Abstract
Background:
Marine brown diatom Chaetoceros calcitrans and green microalga Nannochloropsis oculata are beneficial materials for various applications in the food, nutraceutical, pharmaceutical and cosmeceutical industries.
Objective:
This study investigated cytotoxicity of different crude solvent extracts from C. calcitrans and N. oculata against various cancer cell lines.
Materials and Methods:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was carried out to screen the cytotoxic effects of hexane (Hex), dichloromethane (DCM), ethyl acetate, and methanol extract from C. calcitrans and N. oculata toward various cancer cell lines. Flow cytometry cell cycle was used to determine the cell cycle arrest while the mode of cell death was investigated through acridine orange/propidium iodide (AOPI) staining, Annexin V-Fluorescein Isothiocyanate (FITC) and Terminal deoxynucleotidyl transferase-mediated d-UTP Nick End Labeling (TUNEL) assays. Expression profile of apoptotic and proliferative-related genes was then determined using the multiplex gene expression profiler (GeXP).
Results:
Crude ethyl acetate (CEA) extract of C. calcitrans inhibited growth of MDA-MB-231 cells, with IC50 of 60 μg/mL after 72 h of treatment. Further studies were conducted to determine the mode of cell death at various concentrations of this extract: 30, 60 and 120 μg/mL. The mode of cell death was mainly apoptosis as shown through apoptosis determination test. The expression data from GeXP showed that caspase-4 was upregulated while B-cell leukemia/lymphoma 2(Bcl-2) was down regulated. Thus, caspase-4 induction endoplasmic reticulum death pathway is believed to be one of the mechanisms underlying the induction of apoptosis while Bcl-2 induced S and G2/M cell cycle phase arrest in MDA-MB-231 cells.
Conclusion:
CEA extract of C. calcitrans showed the highest cytotoxicity on MDA-MB-231 via apoptosis.
Keywords: Apoptosis, Chaetoceros calcitrans, crude ethyl acetate extract, gene expression profiler, MDA-MB-231
INTRODUCTION
Marine algae are photosynthetic organisms that are rich in bioactive compounds, minerals, polysaccharides, polyunsaturated fatty acids, and vitamins. Thus, they are well-known as a good source of functional food.[1] Among them, microalgae are gaining popularity among researchers due to their polyunsaturated fatty acids, β-carotenes and other phenolic molecules. Chaetoceros calcitrans, a brown diatom in the Bacillariophyceae, has received attention due to its valuable polyunsaturated fatty acid profile. It is widely cultivated as live feed for bivalves and crustaceans.[2,3] On the other hand, Nannochloropsis oculata is a unicellular green alga that is spherical in shape, with a diameter of about 2-5 μm, belonging to the Eustigmatophyceae. It plays an important role in the food chain, and is commonly used as live feed.[4] A total of 7.6 million deaths from cancer and 12.7 million new cases of cancer were recorded in 2008[5] and breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females world-wide, accounting for 23% (1.38 million) of the total new cancer cases and 14% (458, 400) of the total cancer deaths in 2008.[6] Therapeutic agents derived from plant, animal and marine microorganisms have been widely used in cancer treatment-related studies. Among these, marine algae have attracted considerable attention from researchers since marine compounds show great potential as anticancer drugs.[7,8] Spirulina is the most frequently studied marine algae.[9] In a previous study, notable antioxidant properties were found in both C. calcitrans and N. oculata.[10] The role of free radical scavenging in the prevention of cancer cell formation has been reported previously.[11] Thus, this study was carried out to evaluate the potential cytotoxicity of crude solvent extracts of C. calcitrans and N. oculata on human breast cancer cell lines.
MATERIALS AND METHODS
Marine microalgae cultivation and sample preparation
C. calcitrans (UPMAAHU10) and N. oculata (UPMAAHU20) were provided by the Marine and Aquaculture Laboratory of the Institute of Bioscience, Universiti Putra Malaysia. These microalgae were cultivated in 30 p.p.t of artificial seawater following overnight sterilization of the water with Clorox. Conway medium (main mineral solution, trace metal solution, silicate solution and vitamin solution) was added as a nutrient source for growth. Both microalgae were grown in complete growth media, adjusted to pH 8 with carbon dioxide aeration, under illuminated conditions at room temperature. They were the harvested after 5-7 days (at the stationary phase) by centrifugation (Hitachi High Speed Refrigerated Centrifuge Models CR22G II/CR 21G II) at 8000 rpm, 23°C for 10 min (green microalgae) or 20 min (brown microalgae) and rinsed with distilled water and ammonium sulfate, respectively, before being freeze-dried (freeze-drying system: Labconco 77540) for long-term storage.
