Abstract
Many carbonyl species from either lipid peroxidation or glycoxidation are extremely reactive and can disrupt the function of proteins and enzymes. 4-hydroxynonenal and methylglyoxal are the most abundant and toxic lipid-derived reactive carbonyl species. The presence of these toxics leads to carbonyl stress and cause a significant amount of macromolecular damages in several diseases. Much evidence indicates trapping of reactive carbonyl intermediates may be a useful strategy for inhibiting or decreasing carbonyl stress-associated pathologies. There is no rapid and convenient analytical method available for the assessment of direct carbonyl scavenging capacity, and a very limited number of carbonyl scavengers have been identified to date, their therapeutic potential being highlighted only recently. In this context, we have developed a new and rapid sensitive fluorimetric method for the assessment of reactive carbonyl scavengers without involvement glycoxidation systems. Efficacy of various thiol- and non-thiol-carbonyl scavenger pharmacophores was tested both using this screening assay adapted to 96-well microplates and in cultured cells. The scavenging effects on the formation of Advanced Glycation End-product of Bovine Serum Albumin formed with methylglyoxal, 4-hydroxynonenal and glucose-glycated as molecular models were also examined. Low molecular mass thiols with an α-amino-β-mercaptoethane structure showed the highest degree of inhibitory activity toward both α,β-unsaturated aldehydes and dicarbonyls. Cysteine and cysteamine have the best scavenging ability toward methylglyoxal. WR-1065 which is currently approved for clinical use as a protective agent against radiation and renal toxicity was identified as the best inhibitor of 4-hydroxynonenal.
Keywords: Reactive carbonyl species, Carbonyl scavenger, Fluorescent adduct, Screening assay
Graphical abstract
Reaction of carbonyl compounds with NBD-H to form highly fluorescent derivatives. Fluorescence is strongly inhibited by carbonyl scavengers such as thiols with an α-amino-β-mercaptoethane structure.
Highlights
-
•
We describe a rapid method for assessment of reactive carbonyl scavengers.
-
•
We evaluated the carbonyl scavenger activity of various pharmacophores.
-
•
α-amino-β-mercaptoethane structure showed the highest degree of activity.
Introduction
Reactive carbonyl species (RCS), especially α-dicarbonyl compounds, are key mediators of damage caused by oxidative stress and glycation. RCS, as reactive intermediates of cellular carbonyl stress, originate from a multitude of mechanistically related pathways such as glycation [1], sugar autoxidation [2], lipid peroxidation [3], and UV-photodamage [4]. Cellular carbonyl stress results in protein damage referred to as glycation by spontaneous chemical reaction between RCS, such as reducing sugars and more reactive dicarbonyl compounds, with proteins to cause oxidation of their polypeptide backbone, peptide bond cleavage, protein–protein cross-linking, and a range of amino acid side-chain modifications [5]. Both free and protein-bound carbonyls can also directly affect cellular function through multiple pathways, including enzyme inhibition [6].
Compared to free radicals, lipid-derived RCS are stable and can diffuse within or even escape from the cell and attack targets far from the site of formation. Therefore, these soluble reactive intermediates are not only cytotoxic but also behave as mediators and propagators of oxidative stress and tissue damage, acting as second cytotoxic messengers. Interestingly, RCS can exert their detrimental cellular effects by increasing reactive oxygen species (ROS) production [7], thereby forming a vicious cycle of ROS and RCS production. The α,β-unsaturated aldehydes 4-hydroxynonenal (HNE), acrolein, and dicarbonyls methylglyoxal (MG) are the most abundant and toxic lipid-derived RCS. HNE is, generated through the β-cleavage of hydroperoxides from ω-6 polyunsaturated fatty acids. MG is originated from non-enzymatic dephosphorylation of triosedihydroxyacetone phosphate and glyceraldehyde-3-phosphate as well as the lipid peroxidation, the glucose auto-oxidation and degradation of glycated proteins. These reactive and electrophilic compounds act as key factors in the development and progression of a variety of chronic diseases such as cardiovascular (e.g., atherosclerosis, long-term complications of diabetes) and neurodegenerative diseases, cerebral ischemia, rheumatoid arthritis, and post-ischemic reoxygenation injury [8–10].
Among the different approaches that can be considered to prevent or restrain the RCS-induced damage, that based on a direct trapping of reactive aldehydes seems to be the most promising, and represents a good therapeutic target. Even if this strategy will not completely abolish or contain the oxidative stress, it can be useful to reduce the toxicological consequences of RCS attack and slow down the progression of pathological events.
Currently, there is no convenient analytical method available for the assessment of direct carbonyl scavenging capacity, and a very limited number of inhibitors of cellular carbonyl stress have been identified to date. Carbonyl scavenger effect of various compounds was measured by evaluating their cytoprotective effect [11–14] or their ability to decrease the protein carbonyl content on cultured cells [13–16]. Some inhibitors of glycation interfere with the reaction by trapping intermediate α-dicarbonyls [17–19], whereas other inhibitory substances act merely as antioxidants and transition metal chelators [20]. In vitro AGE fluorescence or immunological quantification of specific AGEs is complicated by the nature of employed glycoxidative reaction systems for screening α-dicarbonyl scavenger.
Carbonyl compounds are sparingly absorbent and do not exhibit native fluorescence. A highly fluorescent, light-stable chromophore with a suitable anchor group is needed for the labelling of carbonyl compounds in order to get a stable and strong fluorescence signal, especially if an intense light source is applied. If the labelling reagent itself is fluorescent, a strong fluorescent background signal will be obtained from the non-converted reagent. Therefore, better results are expected for non-fluorescent labelling reagents that form highly fluorescent carbonyl adducts.
In the present study, we present a convenient, rapid and sensitive fluorimetric method for high-throughput assessment of both reactive monocarbonyls and dicarbonyl scavengers without involvement glycoxidation systems. The method reported here is based on the reaction of carbonyl compounds with 7-hydrazino-4-nitrobenzo-2,1,3-oxadiazole (NBD-H) to form highly fluorescent derivatives via hydrazone formation [21].
To confirm the reliability of this method, various RCS-sequestering compounds and pharmacophores known as therapeutic agents to protect cells against carbonyl stress were also tested on cultured cells and with two other screening assays specifically designed for aliphatic monocarbonyl and dicarbonyls scavengers respectively. A strong correlation between RCS trapping ability measured with the proposed NBD-H assay and the other assays was revealed. Efficacy of other thiol- and non-thiol-carbonyl scavenger pharmacophores from approved drug was then tested using our NBD-H assays. Their inhibitory activity was also evaluated on the formation of AGE using MG, HNE and glucose-glycated BSA as molecular models.
Materials and methods
Reagents and materials
4‐hydroxynonenal was purchased from Cayman (Ann Arbor, MI, USA). Luciferin-luciferase reagent (Biofax A) was purchased from Yelen (Ensues la redone, France). Other reagents are from Sigma-Aldrich (St. Louis, MO, USA) with analytical grade. HNE was pre-diluted in DMSO prior to usage. Other RCS studied are freely soluble in water (100–600 g/l at 20 °C) and were tested in aqueous soluble range concentrations. Screening assays were performed with a microplate spectrofluorimeter TECAN Infinite 200 (TECAN, Männedorf, Switzerland)
Cell culture and cytotoxicity evaluation
X63-Ag8.653 HGPRT deficient mouse Balb/c myeloma cells [22] were cultured in RPMI-1640 or 90% Dulbecco׳s MEM glutamax plus supplemented with 10% heat-inactivated fetal bovine serum (FBS), sodium bicarbonate, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were maintained at 0.1–1.106 cells/ml; optimal split ratio of 1/10–1/20 every 48 h; 15×103 cells per well were incubated 24 h without or with various concentrations of RCS at 37 °C with 10% CO2 and without FBS in 96-well plates.
