Abstract
The 17-year-old male patient presented with fever, weakness, dyspnea and weight loss. His chest radiography demonstrated diffuse reticulonodular density, and high-resolution lung tomography indicated diffuse micronodules and prevalent ground-glass pattern. The findings were consistent with miliary involvement. The patient underwent examinations for rheumatology, immunology, cytology and infectious conditions. His immune system was normal and had no comorbidities or any history of immunosuppressive treatment. Strongyloides stercoralis larvae were noted upon direct inspection of the feces. Clinical and radiological improvement was achieved with albendazole 400 mg/day. This case is being presented since miliary involvement in the lungs caused by S. stercoralis infection in an individual with intact immune system is rare and difficult to diagnosis.
Keywords: Strongyloides stercoralis, Pulmonary infection, Miliary involvement, Immunocompetent
1. Introduction
Strongyloidiasis is an infection caused by Strongyloides stercoralis and is common particularly in tropical and subtropical regions. It is estimated that 30–100 million individuals worldwide are infected with this parasite, which is reported as sporadic cases in Turkey [1]. Strongyloidiasis is the leading helminth infection that may be fatal in immunosuppressed individuals [2]. Those with normal intact immune system are usually asymptomatic or present with mild gastrointestinal or dermatologic symptoms. We determined that pulmonary complaints and miliary involvement in the immunocompetent individual was due to the S. stercoralis infection.
2. The case
A 17-year old male was admitted to hospital with certain complaints that lasted for 15 days such as weakness, fatigue, fever, perspiration, weight loss and dyspnea. He was working as a waiter and had no history of chronic disease or long-term or immunosuppressive drug treatment or recent travel. There was not any specificity in his own and familial history. He did not have any smoking or alcohol consumption habits. He did not describe rash, nausea, vomiting, abdominal pain, diarrhea or constipation.
In his vital findings were as follows: Fever: 37.5 °C, Blood pressure: 120/70 mmHg, Respiratory rate: 18/min, Heart rate: 92. And during the examination of respiratory system bilateral bazillary cracles were heard. No skin laceration, urticaria, petechia or purpura was observed.
Routine laboratory tests were normal except for the erythrocyte sedimentation rate and SGPT; 55 mm/h and 75 mg/dl respectively. Anti-HIV was negative. In his arterial blood gas analysis; PH was 7.39, PO2 was 59.2 mmHg, PCO2 was 35.2 mmHg, HCO3 was 22.7 and oxygen saturation was % 91.5.
Chest X-ray showed bilateral diffuse micronodules and ground-glass appearance. (Figs. 1 and 2)
Fig. 1.
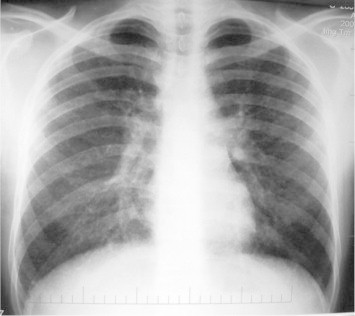
Chest X-ray showing bilateral diffuse micronodules and ground glass appearance.
Fig. 2.
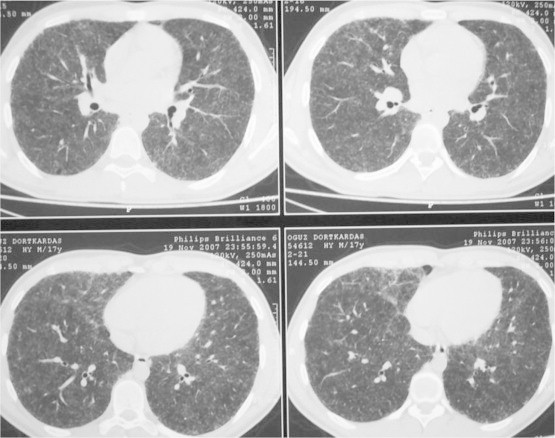
HRCT demonstrating diffuse bilateral micronodular infiltration, clarity in septal signs and ground-glass appearance.
High resolution computed tomography demonstrated diffused bilateral micronodular infiltration, and clarity in septal signs and diffused ground-glass appearance was observed. (Fig. 2)
Acid fast staining and culture of sputum were negative. Tuberculin test was negative. In his peripheral smear eosinophil of %4, lymphocyte of %10, monocyte of %6 and neutrophile of %80 were detected. The blood ACE level was 35, 24-h urine Ca was normal. Serologies of Brusella, cyst hydatid, Salmonella were negative. AntiDS DNA, Antimitochondrial Antibody (AMA) was negative, anti-smooth muscle antibody (ASMA) was positive and p-ANCA, c-ANCA and anti-nuclear antibody (ANA) were found at borderline. IgG was 2280 mg/dl, IgM was 116 mg/dl, total IgE was 142 mg/dl and IgA was 315 mg/dl.