Crude extract preparation
Two grams of freeze-dried powder of C. calcitran or N. oculata were shaken in 500 mL of hexane (Hex), dichloromethane (DCM), ethyl acetate (EA) or methanol (MeOH) for 24 h and filtered through cotton. The solvent was then dried off using a rotary evaporator (EYELA N-1000S-WD, Tokyo Rikakikai Co., Ltd) at 35°C and the dried extract was stored at −20°C for further use. All of the dried crude solvent extract was dissolved in dimethyl sulfoxide (DMSO) (100 mg/mL) before use.
Cell culture maintenance
The parental cell lines, namely human breast cancer estrogen-independent (MDA-MB-231), estrogen-dependent human breast cancer Michigan Cancer Foundation-7 (MCF-7), rat breast cancer (4T1), liver cancer Human hepatocellular carcinoma cell line-2 (HepG 2), cervical cancer (HeLa), prostate cancer (PC3), lung cancer (A549), colorectal cancer (HT29), ovarian cancer (Caov3) and non-tumorigenic fibroblast cells (3T3) were provided by the Laboratory of Molecular Biomedicine, Universiti Putra Malaysia. These cell lines were maintained at pH 7.4 in Roswell Park Memorial Institute (RPMI) 1640 (GIBCO) sterile culture medium supplemented with 10% (v/v) fetal bovine serum (FBS; Deoxyribonucleic acid [iDNA], Singapore) and a 1% penicillin-streptomycin mixture (GIBCO Bethesda Research Laboratories, Inc [BRL], Gaithersburg, MD), All cell lines were cultured under controlled conditions (37°C, 5% CO2 and 100% humidity).
Determination of cytotoxicity
The cytotoxic effect of four solvent extracts from C. calcitrans and N. oculata was assessed on various cancer cell lines: MDA-MB-231, MCF 7, 4T1, HepG2, HeLa, PC3, A549, HT29, Caov3 and 3T3 using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Methyl thiazol tetrazolium (MTT; Sigma-Aldrich, USA) assay. Doxorubicin was used as a positive control at a starting concentration of 60 μg/mL. After 72 h of treatment, the cytotoxic effect was assessed at 570 nm using a Molecular Devices SpectraMax 384 Plus microplate reader (Harlow Scientific, Arlington, MA).
Evaluation on the mode of cell death
MDA-MB-231 cells were treated with different concentrations of extracts (30, 60 and 120 μg/mL) for 72 h. Then extract treated or untreated cells were harvested, washed, and subjected to the following bioassays. Doxorubicin (10 μg/mL) was used as a positive control.
Cell cycle analysis
The harvested cells were fixed with 70% ethanol at −20°C for at least 2 h. Prior to analysis, 25 μL of propidium iodide (PI) (1 mg/ml) and 50 μL of ribonuclease A (1 mg/mL; Sigma) were added to the cells. The DNA content was analyzed using the Becton Dickinson Fluorescence Activated Cell Analyzer FAC Scan and Cell Quest software. Subsequent data analysis was performed using ModFit software (Becton Dickinson, UK).
Acridine orange/PI (AO/PI) staining
Cells were stained with 10 μL each of 20 μg/mL AO (AO; Invitrogen, USA) and 20 μg/mL PI (PI; Invitrogen, USA). Then, 10 μL of the mixture were then loaded onto a microscope slide and observed under an Axiolab fluorescence microscope (Zeiss, Germany).
Annexin V-Fluorescein isothiocyanate (V-FITC) staining
Treated and untreated cells were resuspended in 100 μL binding buffer followed by staining with 1.25 μL annexin V reagent and 10 μL PI (Merck, Germany) in the dark. Then, all the samples were acquired using the Becton Dickinson FAC Scan and the results were analyzed using the Cell Quest Pro software (Becton Dickinson, UK).
Detection of DNA fragmentation using terminal deoxynucleotidyl transferase-mediated d-UTP nick end labeling (TUNEL) assay
The TUNEL assay was carried out using an Apo-BrdU™ Kit (Merck, Germany) according to the standard protocol. Briefly, the cells were initially washed twice with 1 mL of wash buffer. The supernatant was discarded and the cell pellet was resuspended in 50 μl of DNA labeling solution and incubated for 2 h at 37°C. One mL of rinsed buffer was added to rinse off the unbound label and was then removed by centrifuging at 1000 rpm for 10 min. This step was repeated prior to adding 100 μL of antibody solution. This was followed by 1 h of incubation in the dark at room temperature. Lastly, they were incubated together with 500 μL of PI/RNase staining buffer for 30 min at room temperature in the dark before analysis in the flow cytometer (Becton Dickinson, UK).