Caco-2 cells cell line (generous gift of Dr. Massey-Harroche, IBDML, Marseille, France) were routinely grown in Dulbeco׳s modified essential medium (DMEM) or supplemented with 10% foetal calf serum (FCS), 1% l-glutamine and 1% antibiotics (all from Invitrogen) and maintained in a 5% CO2 incubator at 37 °C. For studying toxics or molecules effects, Caco-2 cells were seeded at a density of 20×103 cells per well in 96-well plates and let to differentiate with medium changed every 2 days. After 6–8 days of differentiation, Caco-2 monolayers were incubated 24 h without or with RCS diluted to the appropriate density in DMEM (without FCS or antibiotics).
Cytotoxicity was determined by measurement of intracellular ATP content using the luciferin–luciferase reaction [23]. Briefly, cells were lysed by adding Triton X-100 at a final concentration of 1%. 100 µL of cell lysates were collected and transferred into 96-well plates after 5-min incubation,. One hundred microliters of luciferin–luciferase reagent (Yelen, France) were then immediately added before luminescent signal was quantified in a microplate luminometer TECAN Infinite 200 (TECAN, Männedorf, Switzerland).
We evaluated the protective effects of the carbonyl scavengers by comparing the intracellular ATP content of untreated cells with cells exposed to carbonyl stress±test compounds.
Global carbonyls scavenger evaluation (NBD-H assay)
This assay is base on the reaction of NBD-H with both mono and dicarbonyls compounds via hydrazone formation to form highly fluorescent products. NBD-H solution was prepared at 200 µM in 100 mM phosphate buffer, pH 7.4 with 1 M HCL. RCS (MG and HNE) and carbonyl scavenger solutions were prepared in 100 mM phosphate buffer, pH 7.4. 100 µl of each carbonyl solution (100 µM) was incubated 30 min at 37 °C with 100 µl of phosphate buffer or 100 µl of carbonyl scavenger solutions at various concentrations in 96 wells microplate. 100 µl of NBD-H solution was then added in each well and fluorescence was measured at 560 nm, exciting at 500 nm after 5 min. The % inhibition of NBD-H adduct formation was calculated for each compound from the fluorescence response versus concentration. Each treatment was compared with control without scavenger, and statistical significance between two groups was evaluated using Student׳s t test. One-way analysis of variance (ANOVA) was used for the data presented in Table 2 with post-hoc multiple comparison by a Bonferroni test using the Graph Prism program. The criterion for significance was set at p<0.05.
Table 2.
Scavenging activity of MG and HNE in myeloma cells and screening assays.
| Compounds |
Methylglyoxal |
4-Hydroxynonenal |
||||
|---|---|---|---|---|---|---|
| NBDHa | TRIa | Myeloma cellsb | NBDHa | CHDa | Myeloma cellsb | |
| RCS | 0.1 mM | 0.006 mM | 1 mM | 0.1 mM | 0.1 mM | 0.1 mM |
| L-cysteine | 0.23±0.03 | 0.021±3×10−3 | 600 | 0.18±0.02 | 0.11±0.01 | 0.6 |
| Penicillamine | 0.26±0.03 | 0.01±2×10−3 | 700 | 0.85±0.05 | 0.75±0.05 | 0.88 |
| Glutathione | 2.5±0.03 | >0.6 | 2.1×103 | 0.24±0.03 | 0.27±0.02 | 0.8 |
| N-acetyl-cysteine | 4.2±0.05 | d | 6.2×103 | 0.48±0.04 | 0.56±0.07 | 0.64 |
| Aminoguanidine | 0.74±0.16 | 0.13±7×10−3 | 103 | 8±0.6 | 0.42±0.05 | 1.6 |
| Cysteamine | 0.22±0.03 | 0.17±0.02 | ||||
| Homocysteine | 0.79±0.17 | 0.17±0.02 | ||||
| WR-1065 | 0.8±0.14 | 0.12±0.02 | ||||
| Pyrodoxamine | >10c | >10c | ||||
| Metformin | >10c | >1 | >5×103 | >10c | >10 | >5 |
| Captopril | 6.0±0.5 | 1.0±0.2 | ||||
| Carnosine | >10c | >10c | ||||
| β-Alanine | >10c | >10c | ||||
IC50 values expressed in mmol/l corresponding to 50% of inhibition of carbonyl derivative formation.
IC50 values expressed in mmol/l corresponding to 50% of toxicity induced at 1 mM and 100 µM for MG and HNE respectively. Each data point is the mean±SD from triplicate determinations. Each treatment is compared with control, and statistical significance between two groups is evaluated using Student׳s t test.
p>0.05 versus control in the absence of scavenger.
Fluorescence signal higher than control.
Dicarbonyl scavenger evaluation (TRI assay)
This assay is based on the reaction of α-dicarbonyl compounds with 6-hydroxy-2,4,5 triaminopyrimidine (TRI) to form the corresponding fluorescent pteridinic derivatives [24]. MG used as α-dicarbonyl compound reacts with TRI to form 6,7-dimethylpterin.
MG, carbonyl scavengers and TRI solutions were prepared in 300 mM phosphate buffer (pH 7.4). 100 µl of MG solution (6 µM) was incubated 1 h at 37 °C with 100 µl of phosphate buffer or 100 µl of carbonyl scavenger solutions at various concentrations in 96 wells microplate. 100 µl of TRI solution (60 µM) was then added in each well and fluorescence was measured at 445 nm, exciting at 350 nm after 1 h. The % inhibition of TRI-MG formation is calculated for each compound from the fluorescence response versus concentration.
Monocarbonyls scavenger evaluation (CHD assay)
Aldehydes can be condensed with 1,3 cyclohexanedione (CHD) and ammonium ion to form highly fluorescent and stable water-soluble adduct. CHD can easily react with aliphatic mono-unsaturated aldehydes such as HNE but poorly with α-dialdehydes such as glyoxal and methylglyoxal. CHD is also a good fluorescence labelling reagent for determination of mono-carbonyl compounds [25].
RCS (HNE) and carbonyl scavenger solutions were prepared in 100 mM phosphate buffer, pH 7.4. The derivatization reagent solution was prepared with 0.4 g of ammonium acetate and 13 mg of CHD diluted in 10 ml of 100 mM phosphate buffer, pH 7.4. 100 µl of each carbonyl solution (100 µM) was incubated 60 mn at 37 °C with 100 µl of phosphate buffer or 100 µl of carbonyl scavenger solutions at various concentrations in 96 wells microplate. 100 µl of CHD solution was then added in each well and incubated 60 min at 37 °C. The fluorescence was measured at 460 nm, exciting at 395 nm. The % inhibition of CHD adduct formation was calculated for each compound from the fluorescence response versus concentration.
