Abstract
Pulmonary aspergillomas usually occur in pre-existing lung cavities exhibiting local immunodeficiency. As pulmonary aspergillomas only partially touch the walls of the cavities containing them, they rarely come into contact with the bloodstream, which makes it difficult for antifungal agents to reach them. Although surgical treatment is the optimal strategy for curing the condition, most patients also have pulmonary complications such as tuberculosis and pulmonary fibrosis, which makes this strategy difficult. A 72-year-old male patient complained of recurrent hemoptysis and dyspnea, and a chest X-ray and CT scan demonstrated the existence of a fungus ball in a pulmonary cavity exhibiting fibrosis. Although an examination of the patient's sputum was inconclusive, his increased 1-3-beta-D-glucan level and Aspergillus galactomannan antigen index were suggestive of pulmonary aspergilloma. Since the systemic administration of voriconazole for two months followed by itraconazole for one month was ineffective and surgical treatment was not possible due to the patient's poor respiratory function, liposomal amphotericin B was transbronchially administered directly into the aspergilloma. The patient underwent fiberoptic bronchoscopy, and a yellow fungus ball was observed in the cavity connecting to the right B2bi-beta, a biopsy sample of which was found to contain Aspergillus fumigatus. Nine transbronchial administrations of liposomal amphotericin B were conducted using a transbronchial aspiration cytology needle, which resulted in the aspergilloma disappearing by seven and a half months after the first treatment. This strategy could be suitable for aspergilloma patients with complications because it is safe and rarely causes further complications.
Keywords: Liposomal amphotericin B, Pulmonary aspergilloma, Topical treatment, Transbronchial direct administration
Introduction
Aspergillus is commonly found in all environments and causes a variety of diseases depending on the immunological status of the host and the local condition of the lung [1,2]. Pulmonary aspergillomas usually occur in pre-existing lung cavities exhibiting localized immune deficiency [3]. As pulmonary aspergillomas only partially touch the walls of the cavities containing them, they rarely come into contact with the bloodstream, which is the major reason why the systemic administration of antifungal agents is ineffective at eradicating the condition [4]. Most patients with pulmonary aspergillomas exhibit complications such as tuberculosis and pulmonary fibrosis, which makes curative surgical treatment difficult. We report a case of aspergilloma that was successfully treated via the transbronchial administration of liposomal amphotericin B (L-AMB) directly into the aspergilloma using a transbronchial aspiration cytology (TBAC) needle.
Case report
A 72-year-old male patient complained of recurrent hemoptysis and dyspnea, and a chest X-ray and CT scan (Fig. 1) demonstrated the existence of a fungus ball (longest diameter: 28 mm) in a pulmonary cavity exhibiting idiopathic pulmonary fibrosis (IPF)-induced traction bronchiectasis. Although an examination of the patient's sputum was inconclusive, he exhibited a high 1-3-beta-D-glucan level (53.8 pg/mL) and an Aspergillus galactomannan antigen index of 2.2, which were suggestive of pulmonary aspergilloma. Voriconazole (VRCZ) was systemically administered for two months, before itraconazole (ITCZ) was systemically administered for a further month; however, this did not have any effect on the patient's symptoms or the size of his aspergilloma. Since surgical treatment was not possible due to the patient's poor respiratory function, topical treatment was adopted.
Fig. 1.

Chest X-ray (A) and CT scan (B, C) obtained at the first visit demonstrated the existence of a fungus ball (longest diameter: 28 mm) in a pulmonary cavity exhibiting idiopathic pulmonary fibrosis-induced traction bronchiectasis.
Fiberoptic bronchoscopy (FOB) was performed, and a yellow fungus ball was observed in the cavity connecting to the right B2bi-beta (Fig. 2(A)), a biopsy examination of which detected Aspergillus fumigatus.
Fig. 2.
