Abstract
Progesterone is an ovarian steroid hormone that is essential for normal breast development. The actions of progesterone are largely mediated through binding to its cognate steroid hormone receptor, the progesterone receptor (PR). PR isoforms exist in the nucleus and transcriptionally activate genes necessary for proliferation and survival (classical role). Cytoplasmic or membrane-associated PR exists in the cytoplasm where it participates in protein complexes with signaling molecules and other steroid hormone receptors capable of rapid activation of cytoplasmic protein kinase cascades. This review details the extra nuclear scaffolding actions of PR with c-Src and MEK1, the upstream components of MAP kinase modules.
Keywords: Progesterone receptor, CD domain, Scaffold, Rapid kinase activation, c-Src, MEK1
1. Introduction
Steroid receptors are critical activators of gene transcription in response to binding of their cognate ligands. These transcriptional responses mediate a diverse array of cellular functions, including metabolism, cell cycle progression and survival signaling. The actions of steroid receptors in the nucleus have been well defined, and the required domains necessary for these interactions have been well studied (Fig. 1). However, extra-genomic functions of steroid receptors have suggested that many of these receptors have critical cytoplasmic actions, which are functionally separable from the nuclear actions carried out by the same receptors. The importance of extra-nuclear functions has been recently defined for many steroid receptors, including the estrogen, androgen, glucocorticoid, mineralocorticoid, and progesterone receptors. This review will discuss domains within the progesterone receptor (PR) that are responsible for initiating cellular signaling and scaffolding interactions with cytoplasmic protein kinases.
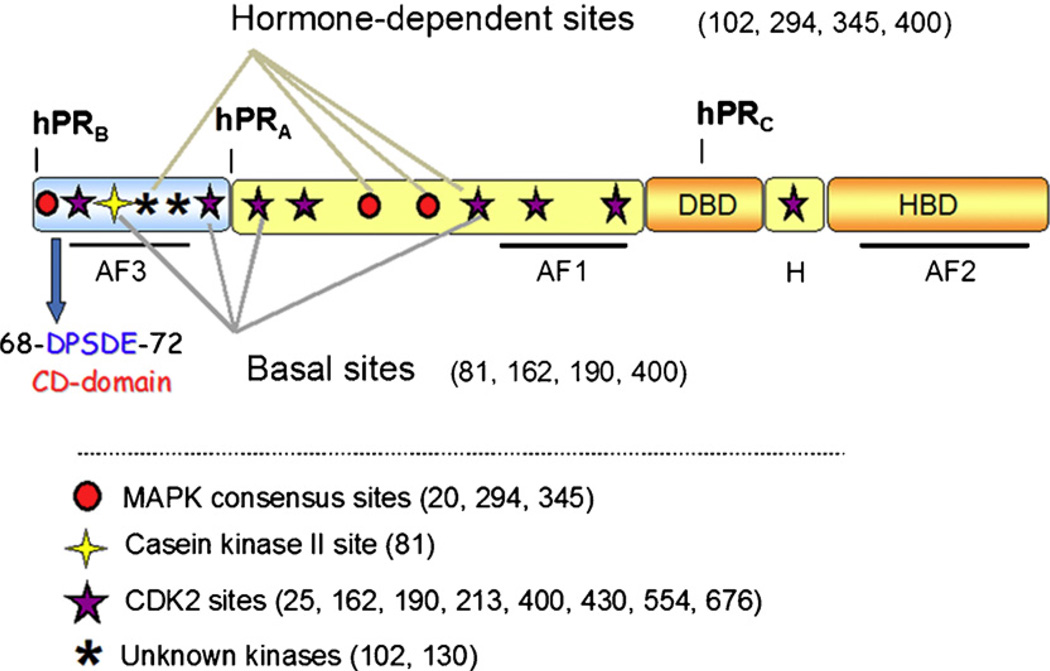
Fig. 1.