Polymerase chain reaction
Total ribonucleic acid (RNA) was extracted from the treated and untreated cells using the RNeasy® Plus Mini Kit (Qiagen, Germany). Following extraction and quantification, RNA from each sample was reverse transcribed to cDNA using a GenomeLab™ gene expression profiler (GeXP) Start Kit (Beckman Coulter, USA) containing Kanamycin resistance (KANr) as an internal control, reverse transcription buffers and reverse transcriptase. It was then mixed with a gene-specific reverse primer mix, RNA and RNase-free water according to the manufacturer's instructions. The RT reaction was then run on a thermal cycler (MJ Research Peltier Thermal Cycler (PTC)-225) using the following program: 48°C for 1 min; 37°C for 5 min; 42°C for 60 min; 95°C for 5 min, hold at 4°C. The PCR mixture containing MgCl2, 5x PCR Master Mix buffer (fluorescently labeled universal forward primer) and Thermo-Start® DNA polymerase (Thermo Fisher Scientific, Pittsburgh, PA) was prepared at 4:4:0.7 with respect to the final volume of 8.7 μL for each sample. This mixture was then mixed well with 9.3 μL of cDNA from each sample and 2 μL of a 200 nM multiplex of gene-specific forward universal primers. The final PCR was performed in an MJ Research PTC-225 thermal cycler starting at 95°C for 10 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, 68°C for 1 min and holding at 4°C.
Genomelab GeXP genetic analysis
The PCR product was mixed with 37.5 μL sample loading solution and 0.5 μL of the DNA size standard-400 (GenomeLab™ GeXP Start Kit, Beckman Coulter, USA) and was overlaid with a layer of mineral oil before transfer to a Capillary Electrophoresis Quantitation(CEQ) 8000 Genetic Analysis System. From the system software, Frag-3 protocol was chosen to allow the PCR products with fluorescently labeled fragments to be separated by capillary gel electrophoresis. This separation was completed according to their product size at 6.0 kV and 50°C for 35 min and after the denaturation step at 90°C for 120 s and 30 s of injection at 2.0 kV. The raw data from the separated product were initially analyzed using the Fragment Analysis module of the GenomeLab GeXP system software. Information related to the product fragment, and the height and area of the peak was imported into the eXpress Analysis module of the eXpress Profiler software. The housekeeping genes used in this experiment were checked for their consistency using this software. Beta-actin was selected as a reference gene for normalizing all data from the targeted genes.
Statistical analysis
GraphPad Prism 4.00 statistical software was used to analyze the data. One-way ANOVA and Tukey HSD post-hoc tests were applied to test for significant differences at P < 0.05. All results were calculated as the mean of three experiments.
RESULTS
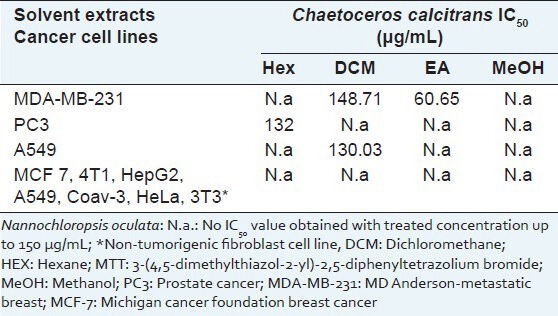
The cytotoxicity of four crude solvent extracts, namely Hex, DCM, EA and MeOH, extracted from C. calcitrans and N. oculata was assessed in various cancer cell lines (MDA-MB-231, MCF 7, 4T1, HepG2, HeLa, PC3, A549, HT29, Caov3 and 3T3) via MTT assay, as summarized in Table 1. None of the solvent extracts from the green microalga N. oculata had a cytotoxic effect on the tested cell lines after 72 h of treatment (results not shown). On the other hand, Crude ethyl acetate (CEA) extract showed the strongest effect on the estrogen receptor-negative breast cancer cell line, MDA-MB-231 (IC50- 60 μg/mL), but weak cytotoxicity (IC50 >150 μg/mL) against the estrogen receptor-positive breast cancer cell line, MCF-7. The cytotoxic effects towards a non-tumorigenic fibroblast cell line were investigated using the same screening solvent extracts as used for the other cancer cell lines. The results showed that solvent extracts exhibited a specific cytotoxic effect on cancer cells but showed no cytototoxicity toward normal cell lines.
Table 1.
Cytotoxicity of solvent extract from Chaetoceros calcitrans and Nannochloropsis oculata against various cancer cells after 72 h continuous exposure as assessed by MTT assay
Cell cycle study
The effect of the CEA extract of C. calcitrans on the cell cycle distribution of MDA-MB-231 cells after 72 h of treatment at different concentrations is summarized in Figure 1. The number of MDA-MB-231 cells in the G0/G1 phase decreased significantly (P < 0.05) from the low to high concentration of extract (30, 60 and 120 μg/mL). The decrease in the number of cells in the G0/G1 phase when treated with 30 μg/mL and 60 μg/mL of extract was accompanied by an increase in the number of cells in the S and G2/M phase. At the highest concentration (120 μg/mL), there was a significant increase in apoptotic cells associated with the sub-G0/G1 phase (P < 0.05, 33.58% increase).
Figure 1.