BSA assay
BSA (50 mg/ml) was incubated 24 h at 37 °C with MG (5 mM) or HNE (5 mM) or glucose (20 mM) in 300 mM phosphate buffer (pH 7.4) in the presence or absence of various compounds. Fluorescence of the advanced glycated end-products [26] was monitored using a microplate spectrofluorimeter. Specific fluorescence was employed for each carbonyl: λex=330/λem=392, λex=345/λem=396, λex=330/λem=394 for MG, HNE and glucose respectively. BSA alone was used as a control. The % inhibition of AGE formation in the test sample versus control was calculated for each inhibitor compounds using Graphpad software. IC50 values are expressed in equivalent of aminoguanidine. One-way analysis of variance (ANOVA) was used for the data presented in Fig. 6.
Fig. 6.
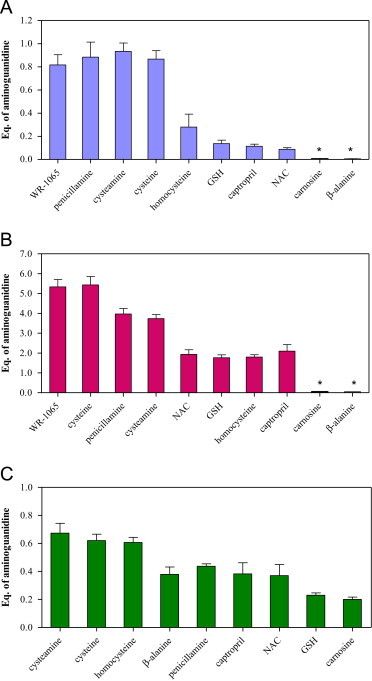
Inhibition of AGE-BSA formation by various compounds. BSA (50 mg/ml) was incubated at 37 °C for 24 h in 0.4 M phosphate buffer, pH 7.4 in the absence and presence of various concentrations of the compounds with (A) 5 mM methylglyoxal, (B) 5 mM 4-Hydroxynonenal, (C) 20 mM glucose. IC50 values of inhibition of AGE-BSA formation are expressed in equivalent of aminoguanidine. Each data point is the mean±SD from triplicate determinations. In addition, one-way ANOVA of the values yielded p<0.01. *p>0.05 versus controls in the absence of scavenger.
High-performance liquid chromatography analysis
Chromatographic analysis was performed using a Waters AllianceTM System equipped with a Waters 2690 XE separation module and a Waters 474 Scanning fluorescence detector controlled by the Waters MilleniumTM Chromatography manager software.
Separation of MG-TRI adduct was achieved at room temperature on a Alltima HP C18 column (250 mm×4.6 mm; 5 µm) with a isocratic flow rate of 0.8 mL min−1. Solvent A is 20 mM phosphate buffer (pH 6.9); solvent B is methanol. The elution programme with linear gradients was the following: 0 min, 10% B; 15 min, 15% B; 16 min 80% B; 20 min 80% B; 21 min 10% B; 27 min reinjection 20 µl. Derivatives were measured at excitation and emission wavelengths of 365 nm and 447 nm, respectively. Quantification is based on peak area.
Results
Cytotoxicity
The cellular toxicity of carbonyl compounds such as G, MG and HNE has been well established in various neuronal cells [27,28], macrophage-derived cell lines [29], and intestinal cells (Caco-2 and HT-29) [30]. Then, we were interested in assessing carbonyl toxicity both on Caco-2 and high sensitive myeloma cell lines, which are extensively used in cell-based assays.
Six mono and dicarbonyl compounds were first subjected to the screening test of cytotoxic activity. Increasing concentrations of all RCS excepted hexanal resulted in a concentration-dependent growth inhibition of the two type of cell culture. The overall cytotoxic potential of the RCS evaluated is evident from data presented in Table 1, which represent the concentration (IC50 value) of the respective carbonyl compounds that inhibited cell viability by 50% as compared to controls. Caco-2 cells are slightly less sensitive toward all carbonyls tested than myeloma. We observed that both cell lines present a retardation of cell growth when exposed to micromolar concentration for all compounds except hexanal which has no cytotoxic effect. Acrolein and HNE are the most cytotoxic on myeloma cell cultures (IC50<10 µM), whereas the toxicity of formaldehyde (IC50≈100 µM) and MG (IC50≈350 µM) are markedly less. Our data are consistent with previous reports describing the toxicities of various aldehydes [31].
Table 1.
Cytotoxicity values of RCS incubated 24 h at 37 °C in mouse myeloma B X63Ag5815 and caco2 cells. Cytotoxicity is determined by measurement of intracellular ATP content using the luciferin–luciferase reaction. Percent viability relative to control is calculated for the various RCS in each cell line and used to calculated IC50 values for growth inhibition.
| Compounds | Cytotoxic concentration IC50 (µM) |
|
|---|---|---|
| Lymphome B | Caco2 | |
| Acrolein | 3–6 | nd |
| Formaldehyde | 62.5–125 | 312–625 |
| Methylglyoxal | 250–500 | 625–1250 |
| 4-HNE | 5–10 | 10–15 |
| glyoxal | 625–1250 | nd |
| hexanal | >10 000 | nd |
Carbonyls scavenging on cell culture
As the cellular toxicity of MG and HNE has been established in our cell experiments and well described in the literature in various cells, we were interested in assessing protection of these carbonyls toxicity by scavengers using mouse myeloma cell culture model. Cells were exposed to MG or HNE at higher concentration than their cytotoxic IC50 values (see Table 1) in the absence or presence of carbonyl scavengers. Hence, cell experiments were carried out with 1 mM of MG and 100 µM of HNE in order to increase cellular toxicity during 24 h exposure. The effectiveness of test compounds was assessed on cell growth. Increasing concentrations of each carbonyl scavenger resulted in increased protection in cell types. No significant cellular toxicity is observed with the tested compounds alone during a 24 h exposure to mouse myeloma cells. Results reported in Table 2, express the concentration (IC50 value) of the scavengers corresponding to 50% of toxicity induced by carbonyls.
Cysteine and penicillamine similarly protect myeloma against growth inhibition by MG and are the most effective. Protective effect of aminoguanidine is slightly less than cysteine or penicillamine but two fold higher than glutathione and more than six fold higher than NAC. Metformin is not protective. Compared to MG toxicity, protective effect of test compounds against HNE toxicity is much weaker (10 fold less in average). Penicillamine and NAC are the most effective against HNE toxicity. This protective effect is likely due to a direct chemical scavenging of the toxic MG and HNE, because pre-incubation of cells with scavengers for 24 h followed by exposure to the RCS in the absence of either compound do not show any protective effect (data not shown).
Screening assays for carbonyl scavengers
We performed screening assays for MG and HNE scavengers using TRI, CHD and NBD-H reagents. These reagents are not naturally fluorescent but their reaction with aldehydes and ketone give highly fluorescent derivatives (Fig. 1). CHD do not react with dicarbonyls, but can easily react with aliphatic monocarbonyls (via Hantzsch reaction) to form highly stable and water-soluble adduct. TRI do not react with monocarbonyls but form fluorescent 6,7-dimethylpterin derivatives with dicarbonyls.
Fig. 1.