Schematic of progesterone receptor. All three PR isoforms (PR-A, PR-B and PR-C) are transcribed from the same gene, containing distal and proximal promoters, and created via differential use of two internal translational start sites. Shown are three transcription activation function (AF) domains, the B-upstream segment (BUS), the DNA-binding domain (DBD), the hinge region (H) and the hormone-binding domain (HBD). PR is phosphorylated basally, as well as in response to hormone. Shown here are the various sites of phosphorylation as determined in vitro and in vivo, and kinases that are likely responsible for phosphorylation at these sites. The putative common docking (CD) domain is located within the BUS, a segment unique to PR-B. This is the proposed site for MEK1 binding to PR.
2. Progesterone receptor structure and function
PR exists in three isoforms as a result of transcription from a single gene and the use of three different translational start sites [1]. PR-B is the full-length form of the protein (116 kDa), PR-A (94 kDa) lacks the initial N-terminal 165 amino acids, coined the B-upstream segment (BUS) as this region is unique to PR-B (Fig. 1), and PR-C (60 kDa) contains an even larger N-terminal truncation that disrupts the DNA-binding domain (DBD). Thus, only PR-A and PR-B contain the critical components for nuclear receptor function, including the ligand-binding domain (LBD), hinge region (H), DNA-binding domain, and two of the three activating function domains (AF). While PR-C is not a functional transcription factor, it acts to inhibit PR-B transactivation in the uterus [2] or potentiate the transcriptional effects of the other PR isoforms in the breast [3]. Although both transcriptionally active PR isoforms (PR-A and PR-B) are commonly co-expressed, studies in PR knockout mice have shown that PR-B is required for mammary gland development, while PR-A is essential for uterine development [4–7]. The isoforms can function independently, or as heterodimers; they have different transcriptional activities and can target different subsets of promoters [8]. Unliganded PR rapidly shuttles between the cytoplasm and nucleus; cytoplasmic PR is associated with chaperones and heat shock proteins, including Hsp70 and Hsp90. Following ligand (progesterone or synthetic progestins, such as R5020) binding, PR is freed from these associations, undergoes dimerization and translocation to the nucleus. Once localized in the nucleus, PR activates transcription of PR-target genes, either directly, through binding to progesterone response elements (PREs), or indirectly through tethering interactions with other transcription factors (AP1, SP1, STATs).
PRs undergo considerable post-translational modification, including phosphorylation, acetylation, sumoylation, and ubiquitination [9,10]. There are at least 14 serine residues in PR that are known to be phosphorylated in vitro or in vivo (Fig. 1) [11–17]. These phosphorylation events can occur basally or in response to activation via hormone binding or kinase activation. PR phosphorylation is thought to be a modifier of receptor function, with phosphorylation effecting subcellular localization [18], transcriptional activity [13,19,20], receptor turnover [10,13,14], protein complex formation and target-gene specificity [18,21]. Kinases that are known to phosphorylate PR either in vitro or in vivo include mitogen-activated protein kinase (MAPK), casein kinase II and cyclin-dependent kinase 2 (cdk2) [14,22].
In addition to the above-mentioned canonical interaction domains that are critical to supporting nuclear gene transcription, kinase interaction domains have been identified within PR (Figs. 1 and 4) and act as critical regions required for rapid activation of cytoplasmic protein kinases in response to progestins [23–25]. This rapid signaling is fully integrated with the genomic actions of PR, as progesterone-activated protein kinases in turn phosphorylate PR and its co-regulatory molecules.
