Abstract
Background:
The compound Hongdoushan capsule (CHC) is widely known as compound herbal preparation and is often used to treat ovarian cancer and breast cancer, and to enhance the body immunity, etc., in clinical practice.
Objective:
To determine simultaneously 10 bioactive components from CHC, namely glycyrrhetinic acid, liquiritin, glycyrrhizin, baccatin III, 10-deacetylbaccatin III, cephalomannine, taxol, ginsenoside Rg1, ginsenoside Re, and ginsenoside Rb1.
Materials and Methods:
A high performance liquid chromatograph method coupled with photodiode array detector was developed and validated for the 1st time. Chromatographic analysis was performed on a SHIMADZU C18 by utilizing a gradient elution program. The mobile phase was acetonitrile (A)-water (B) at a flow rate of 0.8 mL/min.
Results:
The calibration curve was linear over the investigated concentration ranges with the values of r2 higher than 0.9993 for all the 10 bioactive components. The average recovery rates range from 98.4% to 100.5% with relative standard deviations ≤2.9%. The developed method was successfully applied to analyze 10 compounds in six CHC samples from different batches. In addition, the herbal sources of 32 chromatographic peaks were identified through comparative studying on chromatograms of standard, the respective extracts of Hongdoushan, RenShen, GanCao, and CHC.
Conclusion:
All the results imply that the accurate and reproducible method developed has high separation rate and enables the determination of 10 bioactive components in a single run for the quality control of CHC.
Keywords: Bioactive components, compound herbal preparation, compound Hongdoushan capsule, high performance liquid chromatograph-photodiode array, quality control
INTRODUCTION
Compound herbal preparations (CHPs) have been widely used by millions of people throughout the world for many years. Therapeutic effects of CHPs, which may consist of hundreds of phytochemicals, are integrative results of multiple bioactive components, which can be slightly different according to climate, regions of cultivation and seasons of harvest, etc., To guarantee the safety and the efficacy of CHPs in clinical practices, the quality control is necessary, and indispensable.[1,2,3,4] However, it is very difficult under most conditions to identify and simultaneously determine the most of active components in a CHP.[5,6] Undoubtedly, the quality control of the CHP consisting of more than two crude herbs is not only complicated but also challenging. Therefore, more and more attentions have been paid to this field. Several techniques such as gas chromatography,[7,8] high performance liquid chromatograph (HPLC),[9,10,11] and HPLC-photodiode array (PDA)/MS[12,13] have been applied to carrying out simultaneous quantitative analysis of CHPs.
The compound Hongdoushan capsule (CHC) is often used to treat ovarian cancer and breast cancer, and to enhance the body immunity in clinical therapy.[14,15] State Food and Drug Administration[14] records the formula of CHC as follows: 50 kg of barks of Hongdoushan, 166 g of RenShen, and 500 g of GanCao, each of which containing many components that may be relevant to the medicine's putative activity. RenShen, the roots of Panax ginseng, mainly consists of several ginsenosides, such as ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1,[16,17] and has anti-cancer, and immunomodulation activity.[17,18] GanCao, the roots of Glycyrrhiza Uralensis Fich., has activities in clearing away heat and toxic, and painkilling effect.[19] The major active components of GanCao include glycyrrhizin, liquiritin, and glycyrrhetinic acid.[20] Hongdoushan, the bark of Taxus Chinensis Var. Mairei, has been considered to be the most hopeful anticancer natural resources, which possess many components of taxanes, such as taxol, 10-deacetylbaccatin III (10-DAB III), baccatin III and cephalomannine.[21]
To our knowledge, there are no methods available for the simultaneous determination of multiple components in CHC. In this paper, the HPLC coupled with a PDA detector (HPLC-PDA) was firstly established to simultaneously determine 10 components in CHC. This method was successfully applied to the quality assessment of six batches CHCs. Meanwhile, we identified the herb source of 32 main peaks in the chromatogram of CHC.
MATERIALS AND METHODS
Chemicals and reagents
HPLC grade methanol and acetonitrile were purchased from Merck (Germany). Distilled water was prepared to use Milli-Q purification system (Millipore, Bedford, MA, USA). Standard substances of ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, taxol, glycyrrhizin, liquiritin, glycyrrhetinic acid, 10- DAB III, baccatin III and cephalomannine were all obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The purity of the compounds was >98% as determined by HPLC.
Materials and samples
Hongdousan, RenShen, and GanCao samples were collected by the authors. All of the raw medicinal herbs were verified by Changhua Wang (Chongqing Academy of Chinese Materia Medica, China). CHC samples were gifted by Chongqing Sino Bio-Phramaceutal Co., Ltd. (Chongqing, China).