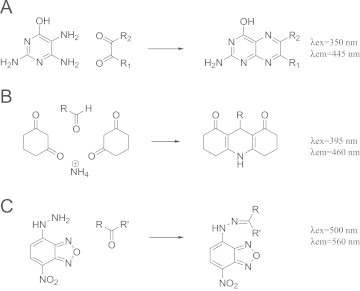
Derivatization of aldehydes with fluorimetric agents. Derivatization of dicarbonyl with TRI to form 6-methylpterin (A), derivatization of monoaldehyde with CHD (B), derivatization of mono and dicarbonyl with NBD-H (C).
In our conditions, the fluorescence response reaches a maximum value after 1 h and 45 min for CHD and TRI respectively. The derivatives products are stable during few hours.
NBD-H is a more convenient probe than TRI and CHD as it react strongly with both mono and dicarbonyl compounds to convert them into fluorescent hydrazones. The fluorescence is easily seen after few seconds and the reaction is complete within 6–8 min, but contrary to TRI or CHD, derivatives products are not so stable. For each assay, RCS and carbonyls were incubated together 1 h before the probe addition. Results are expressed in molar ratio scavenger/carbonyl corresponding to 50% of reagent derivatives inhibition.
Scavenging agents of α,β-dicarbonyls are useful to prevent the formation of AGEs from α,β-dicarbonyl precursors and of α,β-dicarbonyl moieties of glycated proteins [32]. For this purpose, we developed as a first step a screening assay for MG scavengers using TRI reagent. Results in Table 2 show the molar ratio scavenger/MG corresponding to 50% of inhibition of TRI-MG formation. The tested compounds do not react to the same way with MG: penicillamine and cysteine have the highest reactivity toward MG; aminoguanidine is about 10 fold less reactive than penicillamine; metformin and glutathione do not exhibit any MG scavenging ability. No signal is observed for TRI incubated with scavengers alone, except with NAC. Hence, the reaction between MG and TRI was chromatographically tested in various mixtures with fluorometric detection. The derivative products (6,7-dimethylpterin) have fluorescent properties that are not exhibited by TRI or MG alone. The average retention time of TRI-MG derivative is observed at 10.9 min. The signal is diminished in the presence of dicarbonyl scavengers in the same way as results obtained by TRI assay. Fig. 2 shows the chromatograms of derivatized mixture samples prepared according to the TRI assay. 90% of the 6,7-dimethylpterin signal is reduced in the presence of cysteine at 100 µM. NAC do not inhibit the signal of TRI-MG adduct, even at higher concentration (5 mM). An additional unidentified adduct is observed at 15.07 min. Its signal increases with the time (up to 12 h) and reaches about 20% of the 6,7-dimethylpterin signal after 1 h. This additional adduct observed in HPLC experiments explains the high fluorescent signal observed with NAC in the TRI assay. Consequently, the production of fluorescent of unknown origin with NAC precludes its use in the assay with TRI.
Fig. 2.
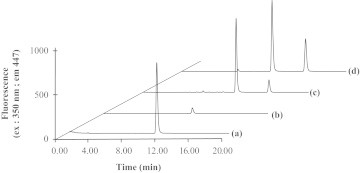
Chromatograms of derivatized mixture samples of TRI (20 µM) and MG (2 µM) after 1 h incubation at room temperature (a); after 1 h incubation in the presence of 100 µM cysteine (b); after 1 h incubation in the presence of 200 µM N-acetylcysteine (c), after 3 h incubation in the presence of 200 µM N-acetylcysteine (d).
Therefore, we performed a screening assay for MG scavenger using NBD-H reagent. Fig. 3a shows the absorbance and fluorescence spectra of NBD-H incubated with MG. NBD-H and MG were reacted for 10 min at 37 °C in pH 7.4 phosphate buffer solution. As shown in Fig. 3a, the absorption spectrum of NBD-MG shows a maximum at 500 nm and the emission spectrum presents a maximum at 560 nm. The fluorescence spectra of NBD-HNE are similar to those exhibited by the NBD-MG derivative. The kinetic of the reaction of NBD-H (200 μmol/L) with 100 μmol/L of MG and HNE was also examined at 37 °C and pH 7.4 (Fig. 3b). The time-course of the appearance of the fluorescence (ex.500 nm; em.560 nm) shows that after 6–8 min of incubation there is no more fluorescence increase. After 6 min a plateau is reaches and the fluorescence intensity is stable for about 60 min. Fluorescence intensity was also monitored when reacting of NBD-H reagent (200 µM) with increasing amounts of HNE and MG (0.05–20 mM) (Fig. 3c). The fluorescence increase observed after reaction of NBD-H with increasing amounts of carbonyl compounds reaches saturation at a molar ratio of carbonyl to NBD-H of approximatively 50:1. This effect could be due to one or more additional adducts as observed by 1H NMR. A complex 1H NMR spectrum was obtained and cannot be unambiguously assigned as the NBD-MG adduct. For a concentration of carbonyls higher than 20 mM, almost no more fluorescence increase appears. A linear plot is observed up to a molar ratio carbonyls/NBD-H of 5 (r2=0.994 and r2=0.993 for MG and HNE respectively). The signal corresponding to the ratio 1:1 reaches about 10% of the maximum intensity observed for the ratio 50:1.
Fig. 3.
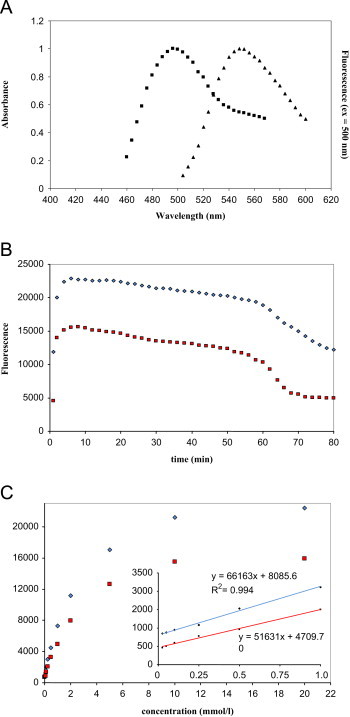
(A) Normalized absorbance and fluorescence spectra of NBD-H (200 µmol/l) after reaction with MG (100 µmol/l) in 100 mM phosphate buffer pH 7.4. (B) Time-course of the appearance of the fluorescence (ex. 500 nm; em. 560 nm) at 37 °C of NBD-H (200 µM) with MG (♦) and HNE (■) at 100 µM. (C) Fluorescence response of MG (♦) and HNE (■) after 10 min incubation at 37 °C with NBD-H with increasing amounts of carbonyls (0.05–20 mM).
Fluorescence response of RCS decreases linearly with the amount of added scavenger. In the case of methylglyoxal (100 µM), the inhibition shows a linear dependance with the amount of penicillamine up to 400 µM (Fig. 4a). The scavenging effect also depends on the time of incubation of scavenger with the RCS. A non-linear decrease is observed with incubation time. The signal is reduced by half for both MG and HNE after 30 min (Fig. 4b).
Fig. 4.