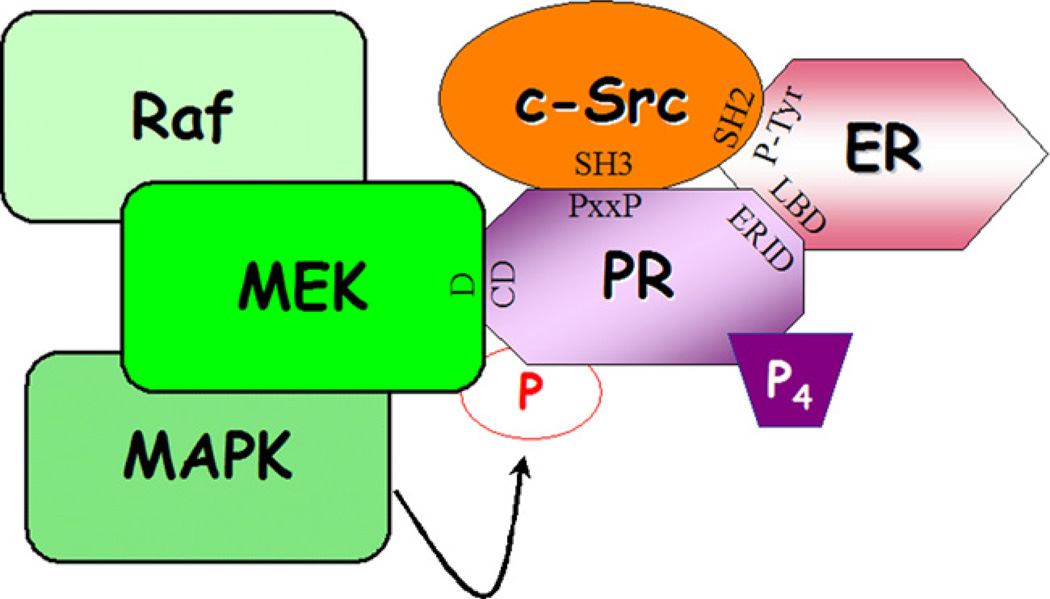
Fig. 4.
Model of PR-scaffolding interactions. Previously reported interactions between PR (PxxP, proline-rich domain) and/or ER (phospho-tyrosine 537) and c-Src (SH3 domain with PR, SH2 domain with ER), as well as interactions between PR (ERID, estrogen receptor interaction domain) and ER (LBD, ligand-binding domain) have been shown to be necessary for progesterone-induced c-Src/MAPK activation [23–25,36,37]. Additionally, complex formation between PR (CD domain) and MEK1 (D domain) may be necessary for MEK1 docking and subsequent PR posttranslational modification and activation.
3. ER/PR-dependent c-Src/MAPK activation mediated by ER-interaction domains of PR
Migliaccio et al. first showed progesterone-induced rapid activation of the MAPK pathway through a c-Src-dependent mechanism in 1998 [24]. In T47D breast cancer cells, following treatment with the synthetic progesterone, R5020, activated c-Src and ERK2 were detected within 2–5 min of hormone treatment. Interestingly, this MAPK activation was blocked following treatment with anti-estrogens, a result that was not anticipated following treatment with a synthetic progesterone. These data implied that there was a requirement for the estrogen receptor in this progesterone-dependent activation of MAPK. Further protein–protein interaction experiments using endogenous (in T47D cells) or exogenous (transfected into COS-7; monkey kidney fibroblast cells) proteins showed that there was an interaction between c-Src, PR and the estrogen receptor (ER). ER appeared to be required for the interaction of PR with c-Src, as well as the subsequent activation of MAPK, as no direct interaction was detected between c-Src and PR under these conditions. These data implicated ER as a linker molecule between c-Src and PR. Further data showed that in T47D cells, MAPK activation following treatment with R5020 approached the levels observed following treatment with epidermal growth factor (EGF), a growth factor known to potently activate Ras/Raf-dependent signaling to MAPK. Importantly, MAPK activation following treatment with progestin was associated with an increase in T47D breast cancer cell growth. In a subsequent report from this group [25], two regions within PR were found to directly interact with ER, ERID-I and –II (ER-interacting domains I and II). Following treatment with synthetic progesterone, ER interaction with the SH2-domain of c-Src mediated activation of MAPK [25]. Interestingly, although they were previously unable to detect an interaction between PR and c-Src in vivo, in this report a direct interaction was detected between these two proteins in vitro that was facilitated by PR’s proline-rich domain (discussed below [23]). However, this interaction was not sufficient to activate downstream components of the MAPK pathway (i.e. ERK2).