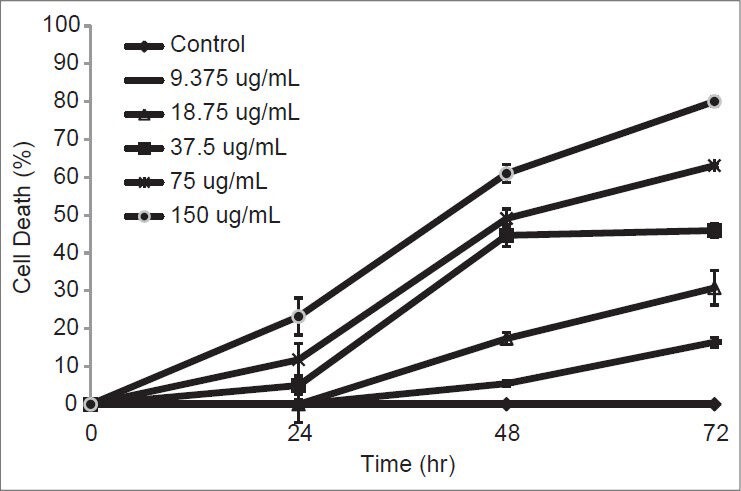
Percentage of cell death on MDA-MB-231 which treated with different concentrations of crude EA extract at 24, 48 and 72 hours
AO/PI study
AO and PI dual staining is a rapid and quantitative way of assessing apoptosis. This method allows the morphological observation of apoptotic cells and distinguishes between apoptotic and necrotic cells.[12] Figure 2 shows viable cells in green and non-viable cells in red under fluorescence microscopy. Results of apoptotic characteristics were shown on cells treated with low concentration; (30 ug/mL) and IC50 (60 ug/mL), which were stained in green at cytoplasm and nucleus. Besides, the blebbing of plasma membrane and chromatin condensed were observed in the nucleus [Figure 2b and c]. High number of cells showed membrane blebbing and some of the necrotic cell were also observed at IC50 (60 ug/mL) treatment where the PI stained the whole cell [Figure 2c]. AO/PI staining can differentiate the viability of cells, but early apoptosis and DNA fragmentation cannot be clearly determined using this method.
Figure 2.
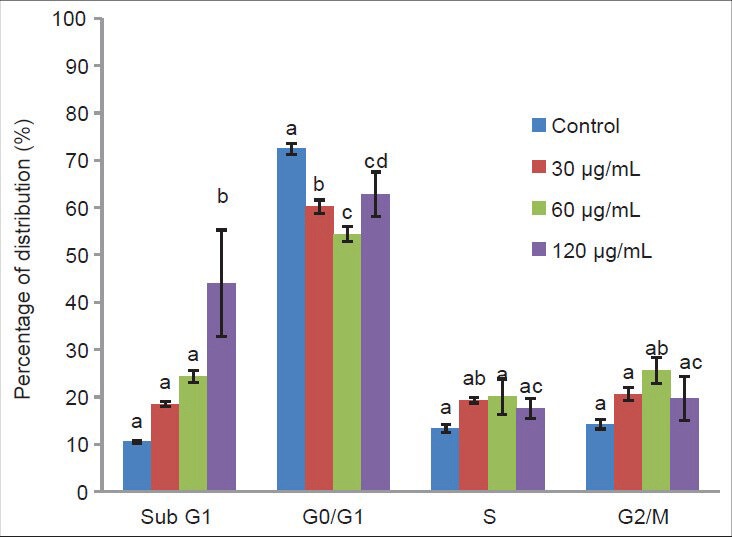
Flow cytometric analysis of apoptosis (sub G1) and cell cycle distribution on the MDA-MB-231 cells treated 72 h with different concentration of Crude ethyl acetate extract from Chaetoceros calcitrans. Data represent percentage mean±standard deviation, n = 3. Data marked with alphabet are significantly different at P < 0.05, in comparison with control culture
Annexin V/PI study
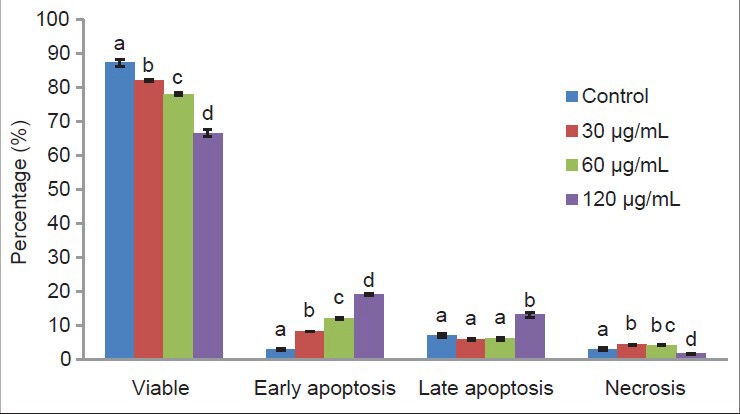
The Annexin V-FITC staining method was used to confirm the presence of early apoptosis in cells. Figure 3 shows the dose-dependent increase in annexin V binding. Specifically, the size of the early apoptosis cell population treated with 30, 60 and 120 μg/mL of CEA extract was significantly enhanced by 8.15 ± 0.08, 11.9 ± 0.24 and 18.9 ± 0.27%, respectively. Meanwhile, under the same treatment, the viable cell population was reduced by 5.3, 9.3 and 20.7%, respectively. However, a large population of late apoptotic cells (13.00 ± 0.83%; P < 0.05) was found after treatment with the high concentration (120 μg/mL) of CEA extract. Early apoptosis was more common than necrosis. This implies that the CEA extract induced cell apoptosis to a greater extent than necrosis. Based on all of the above results, the CEA extract promoted early apoptosis in MDA-MB-231 human breast carcinoma cells.