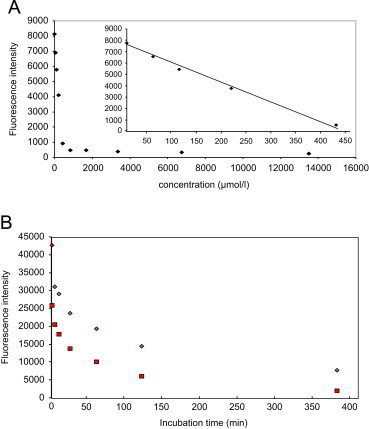
(A) Fluorescence response of MG with various amount of penicillamine according the NBD-H assay. (B) Fluorescence response of MG (♦) and HNE (■) with the incubation time of penicillamine and aminoguanidine respectively.
Results from the NBD-H assay show that cysteine and penicillamine have the best scavenging ability toward MG (Table 2). Aminoguadinine is also good MG scavenger but is about 3 fold less effective than cysteine. Glutathione and NAC do not show strong activity and are more than 11 and 27 fold less effective respectively than cysteine. Interestingly, a good correlation between MG trapping ability measured with the NBD-H and TRI assays is observed (r=0.943). In addition, the correlation coefficient for IC50 values between our cell-based assay and NBD-H assay presents a close interrelation.
Table 2 also express the scavenging ability of reference compounds toward HNE using NBD-H and CHD assays. Molar ratio values for each test compounds are quite similar between the two assays (r=1). Penicillamine is poorly reactive toward HNE and is about 5–7 fold less effective than cysteine which presented the best HNE scavenger ability. Glutathione also exhibits a good scavenging ability and aminoguanidine is a poor HNE scavenger. Elsewhere, the comparability of the screening NBD-H assays and cell culture experiments for reaction of both MG and HNE is reasonably good (r=0.952 and 0.943 respectively). Efficacy of other thiol- and non-thiol-carbonyl pharmacophores was then evaluated for their scavenging capacity toward MG and HNE using our NBD-H assay. Fig. 5 shows their chemical structures. Results with carbonyl scavengers expressed in molar ratio scavenger/carbonyl corresponding to 50% of reagent derivatives inhibition are reported in Table 2. Cysteamine shows high and similar efficiencies toward MG and HNE than cysteine. Homocysteine is more effective toward HNE than MG. WR-1065 do not exhibit a strong MG scavenging ability but is the best HNE scavenger, inhibiting 50% of NBD-H adduct for a molar ratio WR-1065/HNE of 1.2 (p<0.05). Glutathione and NAC also have good scavenging ability toward HNE and are more effective than penicillamine or captopril, which are slightly reactive. The others compounds are not reactive toward HNE. The three best MG scavengers are cysteamine, cysteine and penicillamine (p<0.05). The three best HNE scavengers are WR-1065, cysteamine and homocysteine (p<0.05).
Fig. 5.
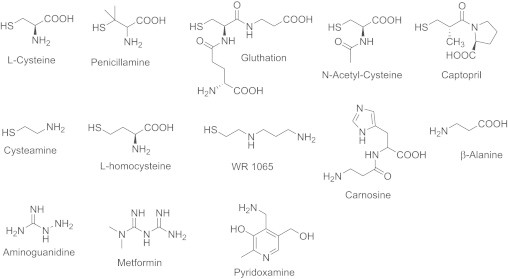
Thiol- and non-thiol pharmacophores used for structure–activity relationship studies on carbonyl scavenger.
Inhibition of AGE formation
Potential inhibition of AGE formation by test compounds was evaluated using MG-, HNE- and glucose-glycated BSA as molecular models by monitoring changes in the intensity of the fluorescence (λex=330–345 nm/λem=392–396 nm) that is commonly used to detect the formation of glycated products [33–35].
Without AGE inhibitors, glycation of BSA (50 mg/ml) with MG (5 mM) or glucose (20 mM) results in rapid production of AGEs, almost within 24 h under the experimental conditions used. The increase in the relative intensity of the fluorescence for glycated BSA is faster with HNE and MG than with glucose during the first 24 h (12 and 4 fold respectively). Hence, compared with HNE and MG, glucose is less active in glycation of BSA. The inhibition effects of various compounds on AGE-BSA formation expressed in equivalent of aminoguanidine are reported in Fig. 6. Of the compounds similarly tested, aminoguanidine is the most effective on the inhibition of MG-glycated. 50% of inhibition is observed for a molar ratio aminoguanidinine/MG of 0.65. Penicillamine, cysteamine and cysteine present a similar activity with IC50 values slightly weaker than aminoguanidine (p<0.05). AGE formation by MG is markedly less inhibited by other compounds except for carnosine and β-alanine, which have no inhibitory effect (p>0.05).
Cysteine and WR-1065 strongly affect AGE formation. 50% of inhibition is observed for a molar ratio cysteine/HNE of 0.55. Effect of cysteine on the inhibition of HNE-glycated is about 1.3 fold higher than penicillamine and cysteamine, and 5 fold higher than aminoguanidine, which show IC50 value of 13.8 mM (p<0.05). The other compounds only fairly inhibit AGE formation by HNE. Carnosine and β-alanine have no inhibitory effect (p>0.05). All tested compounds affect BSA glycated by glucose.
Concerning AGE formation by glucose, aminoguanidine shows the best inhibitory activity with IC50=1.15 mM which is about 5 fold higher than carnosine the weaker inhibitor observed.
Discussion
Many of the carbonyls that are produced result from either lipid peroxidation or glycoxidation are extremely reactive. Unsaturated aldehydes are the most reactive. Reactive carbonyl species induce the carbonyl stress characterized by the formation of adducts and cross-links on proteins, which progressively leads to damages in cells and tissues. Alkenals such as HNE, which are formed during lipid peroxidation, contained a C2–C3 unsaturated bond in addition to the C1 aldehyde, which make the C3 carbon a strong electrophilic center that can undergo Michael addition by nucleophilic groups on proteins, DNA, and lipids thereby causing damage to these molecules. HNE is extremely reactive because of the interaction between the electrophilic double bond, the aldehyde moiety and the hydroxyl group [36]. Reducing sugars such as glucose can form Schiff bases with amino groups on the amino acids lysine and arginine, a reaction know as the Maillard reaction. This can through a series of rearrangements, give rise to fluorescent AGE [1,37]. Oxidation of these glycation products can release dicarbonyls (G and MG), which can also react with proteins, this greatly increases the rate of AGE formation and protein cross-link. Hence, the use of carbonyl scavengers by inhibiting the formation of protein cross-links represents a therapeutically strategy to prevent the RCS-induced damages and carbonyl stress. Most of the carbonyl stress inhibitors used so far have been evaluated in several model using analytical chemistry techniques, which are labor intense, costly, time-consuming, or with slow response time. These methods mainly based on AGE detection [20,38–40], measurement of carbonyl-proteine/peptide adducts [41] and cell viability [10,32], did not highlight a carbonyl-quenching mechanism.
In this context, we have developed a rapid, simple and accurate fluorescent assay adapted to 96 wells microplate, to evaluate the global carbonyl trapping ability in order to design novel agents able to detoxify carbonyl compounds. In general, the fluorescent labelling reagents composed of a highly fluorescent moiety and a tagging moiety, which react with the functional group of analytes to form the fluorescent derivatives, are undesirable because of interference from the fluorescence of the reagents themselves. We have overcome this disadvantage in our method by using NBD-H which is non-fluorescent themselves and react with both mono and dicarbonyls to form highly fluorescent derivatives, and therefore have an advantage as they avoid interfering with the fluorescence of the reagent themselves. In addition, since their excitation and fluorescence wavelengths are at longer wavelengths, detection of carbonyl derivatives has less interference by contaminants, and a highly sensitive detection can be done because of its high reactivity.