4. PR-dependent c-Src/MAPK activation mediated by PR proline-rich domain
Separate work by Boonyaratanakornkit et al. implicated the ER-independent actions of PR in rapid activation of c-Src/MAPK following treatment with R5020. This group found no requirement for direct binding of ER in the PR/c-Src complex [23]. Instead, this paper demonstrated a direct interaction between the SH3 domain of c-Src and an N-terminal proline-rich domain of PR in vitro, and this interaction was confirmed in vivo in T47D cells. Additional in vitro experiments showed that PR and ER independently interact with c-Src through distinct SH3 (PR) and SH2 (ER) domains of c-Src. Using activation of Hck, a member of the Src family kinases, as a model, they showed that purified ligand-bound PR could activate purified Hck in vitro, with no potentiation of activity following the addition of ERα (bound to estradiol), indicating that this effect was independent of ER. Interestingly, in this study progesterone-dependent activation of c-Src potentiated progestin-induced cell growth inhibition of normal mammary epithelial cells, in contrast to what has been observed previously in breast cancer cells (see above). A later report from this group confirmed that in T47D breast cancer cells, R5020 induced a transient increase in cell proliferation that was dependent on the SH3 domain in PR [26]. These differences in the effects of progestins on cell proliferation may be cell type dependent based on the model cell lines used in these assays, and perhaps due to their respective p53 status. Cells in which R5020-dependant activation of MAPK lead to an increase in cell growth (T47D; breast cancer cells) are also mutant for p53 [24,26,27], which may create amore permissive environment for uncontrolled cell growth. In contrast, MCF12A (normal mammary epithethial) cells, which express wild-type p53, were growth-inhibited upon progestin-induced activation of c-Src/MAPK [23]. The presence of wt p53 in these cells may have contributed to enforced check-point control, as progestins induce the p53 target gene, p21. Additionally, Boonyaratanakornkit et al. [23] observed that the potency of MAPK activation was modest (~25% of EGF-induced levels) following treatment with R5020 relative to that induced by EGF. Differences in the strength and duration of MAPK signaling are known to alter the proliferative response; i.e. proliferation is associated with sustained MAPK signaling in fibroblast models [28,29].
Both models suggest that PR plays a significant role in the activation of c-Src and MAPK following treatment with ligand. However, different model systems (in vitro vs. in vivo, T47D vs. MCF12A) were used by the two groups, making direct comparisons difficult. Both models revealed an interaction, at least in vitro, between PR and c-Src through the proline-rich domain of PR, with the disagreement lying in the requirement of this association for ligand-dependent MAPK activation, as well as the subsequent potency of c-Src-dependent MAPK activation. Lastly, the role of ER in progesterone-mediated MAPK activation remains a large inconsistency between the two models.
Work from these groups indicates that PR contains distinct domains (proline-rich, ERID-I and -II) through which it may interact with (either directly or indirectly) membrane-associated or cytoplasmic protein kinases, thereby activating downstream signaling cascades. The presence of numerous protein kinase-interacting and scaffolding domains underscores the significant role that PR plays in extra-nuclear signaling, specifically in activating ERK1/2 MAPKs.