Figure 3.
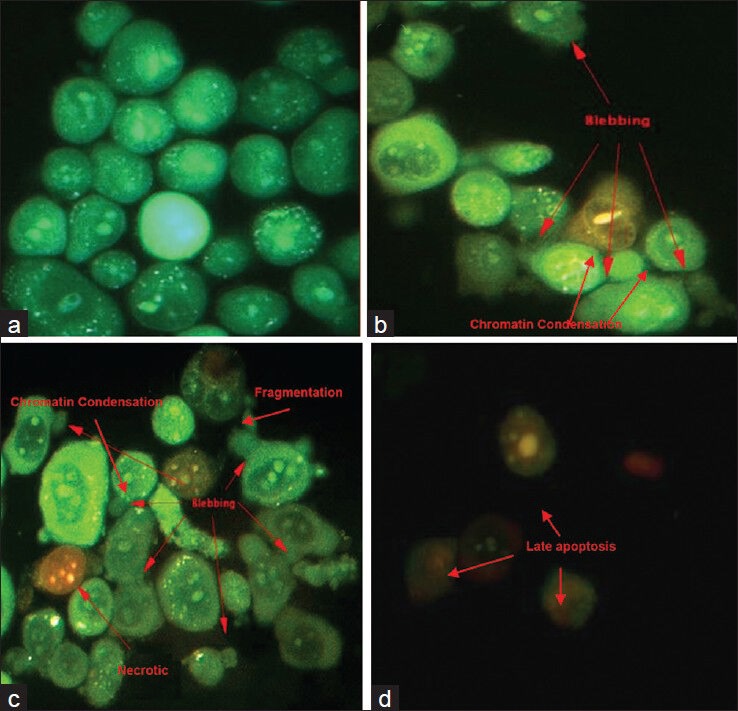
MDA-MB-231 cells with different features characteristic of apoptosis and secondary necrosis as detected by dual staining with AO and PI after 72 hours treatment with EA extract of C. calcitrans. Apoptotic cells showed membrane blebbing, DNA fragmentation and chromatin condensation. Necrotic cells showed diffused chromatin with apparent nucleus membrane disruption. (a) Untreated control; (b) Low concentration (30 ug/mL); (c) IC50 (60 ug/mL); (d) High concentration (120 ug/mL) (magnification: ×400)
TUNEL assay
The induction of DNA fragmentation in MDA-MB-231 cells by 30, 60 and 120 μg/mL of CEA extract after 72 h of treatment was examined via TUNEL detection assay. About half and 90% of the cells that were treated with IC50 and 120 μg/mL showed positive TUNEL labeled, which significantly increased to 43.8 and 89.8%, respectively (in comparison to control; P < 0.001). However, fragmented DNA found in the cell population that was treated with low concentration (30 μg/mL) showed less than 5% of fragmented DNA. At the high concentration of extract, the apoptotic cell population showed significant DNA fragmentation [Figure 4d].
Figure 4.
Flow cytometric analysis of phosphatidylserine-annexin V labeled on the MD Anderson-metastatic breast-231MDA-MB-231 cells treated 72 h with different concentration of Crude ethyl acetate extract from Chaetoceros calcitrans. Data represent percentage mean±standard deviation, n = 3. Data from the same group marked with different alphabet are significantly different at P < 0.05
GeXP analysis
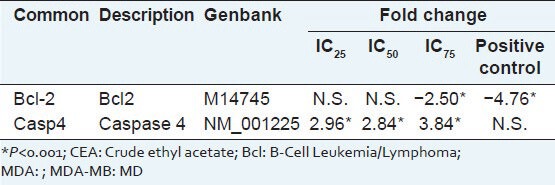
The significant fold changes of multiplex gene expression in MDA-MB-231 cells after 72 h of CEA treatment at the three concentrations are summarized in Table 2. The expression of several genes significantly up regulated when treated with 30 μg/mL CEA extract was caspase-4. Bcl-2 expression was significantly suppressed after treated with 120 μg/mL of CEA extract.
