Abstract
Pneumocystis jiroveci pneumonia (PJP) opportunistically targets immunosuppressed patients, most notably those with advanced HIV/AIDS. Radiologically, PJP typically appears as bilateral diffuse pulmonary infiltrates. Herein an unusual case of an immunocompetent woman developing granulomatous PJP in the absence of evident risk factors is described. PJP may be an under-recognized cause of pulmonary nodules in immune competent individuals.
Keywords: PCP, Pneumocystis, Granuloma
1. Case presentation
A previously well, 49 year old Caucasian female presented to her family physician with increasingly persistent cough and night sweats. She reported a several year history of ongoing morning cough with dark sputum production. Her medical history was unremarkable with the exception of a 20-pack year smoking history and she had no significant risk for endemic mycoses or tuberculosis exposures. Physical exam was non-contributory. Chest imaging revealed a 1.5 cm2 smoothly marginated, non-calcified, non-cavitary left upper lobe pulmonary nodule (Fig. 1). A surveillance bronchoscopy with brushing was negative for malignancy. Despite this, a repeat computed tomography (CT) scan six months after the bronchoscopy identified the lesion to be increasing to 2.3 cm2. Other than a mildly appreciable left superhilar lymph node, the mediastinal structures were unremarkable. This, concurrent with a 30 lb weight loss over the same period, prompted the patient to undergo a left thoracic wedge resection of the left lung. Areas of necrotizing granulomatous inflammation with PJP cysts were identified on histopathology (Fig. 2). No other pathogens, including aerobic organisms, acid-fast bacilli or other fungal species were in either the bronchoalveolar lavage or biopsy. No other pathologic features were apparent. PJP cysts were not present on an expectorated sputum sample collected two days later.
Fig. 1.
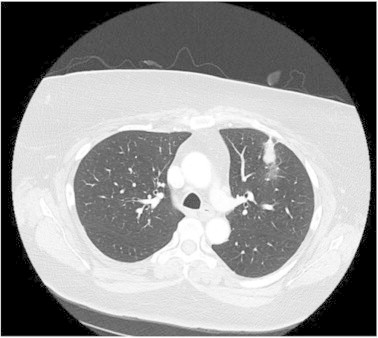
Chest computed tomography scan of the patient demonstrating a well-circumscribed, non-calcified left upper lobe nodule.
Fig. 2.
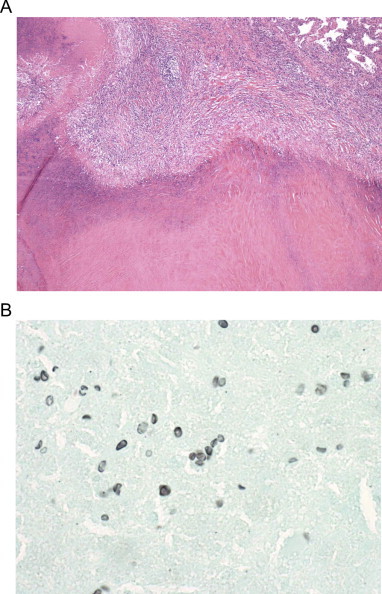
Thoracoscopic lung biopsy of the left upper lobe nodule demonstrates a necrotizing granuloma containing pneumocystis organisms surrounded by a hyal- inized capsule. A. Section of necrotizing granuloma with necrotic centre. Organisms are not visible on this stain. Hematoxylin and eosin stain at 40× magnification B. Organisms with the typical size and shape of Pneumocystis can be seen outlined in black. Grocott's Methenamine Silver stain at 1000× magnification.
An extensive workup was initiated to elicit obvious immunodeficiencies in the patient given that PJP is not expected in an immunocompetent host. She tested negative for HIV-1&2 (by enzyme EIA assay and P24 antigen), HTLV-1&2, hepatitis B and C. Serum immunoglobulins (IgG, IgM, IgA, IgE), lymphocyte subpopulation count (CD3, CD4, CD8), collagen vascular disease markers (ANA, Rheumatoid factor, GBM antibody, ANCA, anti-MPO, PR3) and malignancy investigation (CT of chest and abdomen) were all within normal limits. She had an ability to mount an appropriate immune response, as evidenced by the presence of IgG antibodies for mumps and rubella, diphtheria and tetanus, suggesting appropriate vaccination against such diseases. Laboratory test for complement C3, complement C4, alpha-1 antitrypsin, anti-actin were normal.
While initially no treatment was provided, a lingering cough prompted a course of trimethoprim-sulfamethoxazole (TMP/SMZ) DS 800/160 mg two tablets by mouth thrice daily for three weeks. Symptoms resolved and did not recur through eighteen months of observation nor did any new syndrome to suggest undiagnosed immunodeficiency. Follow up radiographic imaging did not show evidence of recurrence.
2. Discussion
PJP is a type of pneumonia originating from the fungus Pneumocystis jiroveci, which is specific to humans [1]. PJP antibodies can be serologically present in the absence of symptoms and histopathology findings in healthy individuals suggest that Pneunocystis jiroveci is an opportunistic pathogen of ubiquitous distribution and low pathogenicity. Immunocompetent individuals with asymptomatic colonization of pneumocystis have the potential to transmit the fungus to others including immunocompromised individuals [2]. PJP normally presents as an interstitial pneumonia. Granulomatous PJP is unusual, occurring in up to 5% of immunocompromised patients, mostly HIV positive individuals [3,4]. Risk factors for granulomatous PJP include aerosolized pentamidine based PJP prophylaxis, corticosteroid therapy and HIV immune reconstitution disease [5].