Standard solutions
Stock solutions were prepared in methanol at concentrations 362.36 μg/mL for ginsenoside Rg1, 488.92 μg/mL for ginsenoside Re, 429.74 μg/mL for ginsenoside Rb1, 556.93 μg/mL for glycyrrhizin, 340.47 μg/mL for liquiritin, 100.52 μg/mL for glycyrrhetinic acid, 149.30 μg/mL for 10-DAB III, 120.51 μg/mL for baccatin III, 1314.02 μg/mL for taxol, and 122.80 μg/mL for cephalomannine. Then, these stock solutions were mixed balanceable to obtain the combined solutions. Then, the combined solution was diluted step by step to yield a series of standard working solutions with different concentration for linear validation.
Sample preparation
Sample preparation of raw herbs: 10.0 g Hongdoushan, RenShen, and GanCao was dried and powdered, respectively. Then, extracts ware obtained according to the record of the formula of CHC[14] and dissolved in methanol to prepare sample solutions, respectively.
Sample preparation of CHC: 0.3 g sample was accurately weighed into a centrifuge tube and ultrasonically extracted 2 times (2 × 30 min) at room temperature with 10 mL methanol. After centrifuged for 5 min at 9,000 g, the supernatant was moved into 25 mL volumetric flask and the constant volume was obtained with methanol.
All the solutions were filtered through a 0.45 μm membrane filters before injected into HPLC.
Apparatus and HPLC conditions
The HP 1200 Agilent system (Agilent Technologies, Palo Alto, CA, USA) equipped with a dual pump, an auto-sampler and a PDA detector. The analytical column was a SHIMADZU C18 (250 mm × 4.6 mm I.D., Tokyo, Japan) protected with a 4.0 mm × 3.0 mm I.D. Phenomenex guard column (ANPEL Co. Ltd., Shanghai, China). Detection wave length was acquired at: 0-30 min (276 nm), 30-50 min (203 nm), 50-85 min (227 nm); and PDA spectra were recorded from 200 nm to 600 nm. The mobile phase was a gradient prepared from acetonitrile (component A) and water (component B). The elution program was designed as follows: 0-35 min kept at 19%A, 35-50 min linear increased from 19% to 55% A, 50-70 min kept at 55% A, 70-85 min linear decreased from 55% to 19% A. The mobile phase flow rate was 0.8 mL/min. The column temperature was set at 25°C. The injection volume was 5 μL.
Validation studies
Linearity and range
To determine the linear relationship between peak areas and concentration of each component, a range of 3.94-100.52 μg/mL for glycyrrhetinic acid, 8.01-340.47 μg/mL for liquiritin, 18.26-556.93 μg/mL for glycyrrhizin, 4.02-120.51 μg/mL for baccatin III, 4.81-149.30 μg/mL for 10-DAB III, 4.09-122.80 μg/mL for cephalomannine, 44.08-1314.02 μg/mL for taxol, 14.21-362.36 μg/mL for ginsenoside Rg1, 16.03-488.92 μg/mL for ginsenoside Re and 14.09-429.74 μg/mL for ginsenoside Rb1, was tested. Six concentrations for each standard were analyzed in triplicate, generating respective calibration curves. The linearity equations were calculated by linear regression analysis.
Limit of detection and limit of quantification
LOD and LOQ were the concentrations of a component at which its ratios of signal and noise were estimated as 3:1 and 10:1, respectively. They were determined by a series diluted concentration of sample solution by using the described HPLC conditions.
Recovery
The accuracy of the method was measured through the analyst recovery test. Six samples of CHC (No. 1) were spiked with the mixed standards of 10 components. Then the samples were pretreated as described in 2.4 sample preparation.
Precision
Repeatability (intra-day) and intermediate precision (inter-day) were determined through analysis of the CHC sample (No. 1) at 100% level (n = 6) for each of the standards, then the relative standard deviation (RSD) was calculated. The intermediate precision was determined over a period of 3 days, with different analysts.
RESULTS AND DISCUSSION
A HPLC-PDA method for simultaneously determining 10 components in CHC was developed and validated in order to simplify the quality control procedure of CHC. The developed method was successfully used to assess six real samples collected from six batches CHCs produced by Chongqing Sino Bio-Phramaceutal Co., Ltd. (Chongqing, China) and each of these 10 components was detectable. Meanwhile, the herb sources of 32 main peaks in the chromatogram of CHC were identified.