Table 2.
List of upregulated and downregulated apoptotic related genes in CEA-treated MDA-MB-231 cells
DISCUSSION
The use of marine products for therapeutic purposes began in 1979 with many further discoveries in the 1980s.[13,14] Novel marine compounds and promising pharmacological research have been reviewed by many authors.[8,15,16] In this study, various solvent extracts of C. calcitrans and N. oculata were screened against human cancer cell lines using the MTT cytotoxicity assay [Table 1] based on Krebs cycle events in intact mitochondria.[17] This screening test included a control of DMSO-treated cells, to prove that the cytotoxic effect was not caused by DMSO, but by the extract itself.[18,19] The CEA extract had a greater effect on MDA-MB-231 than MCF-7. This is consistent with other studies involving brown algae extracts.[19] The other solvent extracts showed no selective cytotoxicity against the other cancer cell lines tested. Likewise, the CEA extract had no cytotoxic effect on the 3T3 cell line, which is recommended by the US National Institute of Environmental Health Sciences[20] for standard use in cytotoxicity tests.
CEA extract showed poor cytotoxicity on MDA-MB-231 cells after 24 and 48 h of treatment, but increased cytotoxicity at 72 h. Thus, the mode of cell death was further investigated, in which the cells were treated for 72 h with 30, 60 and 120 μg/mL CEA extract. The ability to induce cell cycle arrest in MDA-MB-231 cells after 72 h was determined via cell cycle analysis. Treatment with the CEA extract resulted in the accumulation of cells at the S and G2/M phase at the low concentration and induced apoptosis at the high concentration. This effect was suggested to be dose-dependent and was irreversible at the high concentration.[21] The cell population of sub-G1 was significantly induced by the CEA extract at the high concentration (120 μg/mL), in which the irreparable DNA underwent fragmentation.[22,23] These results imply that the CEA extract would be useful for the suppression of cell proliferation, with a cytostatic effect at lower concentrations (30 and 60 μg/mL), although a cytotoxic effect was induced at the high concentration (120 μg/mL). The apoptosis event was further demonstrated using the AO/PI staining method.
The morphology of MDA-MB-231 cells treated with CEA extract was observed using the AO/PI staining method with the aid of a fluorescence microscope (×400 magnification). At the low concentration (30 μg/mL), apoptotic cells were stained green in the cytoplasm and nucleus, with blebbing of the plasma membrane and chromatin condensation observed in the nucleus, both of which are hallmarks of apoptosis.[24] At 60 μg/mL, other than membrane blebbing, the even spread of chromatin fragments in some of the cells stained with PI indicated that necrotic cells were present;[25] this phenomenon is undesirable due to the inflammatory responses that may be induced as a result.[26] At 120 μg/mL, the cells fluoresced red, mainly from the nucleus, indicating that late apoptosis had occurred.[22,27] Overall, the CEA extract mainly induced apoptosis in MDA-MB-231 cells.
The translocation of phosphatidylserine (PS) from the inner to the outer leaflet within the membrane is an important feature of apoptotic cell death, with the cell membrane remaining intact.[28] By targeting the specific ligand of PS, annexin V analysis was undertaken to investigate the early stages of apoptosis. Cells stained with annexin V-FITC were apoptotic while cells stained with both annexin V-FITC and PI were in late apoptosis, and cells stained with PI alone were necrotic.[29] The results showed a significant increase in early apoptotic cells after treatment with the CEA extract from C. calcitrans. This suggests that the mode of cell death had already switched on towards the apoptosis pathway. In a parallel study, the induction of apoptosis by the CEA extract was evaluated by TUNEL labeling of DNA fragments. Cells labeled with FITC fluoresced green and thus contained broken DNA strands, and total DNA was counter-stained with PI.[30] From the TUNEL assay [Figure 5], high number of cells containing fragmented DNA were found when treated with the high concentration of extract. Taken together, the presence of the sub-G1 peak, the microscopic morphology, and the annexin V and TUNEL assay proved that the CEA extract of C. calcitrans induced apoptosis in MDA-MB-231 cells.
Figure 5.