To date, the scope of carbonyl-sequestering chemistries tested is quite conventional, including compounds comprising nucleophiles directed against the carbonyl group (e.g., hydrazine, amine, and bisulfite) as well as those targeting the double bond of unsaturated carbonyl compounds (e.g., thiol-containing nucleophiles). It is noteworthy that thiol and amino group nucleophilicity enhance carbonyl scavenger potency. Several RCS scavengers have been proposed, including GSH, NAC, pyridoxamine, aminoguanidine and other nucleophilic compounds able to trap RCS intermediates in AGE formation. The most extensively studied in vitro and in vivo is aminoguanidine, which is a scavenging agent of α,β-dicarbonyls being able to prevent the formation of AGEs from α,β-dicarbonyl precursors [42,43]. Effective scavengers tested in our screen are all characterized by at least one nucleophilic center, such as thiol, imidazole, or primary amine group responsible for the scavenging effect. The scavenging mechanism depends on the target aldehyde and on the nucleophilic group of the scavengers. In the case of MG scavenging, compounds bearing two nucleophilic centers are more efficient due to the subsequent more energy favourable 5- and 6-membered ring formation, as observed in Table 2. It was previously observed that the reaction of MG with cysteine or penicillamine under physiological conditions was very fast and reversible, leading to the formation of heterocyclic 2-acetylthioazolidine and 2-acetylthiazole after rearrangement and oxidation reactions [32,44,45]. A difference can be noticed between cysteine and homocysteine due to the additional methylene group in the last compound, leading to the formation of a less favourable 7-membered ring. The same observation can be suggested for glutathione, where cyclization is not expected to occur. WR-1065 exhibited a moderate activity that could be due to the steric hindrance around the secondary amine and its higher pKa value. Aminoguanidine is mono-functional molecule and cannot lead to the formation of an enthalpy-favoured cycle. However, due to the presence in β-position of an additional heteroatom, this compound behaves as highly reactive nucleophiles (β-effect). NAC and captopril exhibit both the lowest reactivity with MG due to the presence of a single nucleophilic sulfuryl group on the molecule.
Concerning HNE scavenging, the reaction scheme is different from the one for MG, i.e. with a one-step process involved. Thus a Michael adduct is formed by the addition of a nucleophilic sulfur or nitrogen atom to the electrophilic carbon-3 of HNE [46]. The most efficient scavengers are the ones bearing the highest number of nucleophilic groups. For instance, WR-1065 bearing three nucleophilic groups exhibits a smaller IC50 value than cysteamine, cysteine or homocysteine, with two reactive centers, following by NAC and captopril with only one reactive sulfuryl group. Compared to cysteine, the presence of a gem dimethyl group in beta-position of the thiol of penicillamine dramatically reduces its quenching ability. Though aminoguanidine bears four potentially reactive nitrogen atoms, these centers are weak nucleophiles due to the protonation of the guanidine group at physiological pH.
It was shown that the reactivity of the SH groups of the investigated thiols with MG decreased in the order cysteine>GSH>NAC [47]. Elsewhere, the scavenging activity of penicillamine toward the dicarbonyl phenylglyoxal was more efficacious than aminoguanidine under physiological conditions in vitro [32]. Our data (Table 2) are in agreement with these findings indicating the requirement of an α-amino-β-mercaptoethane structure for the highest degree of inhibitory activity. Interestingly penicillamine trapp phenylglyoxal more effectively than the corresponding mono-aldehyde HNE, a finding that may be explained by the expected higher electrophilicity of the α-dicarbonyl compound. The α-dicarbonyl adduct is stable in water, whereas the monocarbonyl-derived thiazolidine adducts form reversibly with subsequent release of the aldehyde [48]. This could explain the higher inhibition efficiency of penicillamine toward MG than HNE observed in our screening assay. Cysteamine quenches in 50% MG for a molar ratio cysteamine to MG of 2:1 according our NBD-H assay. The pathophysiological concentration of MG in blood is in the range of 0.8–3 µM [49,50]. As the scavenging effect is dependant on the time of incubation of scavenger with the RCS and considering other RCS targets, it is difficult to estimate the effective concentration of cyteamine into physiological situations. However, at its therapeutic concentration in plasma (>40 µM at range 1–2 h) [51], cysteamine could exhibit a protective effect against MG.
Millimolar concentrations of inhibitory test compounds are required to observe inhibition of the strong glycation activity of MG, HNE and glucose at the millimolar test concentration chosen to create a rapid glycation reaction. Although aminoguanidine shows an unfavourable toxicity profile in vivo, it was chosen as a reference inhibitor of glycation by α-dicarbonyl scavenging [43,52]. We observed that nucleophilic monoamines such as carnosine and beta-alanine do not suppress formation of AGE fluorescence by MG and HNE in our screening system, most probably undergoing preferential glycation themselves. In this study, we have focused our screening efforts on thiol compounds as another class of nucleophilic agents expected to interfere with glycation. The inhibitory effects of penicillamine as observed in the reaction between MG or HNE and BSA may be linked mechanistically to its very strong reactivity towards both mono and dicarbonyls. Cysteine and the decarboxylated derivative cysteamine show also improved inhibitory activity, whereas homocysteine was less effective. The results obtained are in agreement with earlier studies showing that NAC, cysteine and GSH lowered AGE fluorescence [29,53].
In our cell experiments extracellularly applied, MG and HNE are shown to be toxic to mouse myeloma cells in a dose-dependent manner above concentrations of 150 µM and 2 µM respectively. Although concentrations of MG and HNE used in our cell models were much higher than the physiological ones (0.5–1 μM and 0.1–0.2 µM for MG and HNE respectively in plasma from healthy subjects [54,55]), our findings allow for extrapolation of the results to conditions close physiological. The tested carbonyl scavengers are well tolerated and clearly protected myeloma cells against the toxic action of methylglyoxal and HNE.
The scavenging effects of cysteine and penicillamine in cells experiments are more pronounced (ratio scavenger/RCS<1) than in NBD-H assay. As the scavenging effect depend on the kinetic of the reaction between scavenger and RCS, results of the assays can differ widely based on the time of incubation of scavenger with the RCS (30 min in the NBDH assay and 24 h in cells experiments).
As glutathione, cysteine and penicillamine are hydrophilic compounds with log p value of −4.88, −2.79 and −2.15 respectively; reactions probably occur in cell experiments in the extra cellular environment and explain the protective effect of thiols against MG and HNE toxicity. Cysteine has been also reported to protect against acetaldehyde toxicity in vivo by its carbonyl scavenging activity [56], but its catabolism limits its usefulness in vivo. The activity of aminoguanidine toward α,β-dicarbonyls is in part due to the strong nucleophilic amino group that forms a stable Schiff base with the aldehyde function. However, in vivo this mechanism strongly limits the specificity of aminoguanidine, since it is not only active towards cytotoxic aldehydes, but also towards biogenic and physiological aldehydic compounds such as pyridoxal phosphate and pyruvate [57]. Moreover, aminoguanidine is a poor scavenging agent of α,β-unsaturated aldehydes and do not prevent HNE toxicity as demonstrated in our study. α,β-dicarbonyls and α,β-unsaturated aldehydes are key reactive intermediates of cellular carbonyl stress and therefore are both important targets for therapeutic intervention in pathological conditions.