Selection of mobile phase
Two candidate solvent systems abbreviated as SI and SII were tested to separate 10 components simultaneously. In SI, equal gradient elution was selected as methanol, water and acetonitrile (24:42:36, v/v/v).[18] SII was much complicated with acetonitrile as phase A and water as phase B. The gradient elution program was applied as follows: 0-35 min kept at 19% A, 35-50 min linear increased from 19% to 55% A, 50-70 min kept at 55% A, 70-85 min linear decreased from 55% to 19% A. The results showed the separating effect is superior to that of SI. The chromatogram of the standards and samples were shown in Figure 1.
Figure 1.
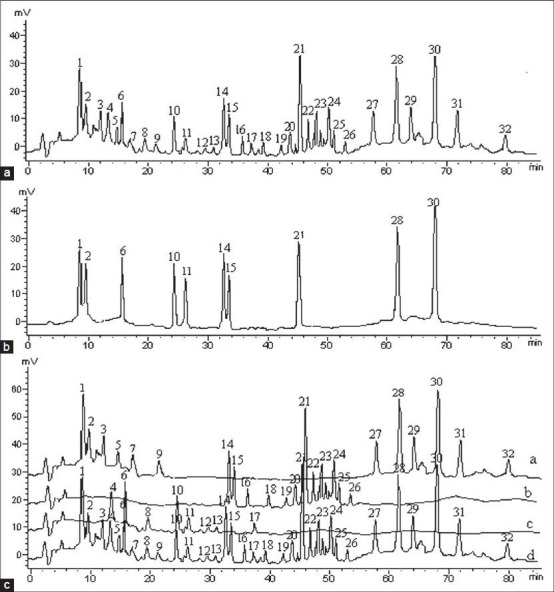
Chromatogram of compound Hongdoushan capsule (CHC) (a) standard compounds (b) and raw herb extracts which make up CHC (c) a: Extract of Taxus Chinensis Var. Mairei, b: Extract of Panax ginseng, c: Glycyrrhiza uralensis Fisch., d: Extracts of CHC. 3-5, 7-9, 12, 13, 16-20, 22-27, 31, 32: Unknown compounds; 1: 10-DAB III; 2: Baccatin III; 6: Liquiritin; 10: Glycyrrhizin; 11: Glycyrrhetinic acid; 14: Ginsenoside Rg1; 15: Ginsenoside Re; 21: Ginsenoside Rb1; 28: Cephalomannine; 30: Taxol
Validation studies
Under the chromatographic conditions described, linearity, LOD, LOQ, accuracy, and precision were studied to validate the developed method.
For linearity, LOD and LOQ, each standard sample with six concentrations were analyzed in triplicate, generating respective calibration curves. The linearity equations were calculated by linear regression analysis. The results were listed in Table 1. The linear relation of peak areas and concentration of each component was found in the range of 3.94-100.52 μg/mL for glycyrrhetinic acid (r2 = 0.9998), 8.01-340.47 μg/mL for liquiritin (r2 = 0.9997), 18.26-556.93 μg/mL for glycyrrhizin (r2 = 0.9998), 4.02-120.51 μg/mL for baccatin III (r2 = 0.9996), 4.81-149.30 μg/mL for 10-DAB III (r2 = 0.9993), 4.09-122.80 μg/mL for cephalomannine (r2 = 0.9999), 44.08-1314.02 μg/mL for taxol (r2 = 0.9999), 14.21-362.36 μg/mL for ginsenoside Rg1 (r2 = 0.9996), 16.03-488.92 μg/mL for ginsenoside Re (r2 = 0.9998) and 14.09-429.74 μg/mL for ginsenoside Rb1 (r2 = 0.9999), respectively. The LOD and the LOQ of glycyrrhetinic acid are 0.0315 μg/mL and 0.1051 μg/mL, respectively, which are lowest among these 10 components. And the LOD and the LOQ of taxol are 1.0514 μg/mL and 3.5047 μg/mL, respectively, which are highest among these 10 components. The low LOD and LOQ values of these 10 components indicate that the HPLC method is enough sensitive to simultaneously determine these 10 bioactive components.
Table 1.
Linearity, LOD, and LOQ of the developed method used to determine 10 components in CHC
The recovery rates were determined by the method of standard addition. Six portions of CHC were spiked with the standards of 10 components. The results were summarized in Table 2. All recovery rates were in the range of 98.4-100.5% with RSD of 1.8-2.9%, which is desirable for quantity determination. Each recovery analysis was repeated 4 times, the precision and accuracy were considered adequate for the validation of the method.
Table 2.
