Mol Immunol. 1981 Apr;18(4):331–339. doi: 10.1016/0161-5890(81)90057-2. [DOI] [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F. Fourth component of human complement: studies of an amine-sensitive site comprised of a thiol component. Biochemistry. 1981 Apr 28;20(9):2394–2402. doi: 10.1021/bi00512a005. [DOI] [PubMed] [Google Scholar]
- Janatova J., Tack B. F., Prahl J. W. Third component of human complement: structural requirements for its function. Biochemistry. 1980 Sep 16;19(19):4479–4485. doi: 10.1021/bi00560a015. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., BIRO C. E. ISOLATION AND DESCRIPTION OF THE FOURTH COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar J. L., Helder A. W., Müller M. A., Goris-Mulder M., Jonker L. S., Brouwer M., Pondman K. W. Physico-chemical and antigenic properties of human C3. Immunochemistry. 1975 May;12(5):359–364. doi: 10.1016/0019-2791(75)90001-4. [DOI] [PubMed] [Google Scholar]
- Moon K. E., Gorski J. P., Hugli T. E. Complete primary structure of human C4a anaphylatoxin. J Biol Chem. 1981 Aug 25;256(16):8685–8692. [PubMed] [Google Scholar]
- Odink K. G., Fey G., Wiebauer K., Diggelmann H. Mouse complement components C3 and C4. Characterization of their messenger RNA and molecular cloning of complementary DNA for C3. J Biol Chem. 1981 Feb 10;256(3):1453–1458. [PubMed] [Google Scholar]
- Ogata R. T., Shreffler D. C., Sepich D. S., Lilly S. P. cDNA clone spanning the alpha-gamma subunit junction in the precursor of the murine fourth complement component (C4). Proc Natl Acad Sci U S A. 1983 Aug;80(16):5061–5065. doi: 10.1073/pnas.80.16.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. L., Shreffler D. C., Capra J. D. Partial amino acid sequences of the murine fourth component of complement (C4): demonstration of homology with human C4 and identification of the amino-terminal subunit in pro-C4. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4275–4278. doi: 10.1073/pnas.77.7.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Gagnon J. Human complement component C4. Structural studies on the fragments derived from C4b by cleavage with C3b inactivator. Biochem J. 1981 Nov 1;199(2):351–357. doi: 10.1042/bj1990351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Sayers C. A., Barrett A. J. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981 May 1;195(2):453–461. doi: 10.1042/bj1950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with alpha 2-macroglobulin. Biochem J. 1981 Jan 1;193(1):129–141. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Hansen H. F., Mortensen S. B., Petersen T. E., Magnusson S. Sequence location of the reactive thiol ester in human alpha 2-macroglobulin. FEBS Lett. 1981 Jan 12;123(1):145–148. doi: 10.1016/0014-5793(81)80039-7. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Hansen H. F. Nascent alpha 2-macroglobulin-trypsin complex: incorporation of amines and water at the thiol esterified Glx-residues of alpha 2-macroglobulin during activation with trypsin. Biochem Biophys Res Commun. 1982 Jul 16;107(1):93–100. doi: 10.1016/0006-291x(82)91674-6. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Lønblad P. B., Stepanik T. M., Petersen T. E., Magnusson S., Jörnvall H. Primary structure of the 'bait' region for proteinases in alpha 2-macroglobulin. Nature of the complex. FEBS Lett. 1981 May 18;127(2):167–173. doi: 10.1016/0014-5793(81)80197-4. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Kristensen T., Wierzbicki D. M., Jones C. M., Lønblad P. B., Magnusson S., Petersen T. E. Primary structure of human alpha 2-macroglobulin. V. The complete structure. J Biol Chem. 1984 Jul 10;259(13):8318–8327. [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Amino acid sequence of the tryptic peptide containing the alkylamine-reactive site from human alpha 2-macroglobulin. Identification of gamma-glutamylmethylamide. J Biol Chem. 1980 Sep 10;255(17):8087–8091. [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Structural characterization of human alpha2-macroglobulin subunits. J Biol Chem. 1979 Jun 10;254(11):4452–4456. [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Janatova J., Gray W. R., Tack B. F. Third component of human complement: localization of the internal thiolester bond. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1054–1058. doi: 10.1073/pnas.79.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Domdey H., Diggelmann H., Fey G. Isolation and analysis of genomic DNA clones encoding the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7077–7081. doi: 10.1073/pnas.79.23.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]