Fiberoptic bronchoscopy was performed and bronchial system was seen as open. Bronchial aspiration and bronchoalveoler lavage cytology was benign. The microbiologic examinations performed in bronchial aspiration for nonspecific culture, fungal cultures, M. tuberculosis, atypical pneumonia and viral factors were found negative. Microscopic examination of the transbronchial biopsy sections revealed most of the pulmonary parenchyma to be replaced by nonnecrotizing granulomata, acute and chronic inflammatory infiltrate, and fibrosis. In the middle of some granulomas parasitic larvae were seen. (Fig. 3)
Fig. 3.
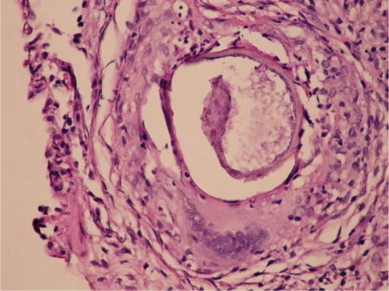
HE×400. In TBB material, at the alveolar area the epithelioid histiocyte groups, the granuloma structures comprised of giant cells and at the middle of some granulomas the parasitic larvae were observed.
When the anamnesis is extended, it was discovered that patient had a history of pika in his childhood, he had walked barefooted on the ground in the restaurant during summer and also had a mussel-eating history. Patient's anti-toxoplasma IgG and IgM, toxocara canis serology was negative. Blood CD4 and CD8 levels were found normal. The abdominal USG, brain BT and eye-ground examinations were normal. Any parasite was not observed in the direct examination of feces and sputum performed three times. No proliferation did occur in sputum or feces cultures. A structure similar to S. stercoralis larva was observed in one of the three samples taken from feces. The patient was consulted with parasitology and received the diagnose of S. stercoralis. Treatment involving albendazol of 400 mg/day and steroid of 0.5 was initiated. In the controls performed at the first month of treatment a clinic improvement was seen but radiology was stable. Bronchoscopy was repeated and transbronchial biopsy showed that the structures within the granuloma were thickened and broken. (Fig. 4)
Fig. 4.
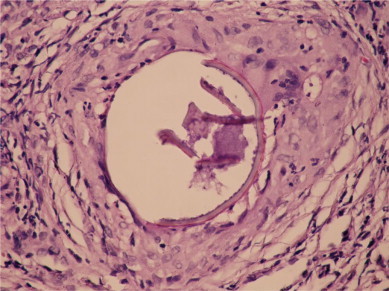
After the treatment, the structures within the granuloma were thickened and broken.
This case is reported since it is rarely seen and hardly diagnosed. Communications between the pathologist and parasitologist allowed the diagnosis.
3. Discussion
Although S. stercoralis can also be seen in temperate climates, it is present mainly in tropical and subtropical climates. Strongyloidiasis affects anywhere from 30 to 100 million people worldwide [3,4] and is endemic in Southeast Asia, Latin America, sub-Saharan Africa, and parts of the southeastern United States [3,5]. In Turkey, it is reported as sporadic cases, particularly in immunosuppressed individuals.
It is seen in regions where the soil is humic, at temperatures above 20 °C and with long-term high humidity. Individuals who work or wander barefoot in adobe, brick or tile manufacturing sites, mines, irrigated farming areas, streams and marsh waterfronts are most commonly infected [1]. The onset of infection is mostly with access of filariform larvae through the skin. It may also result from autoinfection and larvae access through the digestive tract [6,7]. The patient presented herein had barefoot soil contact in a holiday resort as well as history of pica and habit of eating clams. The infection might have occurred through the skin or digestive system.
Larvae accessing the host through the skin travel directly to the lungs via blood vessels. After spilling into the alveolar space, the larvae advance through the trachea and pharynx, where they are swallowed. They are later adsorbed on the duodenal and upper jejunal mucosa. They complete their maturation by approximately two weeks and larvae-containing ovum start to grow within mature females. Rhabditiform larvae emerging from the ovum shortly after pass into the intestinal lumen and are discharged via the excreta [1]. Given the migration pathway followed within the host, the primary signs and symptoms involve the skin, lungs and the gastrointestinal system.
Clinical findings vary depending on the amount of parasites exposed, the immune status of the host and the body part involved. Disseminated disease or hyperinfection may develop in immunosuppressed individuals, in whom mortality rates can be as high as 87% [8]. Individuals with normal immune system may be asymptomatic or may present as acute or chronic vases with pulmonary or gastrointestinal symptoms [1]. Presence of pulmonary symptoms in our patient may be due to the intact immune system and/or non-chronic exposure to the infection.
Reported cases involve patients with AIDS, leukemia, lymphoma, solid organ transplant, long-term corticosteroid use, and chronic pulmonary disease and the symptoms are often of gastrointestinal system origin. Gastrointestinal symptoms of strongyloidiasis include diarrhea, abdominal discomfort, nausea, and anorexia [5,9] Skin involvement is characterized by a migratory, serpiginous, urticarial rash, termed larva currens [5,9]. Our patient had none of these signs or symptoms.