5. PR interacts with MEK1
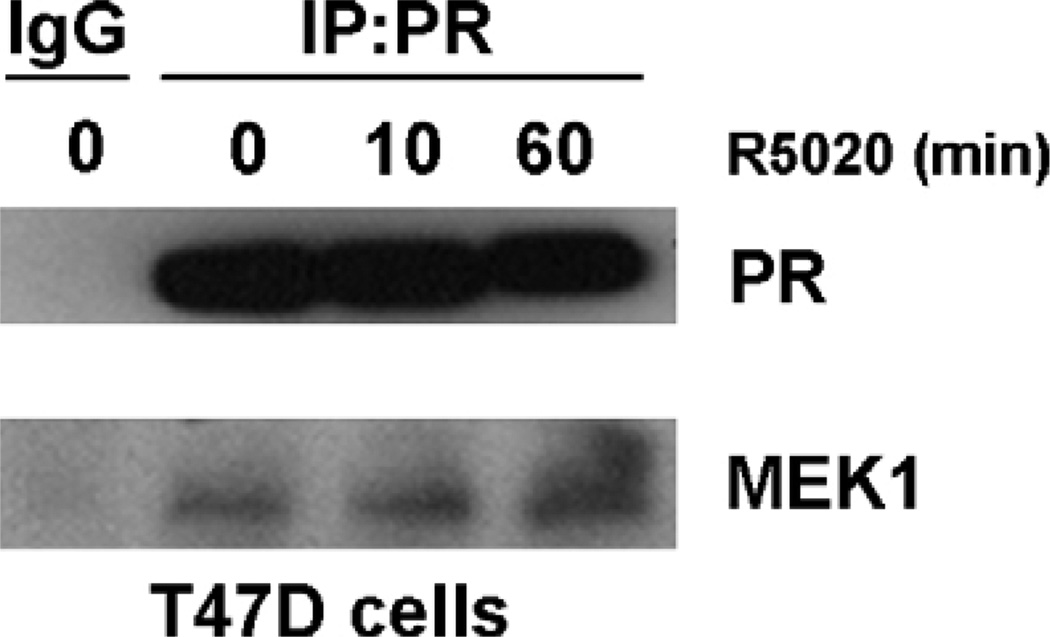
Recent data from our lab shows that PR interacts with an additional component of the MAPK signaling cascade, the upstream kinase activator MEK1. Protein–protein interactions indicate that there is an interaction in vivo between PR and MEK1 in T47D-YB cells, and that this interaction is ligand-independent (Fig. 2). The presence of a PR-MEK1 complex promoted an investigation into putative kinase binding domains that could mediate this interaction. We have identified a putative common-docking (CD) domain in the N-terminal BUS of PR-B (Figs. 1 and 3). CD domains are regions through which MAPKs, such as ERK, interact with their upstream activators, MAPK kinases (MKKs), such as MEK1 [30,31]. MEK1 binding through these domains present on MAPKs, also called cytoplasmic retention signals, may serve to anchor MAPKs in the cytoplasm of unstimulated cells [30]. CD domains are characterized by a cluster of negatively charged amino acids (DxxD/E) that are thought to interact with positively charged amino acids on the opposing protein. These domains mediate interactions not only with MKKs, but MAPK-phosphatases (MKPs) and other kinases downstream of MAPKs that contain the positively charged “D” domain as well [31,32]. CD domains are conserved throughout the MAPK family (Fig. 3), and in part determine the binding specificity of MAPKs with their respective MKKs. Within PR, the putative CD domain, DPSDE (amino acids 68–72; see Figs. 1 and 3), is an exact match to the CD domain of ERK2, suggestive of a mechanism for PR direct binding to MEK1 and/or PR localization to the cytoplasm. We detected an endogenous interaction between PR and MEK1; the necessity of the PR CD domain for mediating this interaction and its functional significance are currently under investigation. PR/MEK1 interactions may stabilize signaling complexes and/or act to recruit MAPK, in order to mediate post-translational phosphorylation events (such as phosphorylation at nearby PR S294 and S345) required for nuclear PR actions. Alternatively, this interaction may provide a scaffold to position MEK1 in proximity to key components of the MAPK signaling pathway (c-Src, EGFR, ERK2) known to be activated by ligand-bound PR.
Fig. 2.
Progesterone receptor interacts with MEK1. Following a 24 h incubation in serum-free media, T47D cells were treated for 0–60 min with 10nM R5020, after which total cell lysates were immunoprecipitated with PR antibody (or rabbit IgG as a control) and the resulting protein complexes were analyzed using Western blotting.
Fig. 3.
CD domains of PR and MAPK family members. The amino acid sequences of the CD domains of PR compared to the members of the MAPK family are shown here. Boxed are the negatively charged amino acids that are predicted to interact with positively charged amino acids located in the D domain of partner binding proteins. Adapted from Tanoue et al. [35].
Another member of the nuclear receptor family, PPARδ, was recently shown to interact with MEK1 through a domain similar in sequence to the putative CD domain identified in PR [33]. This interaction between PPARδ and MEK1 mediates PPARδ nuclear export and, therefore, downregulation of PPARδ and its nuclear actions. The interaction between PR and MEK1 may serve a similar function as a potential mechanism to regulate PR nucleo-cytoplasmic shuttling.