PJP or asymptomatic colonization by P. jiroveci is becoming notably common in immunosuppressed non-HIV patients [6]. TMP/SMZ and potent anti-retroviral therapy have decreased PJP incidence in HIV-positive patients, whereas novel immunosuppressive therapies in treating malignancies and autoimmune inflammatory disorders have increased the incidence of PJP in the HIV negative populations [7] Furthermore, advances in detection by immunofluorescence and molecular assays including PCR have also contributed to the rise of PJP incidence [8]. Several reports of PJP have been described in seemingly immune competent patients [9]. Whether these cases are attributable to unidentified defects in the immune system is as yet unknown.
A literature search was completed using MEDLINE publications from 1946-present and EMBASE publications from 1980-present. “Pneumocystis” or “P. jiroveci” combined with “granuloma” or “granulomatous” were searched. Results were reviewed to assess for association between immunocompetent individuals and granulomatous PJP. Reports either described granulomatous PJP in the immunocompromised population or presentation of interstitial PJP in immunocompetent patients. One report described a nodular presentation of PJP in a hepatitis C positive male [10].
The case described herein is unique in that an immunocompetent individual developed a rare granulomatous presentation of PJP in the absence of any risk factors. This infection was responsible for a sub-acute atypical symptomatic presentation which resolved with therapy. In considering the diagnosis of solitary pulmonary nodules, it is critical to consider a range of diagnostic possibilities (Table 1). The finding of a pulmonary nodule involves investigation for malignancy, infection and connective tissue disorders.
Table 1.
Differential diagnosis for solitary pulmonary nodule [11,12].
|
|
Disclosures
No author has any conflicts that impact the data presented herein. The patient described provided written informed consent for this case to be prepared and published.
References
- 1.Stringer J.R. Pneumocystis. Int J Med Microbiol. 2002;292:391–404. doi: 10.1078/1438-4221-00222. [DOI] [PubMed] [Google Scholar]
- 2.Ponce C.A., Gallo M., Bustamante R., Vargas S.L. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–353. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 3.Travis W.D., Pittaluga S., Lipschik G.Y., Ognibene F.P., Suffredini A.F., Masur H. Atypical pathologic manifestations of pneumocystis carinii pneumonia in the acquired immune deficiency syndrome. Review of 123 lung biopsies from 76 patients with emphasis on cysts, vascular invasion, vasculitis and granulomas. Am J Surg Pathol. 1990;14:615–625. doi: 10.1097/00000478-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hartel P.H., Shilo K., Klassen-Fischer M., Neafie R.C., Ozbudak I.H., Galvin J.R. Granulomatous reaction to Pneumocystis jiroveci: clinicopathologic review of 20 cases. Am J Surg Pathol. 2010;34:730–734. doi: 10.1097/PAS.0b013e3181d9f16a. [DOI] [PubMed] [Google Scholar]
- 5.Tolet A., Duwat H., Daste G., Berry A., Escamilla R., Nevez G. Pneumocystis jiroveci genotypes and granulomatous pneumocystosis. Med Mal Infect. 2006;36:229–231. doi: 10.1016/j.medmal.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Medrano F.J., Montes-Cano M., Conde M., de la Horra C., Respaldiza N., Gasch A. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005;11:245–250. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overgaard U.M., Helweg-Larsen J. Pneumocystis jiroveci pneumonia (PJP) in HIV-1-negative patients: a retrospective study 2002–2004. Scand J Infect Dis. 2007;39:589–595. doi: 10.1080/00365540601150497. [DOI] [PubMed] [Google Scholar]
- 8.Fily F., Lachkar S., Thiberville L., Favennec L., Caron F. Pneumocystis jirovecii colonization and infection among non-HIV infected patients. Med Mal Infect. 2011;41:526–531. doi: 10.1016/j.medmal.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Cano S., Capote F., Pereira A., Calderon E., Castillo J. Pneumocystis carinii pneumonia in patients without predisposing illnesses. Acute episode and follow-up of five cases. Chest. 1993;104:376–381. doi: 10.1378/chest.104.2.376. [DOI] [PubMed] [Google Scholar]
- 10.Harris K., Maroun R., Chalhoub M., Elsayegh D. Unusual presentation of pneumocystis pneumonia in an immunocompetent patient diagnosed by open lung biopsy. Heart Lung Circ. 2012;21:221–224. doi: 10.1016/j.hlc.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Winer-Muram H.T. The solitary pulmonary nodule. Radiology. 2006;239:34–39. doi: 10.1148/radiol.2391050343. [DOI] [PubMed] [Google Scholar]
- 12.Leef J.L., 3rd, Klein J.S. The solitary pulmonary nodule. Radiol Clin North Am. 2002;40:123–143. doi: 10.1016/s0033-8389(03)00113-1. [DOI] [PubMed] [Google Scholar]