Pulmonary symptoms caused by the larvae reaching the lungs are cough, dyspnea, wheezing and hemoptysis [10]. Diagnosis is difficult because many patients have baseline pulmonary complaints [11,12]. It usually presents as pneumonia, alveolar hemorrhage, asthma-like manifestation and pulmonary fibrosis [13]. The reported cases usually involve immunosuppressed patients or those with disseminated disease or the hyperinfection syndrome. Radiological findings of pulmonary Strongyloidiasis are diffuse alveolar opacity, segmental-lobar infiltrates, interstitial infiltrates, abscess-cavity, pleural effusion, ARDS, mediastinal lymphadenopathy, fibrotic alternations and nephrolithiasis [14]. Mass-like appearance with suspected malignancy with radiologic imaging has also been reported [15]. With literature reviews, however, we noted that there have been no previous reports of S. stercoralis infection with miliary involvement. Diffuse, millimetric, micronodular density increase with indefinite borders was observed at bilateral lungs in our patient with high-resolution computed tomography. The patient was therefore investigated thoroughly for conditions with potential miliary involvement, particularly for tuberculosis.
In the clinical diagnosis of the infection, persistent diarrhea, one of the primary signs, should suggest this parasitosis. Sometimes eosinophilia may be the only finding. Definitive diagnosis is established with presence of larvae in the feces, duodenal fluid and sometimes in the phlegm [1]. Although the differential diagnosis of conditions with miliary involvement include parasitic infections, it was difficult to consider parasitosis for our immunocompetent patient presenting with pulmonary symptoms with no gastrointestinal or dermatologic complaints and with normal eosinophil count. Feces was examined only after observing granuloma structures with pathological analysis of the transbronchial biopsy material and detecting parasitic larvae in the midst of the granuloma. Because the patient had no gastrointestinal symptoms or hyperinfection, fecal larvae load was not high either. The diagnosis was possible following successive fecal analyses and consultations with the parasitology and pathology departments. Therefore, the diagnosis calls for high level of suspicion.
This case is presented because detecting S. stercoralis infection and miliary involvement in the lungs in an individual with intact immune system is rare. This is a rare condition and the diagnosis is difficult and is often late.
Conclusion
Although the S. stercoralis is reported as sporadic cases, it should be considered in differential diagnosis.
References
- 1.Ardıc N. Strongyloides stercoralis ve enfeksiyonlarına genel bakış. Mikrobiyol Bul. 2009;43:169–177. [PubMed] [Google Scholar]
- 2.Ok Ü.Z. İmmün sitemi baskılananlardaki barsak parazitozları. Ankem Derg. 2006;20(Ek 2):177–181. [Google Scholar]
- 3.Genta R.M. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen T., Montresor A., Savioli L. Effectively controlling strongyloidiasis [letter] Parasitol Today. 1996;12:164. doi: 10.1016/0169-4758(96)80806-4. [DOI] [PubMed] [Google Scholar]
- 5.Liu L.X., Weller P.F. Strongyloidiasis and other intestinal nematode infections. Infect Dis Clin North Am. 1993;7:655–682. [PubMed] [Google Scholar]
- 6.Garcia L.S. 4th ed. ASM Press; Washington, DC: 2001. Diagnostic medical parasitology. [Google Scholar]
- 7.Çulha G., Savaş L., Önlen Y. Kronik diyare yakınması olan bir hastada Strongyloides stercoralis. Türkiye ParazitDerg. 2006;30:293–295. [PubMed] [Google Scholar]
- 8.Link K., Orenstein R. Bacterial complications of strongyloidiasis: Streptococcus bovis meningitis. South Med J. 1999;92:728–731. doi: 10.1097/00007611-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Grove D.I. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 10.Vijayan V.K. How to diagnose and manage common parasitic pneumonias. Curr Opin Pulm Med. 2007;13:218–224. doi: 10.1097/MCP.0b013e3280f31b58. [DOI] [PubMed] [Google Scholar]
- 11.Berk S.L., Verghese A. Parasitic pneumonia. Semin Respir Infect. 1988;3:172–178. [PubMed] [Google Scholar]
- 12.Smith B., Verghese A., Guiterrez C., Dralle W., Berk S.L. Pulmonary strongyloidiasis. Diagnosis by sputum Gram stain. Am J Med. 1985;79:663–666. doi: 10.1016/0002-9343(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 13.Vijayan V.K. Parazitic lung infections. Curr Opin Pulm Med. 2009;15:274–282. doi: 10.1097/MCP.0b013e328326f3f8. [DOI] [PubMed] [Google Scholar]
- 14.Mokhlesi B., Shulzhenko O., Garimella P.S., Kuma L., Monti C. Pulmonary strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004 January;11(1):6. doi: 10.1097/01.cpm.0000107609.50629.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaprakash B., Sandhya S., Anithakumari K. Pulmonary strongyloidiasis. J Assoc Physicians India. 2009 Jul;57:535–536. [PubMed] [Google Scholar]