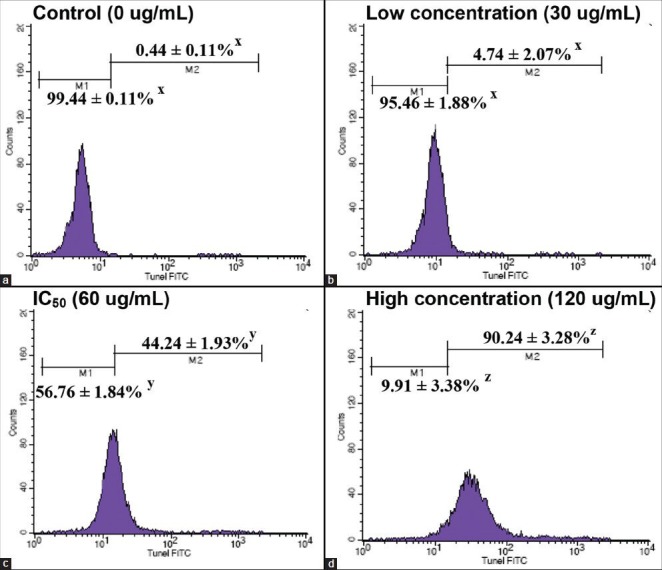
Apoptosis induced by ethyl acetate extract of Chaetoceros calcitrans was determined by the presence of apoptotic deoxyribonucleic acid (DNA) strand breaks detected via Terminal deoxynucleotidyl transferase-mediated d-UTP Nick End Labeling TUNEL staining flow cytometry is shown. DNA fragmentation is obviously increased from low concentration treatment (b) to high concentration of treatment (d), compared with the control (a). Data represent percentage mean±standard deviation (n = 3), *P < 0.05
The expression of apoptosis-related genes was evaluated using a multiplex GeXp system. Expression of caspases-4 was significantly up regulated at all concentrations, which is believed to initiate apoptosis through the endoplasmic reticulum death pathway.[31] The expression of the anti-apoptotic factor, Bcl-2, which also plays an important role in the mitochondria-mediated apoptosis,[32] was effectively suppressed at 120 μg/mL and Bcl-2 suppression are thought to be involved in S and G2/M cell cycle phases arrest.[33,34]
CONCLUSION
Among all the crude solvent extracts tested, the CEA extract of C. calcitrans was the most cytotoxic towards MDA-MB-231, a hormone-independent human breast cancer cell line, with an IC50 of 60 μg/mL. The mode of cell death induced by the extract was mainly apoptosis, as confirmed by the AO/PI staining, annexin V-FITC and TUNEL assay. The induction of apoptosis was found to be dose-dependent. The effects of the CEA extract of C. calcitrans on gene expression were dose-dependent, and the caspase induction pathway is believed to be one of the mechanisms underlying the induction of apoptosis by the CEA extract. Further, study is required to isolate and identify the bioactive compounds from C. calcitrans and in vivo study to further evaluate the potential anti-tumor activity of the extract for potential development as an anticancer agent.
ACKNOWLEDGMENTS
This project was supported by the Agri-Science Fund Grant from the Ministry of Agriculture, Malaysia (Project No. 5450384).
Footnotes
Source of Support: Agri-Science Fund Grant from the Ministry of Agriculture, Malaysia (Project No. 5450384)
Conflict of Interest: None declared.
REFERENCES
- 1.Kornprobst JM. Substances Naturelles D’origine Marine: chimiodiversité, Pharmacodiversité, Biotechnologie. Paris: Lavoisier Publisher; 2005. Spongiaires (Eponges) pp. 601–84. [Google Scholar]
- 2.Tolga G, Yaşar D, Şevket G. Effects of light path lengths and initial culture density on the cultivation of Chaetoceros muelleri (Lemmermann, 1898) Aquaculture. 2003;217:431–6. [Google Scholar]
- 3.Moreau D, Tomasoni C, Jacquot C, Kaas R, Le Guedes R, Cadoret JP, et al. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ Toxicol Pharmacol. 2006;22:97–103. doi: 10.1016/j.etap.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Gwo JC, Chiu JY, Chou CC, Cheng HY. Cryopreservation of a marine microalga, Nannochloropsisoculata (Eustigmatophyceae) Cryobiology. 2005;50:338–43. doi: 10.1016/j.cryobiol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. [Accessed Date 2012 August 11]. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Available from: http://globocan.iarc.fr . [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AM, Gustafson KR. Marine pharmacology in 2003-2004: Anti-tumour and cytotoxic compounds. Eur J Cancer. 2006;42:2241–70. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Mayer AM, Gustafson KR. Marine pharmacology in 2005-2006: Antitumour and cytotoxic compounds. Eur J Cancer. 2008;44:2357–87. doi: 10.1016/j.ejca.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishima T, Murata J, Toyoshima M, Fujii H, Nakajima M, Hayashi T, et al. Inhibition of tumor invasion and metastasis by calcium spirulan (Ca-SP), a novel sulfated polysaccharide derived from a blue-green alga, Spirulina platensis. Clin Exp Metastasis. 1998;16:541–50. doi: 10.1023/a:1006594318633. [DOI] [PubMed] [Google Scholar]
- 10.Goh SH, Loh SP, Yusoff FM. A comparison of the antioxidant properties and total phenolic content in a diatom, Chaetoceros sp. and a green microalga, Nannochloropsis sp. J Agric Sci. 2010;2:123–30. [Google Scholar]
- 11.Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006;44:1065–74. doi: 10.1016/j.fct.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Bank HL. Rapid assessment of islet viability with acridine orange and propidium iodide. in vitro Cell Dev Biol. 1988;24(Suppl 4):266–73. doi: 10.1007/BF02628826. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe HA, Levring T, Tanaka Y. Marine Algae in Pharmaceutical Science. New York: Walter de Gruyter; 1979. Marine algae and their products and constituents in pharmacy; pp. 25–119. [Google Scholar]