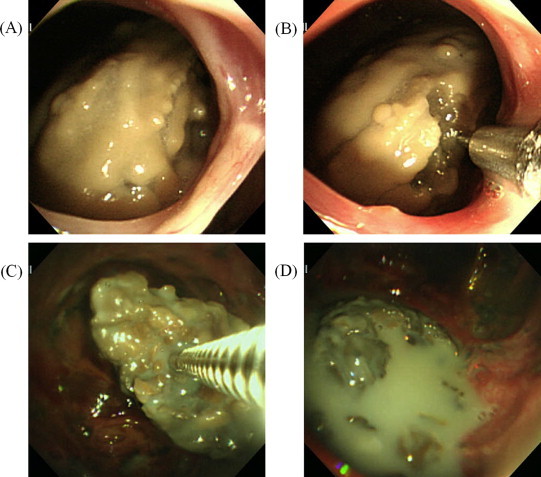
Endoscopic findings of the aspergilloma. Just prior to the first administration of L-AMB, the fungus ball was covered with a yellowish mucinous liquid layer (A), into which L-AMB was administered through a transbronchial aspiration cytology needle (B). An image taken during the sixth round of treatment shows a bare brown aspergilloma without any yellowish coating (C), which broke into fragments after the cavity that contained it had been soaked in L-AMB solution (D).
Since the fungus ball was visible during the FOB, L-AMB was transbronchially administered directly into the aspergilloma using a TBAC needle. One hundred mg/body (2.5 mg/kg) were administered during each treatment, which was equivalent to the dose that would have been administered during systemic therapy. The L-AMB was dissolved in distilled water at a concentration of 10 mg/mL and was administered through a TBAC needle (Fig. 2(B)) at a dose of 0.5 mL per instillation, with each instillation site being different from the previous sites in order to ensure the diffuse and appropriate permeation of L-AMB into the fungus ball. After the procedure, the patient was asked to adopt a right-sided posture for 1 h. The procedure was conducted once a week in the outpatient department for four weeks, and after its safety had been confirmed the L-AMB dose was increased to 200 mg/body, and the procedure was conducted a further three times. By the sixth round of treatment, the fungus ball had diminished in size and turned brown (Fig. 2(C)), and the breakage of the aspergilloma into several parts was observed due to an increase in the internal pressure of the aspergilloma caused by the direct administration of L-AMB (Fig. 2(D)). Surprisingly, during the subsequent treatment period the aspergilloma fragments re-assembled into a single structured fungus ball. At three months after the seventh treatment round, the diameter of the aspergilloma had decreased to 14 mm (Fig. 3(A, B)). Then, the L-AMB dose was reduced to its initial level due to the shrinkage of the fungus ball, and two further rounds of treatment were performed. In the end, the aspergilloma disappeared at two months after the ninth round of treatment; i.e., seven and a half months after the start of treatment (Fig. 3(C, D)).
Fig. 3.

Chest CT scan obtained three months after the seventh administration of L-AMB into the aspergilloma demonstrating the shrinkage of the aspergilloma (longest diameter: 14 mm) (A, B). The aspergilloma disappeared two months after the ninth round of treatment (C, D); i.e., seven and a half months after the initial treatment.
The patient's 1-3-beta-D-glucan level gradually decreased to 28.0 pg/mL, and his Aspergillus galactomannan antigen index was 0.4 at three months after the start of treatment.
During the study period, the fibrotic pulmonary cavity enlarged (Figs. 1 and 3), and the patient's pulmonary function deteriorated in accordance with the progression of his IPF. Chemically-induced bronchitis and drug-induced interstitial lung disease were considered to be potential side effects of the abovementioned treatment regimen, but neither of these conditions developed. In addition, no L-AMB-related renal dysfunction or hypokalemia were observed.
The abovementioned treatment was so effective that the patient's hemoptysis disappeared within two weeks and his aspergilloma shrank within three months and had completely disappeared within seven months.
Discussion
Aspergillus is a ubiquitous fungus, and all human beings breath in its conidia during everyday life. However, any conidia that attach to the lower respiratory tract are removed by mucociliary clearance, and those that reach the alveoli are phagocytosed by alveolar macrophages [5]. Furthermore, even when the conidia sprout hyphae they are sterilized by neutrophils [6], resulting in healthy hosts escaping from fungal infection. Aspergillus can cause a variety of diseases depending on both the immunological status of the host and the local condition of the lung [1,2]. Pulmonary aspergillomas usually occur in pre-existing lung cavities exhibiting local immunodeficiency, such as those caused by tuberculosis, bronchiectasis, emphysema, pneumoconiosis, sarcoidosis, and interstitial pneumonia [3].