6. PR as a scaffold protein
The presence of kinase binding domains within PR (praline-rich, CD domain) suggests that PR may serve as a scaffold protein, providing docking interactions that coordinate the activation of downstream signaling pathways with PR trans-activation (Fig. 4). Clearly, these protein complexes provide a mechanism for signaling specificity that is dictated by the presence of PR [34]. Alternatively, PR complexes that contain either c-Src or MEK1 may require PR as a critical component of a larger protein complex whose assembly is required for sufficient kinase activation and signaling. These data suggest that multimeric protein complexes that are assembled to facilitate kinase signaling may be composed of amore diverse array of proteins than was previously thought. Moreover, the finding that ER is a part of this complex [24,25] suggests that the view of nuclear receptors functioning independently from one another needs revisiting. The identification of additional PR-interacting proteins will shed some light on the proposed model of PR as a scaffold protein. The scaffolding properties of steroid hormone receptors are predicted to be relevant to breast cancer progression characterized by high protein kinase activities, underscoring the need to fully characterize the crucial domains. Current therapies aimed at blocking the transcriptional action of these receptors may fail to target their extra-nuclear (i.e. scaffolding) actions.
References
- 1.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PRC), forms heterodimers with PRB) but interferes in PRB)- DNA binding. J Steroid Biochem Mol Biol. 1997;62(4):287–297. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 3.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 4.Conneely OM, Mulac-Jericevic B, Lydon JP, De FJ, Mayo Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179(1/2):97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 5.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 6.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100(17):9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci USA. 1998;95(2):696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 9.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation de-represses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 10.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97(3):1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao KV, Peralta WD, Greene GL, Fox CF. Cellular progesterone receptor phosphorylation in response to ligands activating protein kinases. Biochem Biophys Res Commun. 1987;146(3):1357–1365. doi: 10.1016/0006-291x(87)90799-6. [DOI] [PubMed] [Google Scholar]
- 12.Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276(11):8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 13.Takimoto GS, Hovland AR, Tasset DM, Melville MY, Tung L, Horwitz KB. Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem. 1996;271(23):13308–13316. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- 14.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319(Pt 3):657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Beck CA, Poletti A, Clement JPt, Prendergast P, Yip TT, et al. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11(6):823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Beck CA, Poletti A, Edwards DP, Weigel NL. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J Biol Chem. 1994;269(49):31034–31040. [PubMed] [Google Scholar]
- 17.Zhang Y, Beck CA, Poletti A, Edwards DP, Weigel NL. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol Endocrinol. 1995;9(8):1029–1040. doi: 10.1210/mend.9.8.7476977. [DOI] [PubMed] [Google Scholar]
- 18.Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17(4):628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- 19.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase2. Mol Cell Biol. 2004;24(24) doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21(18):6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as aprogesterone receptor coactivator. Mol Cell Biol. 2005;25(1):264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigel NL, Bai W, Zhang Y, Beck CA, Edwards DP, Poletti A. Phosphorylation and progesterone receptor function. J Steroid Biochem Mol Biol. 1995;53(1-6):509–514. doi: 10.1016/0960-0760(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 23.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8(2):269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17(7):2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, et al. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23(6):1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21(2):359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 27.Wasielewski M, Elstrodt F, Klijn JG, Berns EM, Schutte M. Thirteen newp53 gene mutants identified among 41 human breast cancer cell lines. Breast Cancer Res Treat. 2006;99(1):97–101. doi: 10.1007/s10549-006-9186-z. [DOI] [PubMed] [Google Scholar]
- 28.Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol Cell Biol. 2004;24(1):144–153. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4(8):556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 30.Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J Biol Chem. 1999;274(43):30349–30352. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]
- 31.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2(2):110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 32.Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch Biochem Biophys. 2006;449(1/2):8–16. doi: 10.1016/j.abb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2007;27(3):803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22(4):823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanoue T, Nishida E. Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol Ther. 2002;93(2/3):193–202. doi: 10.1016/s0163-7258(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 36.Arnold SF, Obourn JD, Jaffe H, Notides AC. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995;9(1):24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- 37.Arnold SF, Vorojeikina DP, Notides AC. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. J Biol Chem. 1995;270(50):30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]