Much attention needs to be dedicated to elucidating the complex kinetics and mechanism of the reaction of cysteine, penicillamine, aminoguanidine and derivatives with several α-dicarbonyls and unsaturated aldehydes. In our simplified approach, these carbonyls are most probably detoxified by direct chemical trapping, establishing the usefulness of carbonyl scavengers for protection of cells against carbonyl stress. The screening method presented here will allow identification and further optimization of both mono- and di-carbonyl scavenger pharmacophores. A good correlation is observed between our screening methods and cell experiments for all RCS tested. For MG, NBD-H and TRI assay give similar correlation with cell experiments (r=0.952 and r=0.947 respectively). For HNE, NBD-H shows also similar correlation than CHD with cell experiments (r=0.970 and r=0.973 respectively) showing cysteine as the best quencher of both MG and HNE. Although the structures of the NBDH adducts have not been clearly established, the method described is simple, sensitive and reproducible and could be used for preliminary screening of candidate scavengers of RCS.
Acknowledgments
This research was supported by a grant from the FEDER and PACA Regional Council (APRF research program; AdiabaOx 2008-13851).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Thornalley P.J., Langborg A., Minhas H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochemistry. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff S.P., Bascal Z.A., Hunt J.V. Autoxidative glycosylation: free radicals and glycation theory. Prog. Clin. Biol. Res. 1989;304:259–275. [PubMed] [Google Scholar]
- 3.Fu M.X., Requena J.R., Jenkins A.J., Lyons T.J., Baynes J.W., Thorpe S.R. The advanced glycation end product, Nε-(carboxymethyl)lysine, is a product of both lipid peroxidtion and glycoxidation reactions. J. Biol. Chem. 1996;271:9982–9999. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 4.Mizutari K., Ono T., Ikeda K., Kayashima K., Horiuchi S. Photo-enhanced modification of human skin elastin in actinic elastosis by Nε-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J. Invest. Dermatol. 1997;108:797–802. doi: 10.1111/1523-1747.ep12292244. [DOI] [PubMed] [Google Scholar]
- 5.Stadtman E.R., Levine R.L. Chemical modification of proteins by reactive oxygen species, In: I. Dalle-Donne, A. Scaloni and D.A. Butterfield, (Eds.), Redox Proteomics:From protein Modifications to Cellular Dysfunction and Disease. John Wiley & Sons, Inc., Hoboken, New Jersey. 2006:3–23. [Google Scholar]
- 6.Lesgards J.F., Gauthier C., Iovanna J., Vidal N., Dolla A., Stocker P. Effect of reactive oxygen and carbonyls species on crucial cellular antioxidant enzymes. Chem. Biol. Interact. 2011;190:28–34. doi: 10.1016/j.cbi.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Lander H.M., Tauras J.M., Ogiste J.S., Hori O., Moss R.A., Schmidt A.M. Activation of the receptor of advanced glycation end products triggers a p21ras-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 1995;270:10017–10026. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radical. Biol. Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 9.Poli G G., Schaur J. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi S., Shinpo K., Moriwaka F., Makita Z., Miyata T., Tashiro K. Neurotoxicity of methylglyoxal and 3-deoxyglucosome on cultured cortical neurons: synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases. J. Neurosci. Res. 1999;57:280–289. doi: 10.1002/(SICI)1097-4547(19990715)57:2<280::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Mehta R., O'brien P.J. Therapeutic intracellular targets for preventing carbonyl cell death with B vitamins or drugs, In: H. Weiner, E. Maser, R. Lindahl and B Plapp, (Eds.),Enzymology and Molecular Biology of Carbonyl, Metabolism, 13th ed. West Lafayette, Indiana, USA, Purdue University. 2007:1003–1010. [Google Scholar]
- 12.Mehta R., Wong L., O׳Brien P.J. Cytoprotective mechanism of carbonyl scavenging drugs in isolated rat hepatocyte. Chem. Biol. Interact. 2009;178:317–323. doi: 10.1016/j.cbi.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Burcham P.C., Pyke S.M. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehydes scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- 14.Galvanic S., Coatrieux C., Elbaz M., Grazide M.H., Thiers J.C., Parini A., Uchida K., Kamar N., Rostaing L., Baltas M., Salvayre R., Negre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radical. Biol. Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Belkheiri N., Bouguerne B., Bedos-Belval F., Duran H., Bernis C., Salvayre R., Nègre-Salvayre A., Baltas M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010;45:3019–3026. doi: 10.1016/j.ejmech.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y.J., Carini M., Butterfield D.A. Protein Carbonylation. Antioxid. Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoda H., Miyata S., Liu B.F., Yamada H., Ohara T., Suzuki K., Oimomi M., Kasuga M. Inhibitory effects of tenilsetam on the Maillard reaction. Endocrinology. 1997;138:1886–1892. doi: 10.1210/endo.138.5.5151. [DOI] [PubMed] [Google Scholar]
- 18.Onorato J.M., Jenkins A.J., Thorpe S.R., Baynes J.W. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. J. Biol. Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 19.Ruggiero-Lopez D., Lecomte M., Moinet G., Patereau G., Lagarde M., Wiernsperger N. Reaction of metformin with dicarbonyl compounds: possible implications in the inhibition of advanced glycation end product formation. Biochem. Pharmacol. 1999;58:1765–1773. doi: 10.1016/s0006-2952(99)00263-4. [DOI] [PubMed] [Google Scholar]
- 20.Rahbar S., Figarola J.L. Novel inhibitors of advanced glycation endproducts. Arch. Biochem. Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi H., Suzuki Y. High-performance liquid chromatography of aliphatic aldehydes by means of post-column extraction with fluorometric detection. J. Chromatogr. A. 1988;457:299–307. [Google Scholar]
- 22.Kearney J.F., Radbruch A., Liesegang B., Rajesky K.J. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. Immunology. 1979;123:1548–1550. [PubMed] [Google Scholar]
- 23.Salmi C., Loncle C., Vidal N., Letourneux Y., Fantini J., Maresca M., Taieb N., Pagès J.M., Brunel J.M. Squalamine: An appropriate strategy against the emergence of multidrug resistant gram-negative bacteria. PloS One. 2008;3:e2765. doi: 10.1371/journal.pone.0002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinosa-mansilla A., Duran-Mers I., Salinas F. High-performance liquid chromatographic-fluometric determination of glyoxal, methylglyoxal, and diacetyl in urine by prederivatization to pteridinic rings. Anal. Biochem. 1998;255:263–273. doi: 10.1006/abio.1997.2470. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka M., Imado N., Maki T., Banno K., Sato T. Determination of free aliphatic aldehydes in plasma by high-performance liquid chromatography of the 1,3-cyclohexanedione derivatives. Chromatographia. 1996;43:501–506. [Google Scholar]