Pulmonary aspergillomas are classified into simple and complex aspergillomas [7], and the latter type is more prevalent because it is associated with underlying diseases. Surgery such as cavernostomy with muscle transposition, partial resection, segmentectomy, or lobectomy [9–11] is recommended as a curative treatment [8]. Although less invasive surgical strategies such as cavernostomy have been developed, underlying diseases can make the optimal surgical procedure very difficult.
For those patients who are unsuitable for surgery, amphotericin B (AMPH-B), L-AMB, VRCZ, ITCZ, and micafungin sodium are utilized as systemic antifungal agents because they are effective against invasive aspergillosis and chronic necrotizing pulmonary aspergillosis [12–14]; however, there is no evidence from randomized controlled studies to support the use of these drugs against aspergillomas, with some reports suggesting that systemic AMPH-B administration is ineffective [15] and oral ITCZ only achieves limited outcomes [16]. The optimal treatment duration has not been established and varies from several months to years, even in cases in which treatment is effective. The limited response rates of systemic antifungals are due to poor drug delivery to saprophytic fungus balls [4], and severe side effects can sometimes lead to treatment cessation. However, all treatments should aim to cure the condition for the reasons outlined below. Balls of fungal mycelia are not static and can invade the surrounding lung tissue, leading to chronic necrotizing pulmonary aspergillosis [3], although spontaneous aspergilloma lysis occurs in 7–10% of cases [17]. Furthermore, hemoptysis of bronchial arterial origin can arise and is sometimes lethal in partially treated cases, with the mortality rate ranging from 2 to 26% [18].
When systemic antifungal agents fail to eradicate an aspergilloma, resulting in continuing hemoptysis and fever, topical treatment with antifungals should be considered [19] and could be a viable option in patients with life-threatening aspergilloma-induced hemoptysis who exhibit risk factors for a poor prognosis [20]. There are two approaches that can be employed to reach aspergillomas during topical treatment, the transbronchial and percutaneous approaches. Both methods involve the instillation of antifungals into the target cavity to soak the fungus ball. Percutaneous approaches have been vigorously investigated [21–23]; however, they can sometimes cause fungal spread into the thoracic space, resulting in fungal empyema, which should be carefully avoided. The most commonly used antifungal agent is AMPH-B, but its reported efficacy varies from study to study, ranging from 65 to 80% [19,21–23]. Although topical treatments have been described by several investigators, no evidence-based conclusion regarding the optimal approaches and antifungals have been established.
We adopted a transbronchial approach in the current case since the fungus ball was visible during FOB. There is one previous report about the instillation of AMPH-B into an aspergilloma-containing cavity using the balloon occlusion technique [24]. Since AMPH-B can irritate bronchi and can cause chemically-induced bronchitis or drug-induced interstitial lung disease, this method is not applicable to patients with underlying IPF, as it can lead to the acute exacerbation of their IPF.
Therefore, we decided to transbronchially administer L-AMB directly into the aspergilloma in order to ensure effective drug delivery. L-AMB is a unilamellar liposomal formulation of AMPH-B, in which AMPH-B is securely incorporated within a liposomal bilayer, which disintegrates when it comes into contact with fungal cell walls and releases AMPH-B at sites expressing ergosterol [25,26]. L-AMB does not diffuse through blood vessel endothelia, which prevents it damaging normal tissues. On the other hand, at infection sites exhibiting increased permeability it spreads through the endothelium toward the fungal surface, which is advantageous for systemic drug delivery [27]. L-AMB is considered to be less irritable to bronchi and lung tissue as it has no detrimental effects on the surface activity of surfactants when administered topically [28].