Intra-day, inter-day precision and recovery of the developed method
The precision was evaluated by repeated assay (intra-day analysis, n = 3; inter-day analysis, n = 6) for 10 components. The RSDs of the intra-assay were in the range of 0.8-2.1% and inter-day precision for each component at one concentration level was also studied with RSDs in the range of 0.7-2.7% as listed in Table 2. All these data show that the developed method has an accepted degree of precision.
Determination of the 10 components in samples
Six real samples were collected from six batches CHCs produced by Chongqing Sino Bio-Phramaceutal Co., Ltd (Chongqing, China) and the content of the 10 components were determined by the established HPLC-PDA method. All of the 10 components in different samples were reliably identified using the new method and the results were presented in Table 3. Among the 10 components, the contents of glycyrrhizin, taxol and liquiritin were found at the high level in the analyzed samples, whereas the content of glycyrrhetinic acid was lowest. The contents of glycyrrhetinic acid, glycyrrhizin and liquiritin from GanCao were stable in six samples (RSD < 2.0%). But the contents of 10-deacetylbaccatin III and baccatin III from the bark of T. Chinensis Var. Mairei and the contents of ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 from RenShen were fluctuant significantly (RSD > 8.2%). It is suggested that the contents of the main activity components in different batches CHCs have great variance and taxol should not be looked as sole index to evaluate the quality of CHC.
Table 3.
Contents of the 10 components in the six CHC samples from different batches (mg/g, n=3)
Herb source identification of main constituents in CHC
Through comparative studying on chromatographic peak in chromatogram [Figure 1] of standard, the respective extracts of Hongdoushan, RenShen, GanCao, and CHC, The herbal sources of 32 chromatographic peaks were identified. Among them, peaks 1, 2, 3, 5, 7, 9, 27, 28, 29, 30, 31, and 32 derived from Hongdoushan, peaks 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, and 26 derived from RenShen, peaks 4, 6, 8, 10, 11, 12, 13 and 17 derived from GanCao.
CONCLUSIONS
In conclusion, a rapid, sensitive and reliable method was established to assess the quality of CHC. By a combination of powerful tools (HPLC-PDA), 10 components have been quantified simultaneously for the 1st time. Among these 10 components, 9 components except for taxol were quantificationally analyzed for the 1st time in CHC. Meanwhile, the herbal resources of 32 main peaks in the chromatogram of CHC were identified. Among these 10 components, ginsenoside Rg1, ginsenoside Re, and ginsenoside Rb1 are the main components in RenShen, which are mainly responsible for the anti-tumor activity, the anti-fatigue activity and the immunomodulatory activity of RenShen; glycyrrhizin and liquiritin from GanCao, as flavonoids, have antibacterium activity, while glycyrrhetinic acid belonging to triterpenoids plays the anti-inflammatory role in GanCao; taxol, 10-deacetylbaccatin III, baccatin III, and cephalomannine are from the bark of T. Chinensis Var. Mairei, in which 10-deacetylbaccatin III and baccatin III are the precursors of taxol, and taxol and cephalomannine are the main components with anti-tumor activities in this formula. It is suggested that, by the developed method, we could simultaneously determine the main active components from RenShen, GanCao, and Hongdoushan, respectively. Hence, the method is helpful to improve the quality control of CHC and lays the ground for studying on the biologic activity of CHC. Further, studies are necessary to find the factors resulting in the quality fluctuation of CHC in order to guarantee the quality stability of CHC.
ACKNOWLEDGMENTS
This work was financially supported by the Fundamental Research Funds for the Central Universities (Project No.CQDXWL-2012-130). The Authors declare that there is no conflict of interest.
Footnotes
Source of Support: This work was financially supported by the Fundamental Research Funds for the Central Universities (Project No.CQDXWL-2012-130)
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: 2002. World Health Organization. In: WHO Traditional Medicine Strategy 2002-2005. http://apps.who.int/medicinedocs/pdf/s2297e/s2297e.pdf . [Google Scholar]
- 2.Center for Drug Evaluationand Research (CDER). Food and Drug Administration. In: Guidance for Industry Botanical Drug Products. 2004. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070491.pdf .