- 26.Rahbar S., Yerneni K.K., Scott S., Gonzales N., lalezari I. Novel inhibitors of advanced glycation endproducts (part II) Mol. Cell Biol. Res. Commun. 2000;3:360–366. doi: 10.1006/mcbr.2000.0239. [DOI] [PubMed] [Google Scholar]
- 27.Shinop K., Kikuchi S., Sasaki H., Ogata A., Moriwaka F., Tashiro K. Selective vulnerability of spinal motor neurons to reactive dicarbonyl compounds, intermediate products of glycation, in vitro: implication of inefficient glutathione system in spinal motor neurons. Brain Res. 2000;861:151–159. doi: 10.1016/s0006-8993(00)02047-3. [DOI] [PubMed] [Google Scholar]
- 28.Guiotto A., Calderan A., Ruzza P., Osler A., Rubini C., Jo D.G., Mattson M.P., Borin G. Synthesis and evaluation of neuroprotective α,β-unsaturated aldehyde scavenger histidyl-containing analogues of carnosine. J. Med. Chem. 2005;48:6156–6161. doi: 10.1021/jm050507q. [DOI] [PubMed] [Google Scholar]
- 29.Okado A., Kawasaki Y., Hasuike Y., Takahashi M., Teshima T., Fujii J., Taniguchi N. Induction of apoptotic cell death by methylglyoxal and 3-deoxyglucosone in macrophage-derived cell lines. Biochem. Biophys. Res. Commun. 1996;225:219–224. doi: 10.1006/bbrc.1996.1157. [DOI] [PubMed] [Google Scholar]
- 30.kuntz S., Kunz C., Rudloff S. Carbonyl compounds methylglyoxal and glyoxal affect interleukin-8 secretion in intestinal cells by superoxide anion generation and activation of MAPK p38. Mol. Nutr. Food Res. 2010;54:1458–1467. doi: 10.1002/mnfr.200900408. [DOI] [PubMed] [Google Scholar]
- 31.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 32.Wondrak G.T., Cervantes-Laurean D., Roberts M.J., Qasem J.G., Kim M., Jacobson E.L., Jacobson M.K. Identification of alpha-dicarbonyl scavengers for cellular protection against carbonyl stress. Biochem. Pharmacol. 2002;63:361–373. doi: 10.1016/s0006-2952(01)00915-7. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer E., Alegria A., Farré R., Clemente G., Calvo C. Fluorescence, browning index and color in infant formulas during storage. J. Agric. Food. Chem. 2005;53:4911–4917. doi: 10.1021/jf0403585. [DOI] [PubMed] [Google Scholar]
- 34.Atiacevich S.B., Buera M.P. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006;95:423–430. [Google Scholar]
- 35.Liu X., Metzger L.E. Application of fluorescence spectroscopy for monitoring changes in nonfat dry milk during storage. J. Dairy Sci. 2007;90:24–37. doi: 10.3168/jds.S0022-0302(07)72605-X. [DOI] [PubMed] [Google Scholar]
- 36.Witz G. Biological interactions of alpha, beta-unsaturated aldehydes. Free Radical. Biol. Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 37.Beal M.F. Oxidatively modified proteins in aging and disease. Free Radical. Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 38.Makita Z., Radoff S., Rayfield E.J., Yang Z., Skolnik E., Delaney V., Friedman E.A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. New Eng. J. Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 39.Papanastasiou P., Grass L., Rodela H., Patrikarea A., Oneopoulos D., Diamandis E.P. Immunological quantification of AGE׳s in serum of patients on haemodialysis or peritoneal dialysis. Kidney Int. 1994;46:216–222. doi: 10.1038/ki.1994.262. [DOI] [PubMed] [Google Scholar]
- 40.Much G., Keis R., Wessels A., Riederer P., Bahner U., Heidland A., Niwa T., Lemke H.D., Schinzel R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997;35:669–677. doi: 10.1515/cclm.1997.35.9.669. [DOI] [PubMed] [Google Scholar]
- 41.Orioli M., Aldini G., Benfatto M.C., Facino R.M., Carini M. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC–MS/MS profiling in Zucker obese rats. Anal. Chem. 2007;79:9174–9184. doi: 10.1021/ac7016184. [DOI] [PubMed] [Google Scholar]
- 42.Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 43.Thornalley P.J., Yurek-George A., Argirov O.K. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000;60:55–65. doi: 10.1016/s0006-2952(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 44.Lo T.W., Westwood M.E., McLellan A.C., Selwood T., Thornalley P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- 45.Kalapos M.P. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 2008;171:251–271. doi: 10.1016/j.cbi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Kaminskas L.M., Pyke S.M., Burcham P.C. Hydrazinophthalazine drugs efficiently trap the toxic short-chain 2-alkenals acrolein and crotonaldehyde. Org. Biomol. Chem. 2004;2:2578–2584. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- 47.Aćimović J.M., Stanimirović B.D, Todorović N., Jovanović V.B., Mandić L.M. Influence of the microenvironment of thiol groups in low molecular mass thiols and serum albumin on the reaction with methylglyoxal. Chem. Biol. Interact. 2010;188:21–30. doi: 10.1016/j.cbi.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Deshmukh K., Nimni M.A. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. II. Functional groups involved in the interaction process. J. Biol. Chem. 1969;244:1787–1795. [PubMed] [Google Scholar]
- 49.Han Y., Randell E., Vasdev S., Gill V., Gadag V., Newhook L.A., Grant M., Hagerty D. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol. Cell. Biochem. 2007;305:123–131. doi: 10.1007/s11010-007-9535-1. [DOI] [PubMed] [Google Scholar]
- 50.Mirza M.A., Kandhro A.J., Memon S.Q., Khuhawar M.Y., Arain R. Determination of glyoxal and methylglyoxal in the serum of diabetic patients by MEKC using stilbenediamine as derivatizing reagent. Electrophoresis. 2007;28:3940–3947. doi: 10.1002/elps.200700129. [DOI] [PubMed] [Google Scholar]
- 51.Belldina E.B., Huang M.Y., Schneider J.A., Brundage R.C., Tracy T.S. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br. J. Clin Pharmacol. 2003;56:520–525. doi: 10.1046/j.1365-2125.2003.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelstein D., Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- 53.Sharma K.K., Santhoshkumar P. Lens aging: effects of crystallins. Biochim. Biophysi. Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zardaril A., Khuhawar M.Y., Laghari A.J. Capillary GC analysis of glyoxal and methylglyoxal in the serum and urine of diabetic patients after use of 2,3-diamino-2,3-dimethylbutane as derivatizing reagent. Chromatographia. 2009;70:891–897. [Google Scholar]
- 55.Siems W., Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol. Asp. Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 56.Hirayama C., Kishimoto Y., Wakushima T., Murawaki Y. Mechanism of the protective action of thiol compounds in ethanol-induced liver injury. Biochem. Pharmacol. 1983;32:321–325. doi: 10.1016/0006-2952(83)90562-2. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi T., Sugiura M., Hamada Y., Miwa I. In vivo formation of a Schiff base of aminoguanidine with pyridoxal phosphate. Biochem. Pharmacol. 1998;55:1667–1671. doi: 10.1016/s0006-2952(98)00010-0. [DOI] [PubMed] [Google Scholar]