In the present case, we decided to directly inject the L-AMB into the fungus ball rather than employ intracavitary instillation since the direct injection method ensures that the L-AMB binds to the cell walls of the fungus, leading to the disintegration of the fungus ball.
Surprisingly, after the fungus ball had been broken into fragments by the L-AMB treatment (Fig. 2(D)) the remaining fragments recombined into a structured fungus ball each time. This suggests that there is a tendency towards fungus ball formation in pulmonary Aspergillus infections and provides clues regarding the mechanism responsible for this phenomenon.
The creation of pulmonary aspergillomas is said to start with the attachment and proliferation of fungi on the pulmonary or bronchus wall due to localized immunodeficiency [1–3]. During the initial phase, the thickening of the pulmonary wall and the detachment of parts of the wall into the cavity are observed, and the detached necrotic fragments then act as the nucleus for the creation of a fungus ball [1–3,6]. Taking this information into account, directly administering a drug into a fungus ball might both mechanically destroy it and invade the fungal structure, resulting in smaller segments being left intact each time, although these intact segments act as the nucleus for the formation of a smaller fungus ball. When the fungus ball becomes small enough to allow L-AMB to fully diffuse through the broken fragments, the remaining fragments are too small to act as a fungus ball nucleus, resulting in the disappearance of the fungus ball.
The treatment strategy employed in the present case did not result in the proliferation of Aspergillus from the original cavity to other bronchi or alveoli, and chemically-induced bronchitis and pneumonia, which have been reported to occur during AMPH-B instillation, were not observed either.
The treatment strategy described in this report seems to be suitable for patients with complications, especially those with pulmonary fibrosis, in terms of both the effectiveness of drug delivery and the scarcity of side effects.
Conflicts of interest statement
None of the authors have any conflicts of interest to declare.
References
- 1.Hope W.W., Walsh T.J., Denning D.W. The invasive and saprophytic syndromes due to Aspergillus spp. Med Mycol. 2005;43(Suppl. 1):S207–S238. doi: 10.1080/13693780400025179. [DOI] [PubMed] [Google Scholar]
- 2.Daly P., Kavanagh K. Pulmonary aspergillosis: clinical presentation, diagnosis and therapy. Br J Biomed Sci. 2001;58:197–205. [PubMed] [Google Scholar]
- 3.Denning D.W. Chronic forms of pulmonary aspergillosis. Clin Microbiol Infect. 2001;7(Suppl. 2):25–31. doi: 10.1111/j.1469-0691.2001.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 4.Israel H.L., Ostrow A. Sarcoidosis and aspergilloma. Am J Med. 1969;47:243–250. doi: 10.1016/0002-9343(69)90150-8. [DOI] [PubMed] [Google Scholar]
- 5.Philippe B., Ibrahim-Granet O., Prévost M.C., Gougerot-Pocidalo M.A., Sanchez Perez M., Van der Meeren A. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoham S., Levitz S.M. The immune response to fungal infections. Br J Haematol. 2005;129:569–582. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 7.Belcher J.R., Plummer N.S. Surgery in broncho-pulmonary aspergillosis. Br J Dis Chest. 1960;54:335–341. [Google Scholar]
- 8.Stevens D.A., Kan V.L., Judson M.A., Morrison V.A., Dummer S., Denning D.W. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:696–709. doi: 10.1086/313756. [DOI] [PubMed] [Google Scholar]
- 9.Shirakusa T., Ueda H., Saito T., Matsuba K., Kouno J., Hirota N. Surgical treatment of pulmonary aspergilloma and Aspergillus empyema. Ann Thorac Surg. 1989;48:779–782. doi: 10.1016/0003-4975(89)90670-x. [DOI] [PubMed] [Google Scholar]