- 14.Fautin DG. Biomedical Importance of Marine Organisms. San Francisco: California Academy of Sciences; 1988. New pharmaceuticals from cultured blue-green algae; pp. 143–50. [Google Scholar]
- 15.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4(Suppl 2):333–42. [PubMed] [Google Scholar]
- 16.Mayer AM, Hamann MT. Marine pharmacology in 2001 - 2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:265–86. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Huang HL, Wu SL, Liao HF, Jiang CM, Huang RL, Chen YY, et al. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. J Agric Food Chem. 2005;53:1776–81. doi: 10.1021/jf049445n. [DOI] [PubMed] [Google Scholar]
- 19.Khanavi M, Nabavi M, Sadati N, Shams Ardekani M, Sohrabipour J, Nabavi SM, et al. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43:31–7. [PubMed] [Google Scholar]
- 20.NIH Publication No. 01-4499. U.S. Public Health Service:NIEHS, Research Triangle Park, N; 2001. National Institute of Environmental Health Sciences (NIEHS). Report of the International Workshop on In Vitro Methods for Assessing Acute Systemic Toxicity. [Google Scholar]
- 21.Dimas K, Papadaki M, Tsimplouli C, Hatziantoniou S, Alevizopoulos K, Pantazis P, et al. Labd-14-ene-8,13-diol (sclareol) induces cell cycle arrest and apoptosis in human breast cancer cells and enhances the activity of anticancer drugs. Biomed Pharmacother. 2006;60:127–33. doi: 10.1016/j.biopha.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen WY, Wu CC, Lan YH, Chang FR, Teng CM, Wu YC. Goniothalamin induces cell cycle-specific apoptosis by modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol. 2005;522:20–9. doi: 10.1016/j.ejphar.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Ding L, Liu B, Qi LL, Zhou QY, Hou Q, Li J, et al. Anti-proliferation, cell cycle arrest and apoptosis induced by a natural xanthone from Gentianopsis paludosa Ma, in human promyelocytic leukemia cell line HL-60 cells. Toxicol in vitro. 2009;23:408–17. doi: 10.1016/j.tiv.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Chan KM, Rajab NF, Ishak MH, Ali AM, Yusoff K, Din LB, et al. Goniothalamin induces apoptosis in vascular smooth muscle cells. Chem Biol Interact. 2006;159:129–40. doi: 10.1016/j.cbi.2005.10.107. [DOI] [PubMed] [Google Scholar]
- 26.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanke HJ, van der Linden PW, Langerak J. Alternative fluorochromes to ethidium bromide for automated read out of cytotoxicity tests. J Immunol Methods. 1982;52:91–6. doi: 10.1016/0022-1759(82)90353-2. [DOI] [PubMed] [Google Scholar]
- 28.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 29.Lee JY, Shin JW, Lee EH, Baek SH, Ku SW, Kim JU. Comparison of the effects of propofol and pentobarbital on hydrogen peroxide-stimulated hepatic SNU761 cells. Korean J Anesthesiol. 2010;58(Suppl 3):277–82. doi: 10.4097/kjae.2010.58.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pec MK, Aguirre A, Moser-Thier K, Fernández JJ, Souto ML, Dorta J, et al. Induction of apoptosis in estrogen dependent and independent breast cancer cells by the marine terpenoid dehydrothyrsiferol. Biochem Pharmacol. 2003;65:1451–61. doi: 10.1016/s0006-2952(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier N, Casamayor-Pallejà M, De Luca K, Mondière P, Saltel F, Jurdic P, et al. The endoplasmic reticulum is a key component of the plasma cell death pathway. J Immunol. 2006;176:1340–7. doi: 10.4049/jimmunol.176.3.1340. [DOI] [PubMed] [Google Scholar]
- 32.Tomita Y, Marchenko N, Erster S, Nemajerova A, Dehner A, Klein C, et al. WT p53, but not tumor-derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization. J Biol Chem. 2006;281:8600–6. doi: 10.1074/jbc.M507611200. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Kim DH, Kim TD, Park BJ, Park HD, Park JI, et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004;216:175–81. doi: 10.1016/j.canlet.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Mandal S, Bérubé G, Asselin E, Mohammad I, Richardson VJ, Gupta A, et al. A novel series of potent cytotoxic agents targeting G2/M phase of the cell cycle and demonstrating cell killing by apoptosis in human breast cancer cells. Bioorg Med Chem Lett. 2007;17:4955–60. doi: 10.1016/j.bmcl.2007.06.033. [DOI] [PubMed] [Google Scholar]