- 3.Chang YX, Ding XP, Qi J, Cao J, Kang LY, Zhu DN, et al. The antioxidant-activity-integrated fingerprint: An advantageous tool for the evaluation of quality of herbal medicines. J Chromatogr A. 2008;1208:76–82. doi: 10.1016/j.chroma.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Wei YH, Qi LW, Li P, Luo HW, Yi L, Sheng LH. Improved quality control method for Fufang Danshen preparations through simultaneous determination of phenolic acids, saponins and diterpenoid quinones by HPLC coupled with diode array and evaporative light scattering detectors. J Pharm Biomed Anal. 2007;45:775–84. doi: 10.1016/j.jpba.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Wang L, Yu X, Ye J. Development of the chromatographic fingerprint of herbal preparations Shuang-Huang-Lian oral liquid. J Pharm Biomed Anal. 2006;41:845–56. doi: 10.1016/j.jpba.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen Hoai N, Dejaegher B, Tistaert C, Nguyen Thi Hong V, Rivière C, Chataigné G, et al. Development of HPLC fingerprints for Mallotus species extracts and evaluation of the peaks responsible for their antioxidant activity. J Pharm Biomed Anal. 2009;50:753–63. doi: 10.1016/j.jpba.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Yu BS, Lai SG, Tan QL. Simultaneous determination of cinnamaldehyde, eugenol and paeonol in traditional Chinese medicinal preparations by capillary GC-FID. Chem Pharm Bull (Tokyo) 2006;54:114–6. doi: 10.1248/cpb.54.114. [DOI] [PubMed] [Google Scholar]
- 8.Abdelwahab SI, Abdul AB, Elhassan MM, Mohan S, Ibrahim MY, Mariod AA, et al. GC/MS determination of bioactive components and antibacterial properties of Goniothalamus umbrosus extracts. Afr J Biotechnol. 2009;8:3336–40. [Google Scholar]
- 9.Ji YB, Xu QS, Hu YZ, Heyden YV. Development, optimization and validation of a fingerprint of Ginkgo biloba extracts by high-performance liquid chromatography. J Chromatogr A. 2005;1066:97–104. doi: 10.1016/j.chroma.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Xu Z, Zhou H, Cao G, Cong XD, Zhang Y, et al. Simultaneous determination of four bioactive compounds in Verbena officinalis L. by using high-performance liquid chromatography. Pharmacogn Mag. 2012;8:162–5. doi: 10.4103/0973-1296.96575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He D, Chen B, Tian Q, Yao S. Simultaneous determination of five anthraquinones in medicinal plants and pharmaceutical preparations by HPLC with fluorescence detection. J Pharm Biomed Anal. 2009;49:1123–7. doi: 10.1016/j.jpba.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Guo JM, Xue CF, Duan JA, Shang EX, Qian DW, Tang YP, et al. Fast characterization of constituents in HuangKui capsules using UPLC-QTOF-MS with collision energy and massfragment software. Chromatographia. 2011;73:447–56. [Google Scholar]
- 13.Karioti A, Furlan C, Vincieri FF, Bilia AR. Analysis of the constituents and quality control of Viola odorata aqueous preparations by HPLC-DAD and HPLC-ESI-MS. Anal Bioanal Chem. 2011;399:1715–23. doi: 10.1007/s00216-010-4473-2. [DOI] [PubMed] [Google Scholar]
- 14.SFDA, State Drug Administration National Drug Standards (Trial Implementation) promulgated. State approval number: Z20026350, China [Google Scholar]
- 15.Gautam A, Koshkina N. Paclitaxel (taxol) and taxoid derivates for lung cancer treatment: Potential for aerosol delivery. Curr Cancer Drug Targets. 2003;3:287–96. doi: 10.2174/1568009033481912. [DOI] [PubMed] [Google Scholar]
- 16.Tian Y, Lu YY, Xie J, Cheng Y, Qi RB, Wu YJ, et al. Rapid determination of ginsenoside Rg1, Re and Rb1 in Ginseng samples by capillary electrophoresis. Anal Method. 2009;1:203–7. doi: 10.1039/b9ay00043g. [DOI] [PubMed] [Google Scholar]
- 17.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–18. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 18.Block KI, Mead MN. Immune system effects of Echinacea, Ginseng, and Astragalus: A review. Integr Cancer Ther. 2003;2:247–67. doi: 10.1177/1534735403256419. [DOI] [PubMed] [Google Scholar]
- 19.Chinese Pharmacopoeia. Part. I. Beijing: China Medical Science and Technology Press; 2010. National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People's Republic of China; p. 81. [Google Scholar]
- 20.Nomura T, Fukai T, Akiyama T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. 2002;74:1199–206. [Google Scholar]
- 21.Parmar VS, Jha A, Bisht KS, Taneja P, Singh SK, Kumar A, et al. Constituents of the yew trees. Phytochemistry. 1999;50:1267–304. doi: 10.1016/s0031-9422(98)00702-x. [DOI] [PubMed] [Google Scholar]