- 10.Oakley R.E., Petrou M., Goldstraw P. Indications and outcome of surgery for pulmonary aspergilloma. Thorax. 1997;52:813–815. doi: 10.1136/thx.52.9.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J.C., Chang Y.L., Luh S.P., Lee J.M., Lee Y.C. Surgical treatment for pulmonary aspergilloma: a 28 year experience. Thorax. 1997;52:810–813. doi: 10.1136/thx.52.9.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbrecht R., Denning D.W., Patterson T.F., Bennett J.E., Greene R.E., Oestmann J.W. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 13.Kohno S., Masaoka T., Yamaguchi H., Mori T., Urabe A., Ito A. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand J Infect Dis. 2004;36:372–379. doi: 10.1080/00365540410020406. [DOI] [PubMed] [Google Scholar]
- 14.Jain L.R., Denning D.W. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J Infect. 2006;52:e133–e137. doi: 10.1016/j.jinf.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Hammerman K.J., Sarosi G.A., Tosh F.E. Amphotericin B in the treatment of saprophytic forms of pulmonary aspergillosis. Am Rev Respir Dis. 1974;109:57–62. doi: 10.1164/arrd.1974.109.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Campbell J.H., Winter J.H., Richardson M.D., Shankland G.S., Banham S.W. Treatment of pulmonary aspergilloma with itraconazole. Thorax. 1991;46:839–841. doi: 10.1136/thx.46.11.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerman K.J., Christianson C.S., Huntington I., Hurst G.A., Zelman M., Tosh F.E. Spontaneous lysis of aspergillomata. Chest. 1973;64:697–699. doi: 10.1378/chest.64.6.697. [DOI] [PubMed] [Google Scholar]
- 18.Glimp R.A., Bayer A.S. Pulmonary aspergilloma. Diagnostic and therapeutic considerations. Arch Intern Med. 1983;143:303–308. doi: 10.1001/archinte.143.2.303. [DOI] [PubMed] [Google Scholar]
- 19.Yamada H., Kohno S., Koga H., Maesaki S., Kaku M. Topical treatment of pulmonary aspergilloma by antifungals. Relationship between duration of the disease and efficacy of therapy. Chest. 1993;103:1421–1425. doi: 10.1378/chest.103.5.1421. [DOI] [PubMed] [Google Scholar]
- 20.Rumbak M., Kohler G., Eastringe C., Winer-Muram H., Gavant M. Topical treatment of life threatening haemoptysis from aspergillomas. Thorax. 1996;51:253–255. doi: 10.1136/thx.51.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munk P.L., Vellet A.D., Rankin R.N., Müller N.L., Ahmad D. Intracavitary aspergilloma: transthoracic percutaneous injection of amphotericin gelatin solution. Radiology. 1993;188:821–823. doi: 10.1148/radiology.188.3.8351355. [DOI] [PubMed] [Google Scholar]
- 22.Jackson M., Flower C.D., Shneerson J.M. Treatment of symptomatic pulmonary aspergilloma with intracavitary instillation of amphotericin B through an indwelling catheter. Thorax. 1993;48:928–930. doi: 10.1136/thx.48.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giron J., Poey C., Fajadet P., Sans N., Fourcade D., Senac J.P. CT-guided percutaneous treatment of inoperable pulmonary aspergillomas: a study of 40 cases. Eur J Radiol. 1998;28:235–242. doi: 10.1016/s0720-048x(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki Y., Yokomura I., Ueda M., Hashimoto S., Mizobuchi K., Arimoto T. Transbronchial intracavitary infusion therapy with balloon catheter for pulmonary aspergilloma. J Jpn Soc Bronchol. 1996;18:584–589. [Google Scholar]
- 25.Adler-Moore J.P., Proffitt R.T. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J Liposome Res. 1993;3:429–450. [Google Scholar]
- 26.Adler-Moore J., Proffitt R.T. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002;49(Suppl. 1):21–30. doi: 10.1093/jac/49.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 27.Adler-Moore J.P. In vivo and in vitro evidence for reduced toxicity and mode of action of AmBisome™. Bone Marrow Transpl. 1993;12(Suppl. 4):S146. [Google Scholar]
- 28.Ruijgrok E.J., Vulto A.G., Van Etten E.W. Efficacy of aerosolized amphotericin B desoxycholate and liposomal amphotericin B in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. J Antimicrob Chemother. 2001;48:89–95. doi: 10.1093/jac/48.1.89. [DOI] [PubMed] [Google Scholar]


