The need for soft and elastic scaffold materials to resemble the elastic nature of soft tissues has recently driven the development of various biodegradable elastomeric polymers in tissue engineering and drug delivery. [1–10] As a substitute for the native extracellular matrix (ECM) of a target tissue or organ, the ideal scaffold material should be not only soft and elastic, but also amenable to surface biofunctionalization to mediate cell and tissue responses. [6, 8, 10–12] A number of biodegradable elastomeric polymers such as poly(glycerolsebacate) (PGS), [2, 9] poly(ε-caprolactone) (PCL), [2, 9] and citrate-based biodegradable elastomers (CABEs) such as poly (1, 8-octanediol citrate) (POC), [1, 4, 7] crosslinked urethane-doped polyester elastomers (CUPE), [5] poly(alkylene maleate citrates) (PAMCs), [13] and biodegradable photoluminescent polymers (BPLPs) [6] have been synthesized to demonstrate their potential in tissue engineering. However, most of these materials are mechanically weak, with a tensile strength at dry state typically no more than 10 MPa, [1–4, 7, 9] which is significantly lower than that of human anterior cruciate ligaments (38 MPa). Unfortunately, these materials become even weaker when fabricated into porous scaffolds and/or used in vivo in physiological wet conditions significantly limiting their use in various tissue engineering applications. [3]
Effectively balancing the mechanical properties, biofunctionalizition, and biodegradation of the above polymers, especially site- or ligand-specific biofunctionalization to regulate cell/tissue-biomaterial interactions, has been a challenge. [14] In terms of addressing concerns on the weak mechanical strength of the existing elastomers, the introduction of urethane or amine groups into polyesters has proved to be an effective way for improving mechanical strength of polyester elastomers. [2, 5, 6, 9, 15] Increasing the cross-linking density may serve as another strategy. [1, 7] However, improving mechanical properties by increasing polymer cross-linking densities and introducing urethane/urea bonds in elastomers sacrafices the limited functional groups for future bioconjugation/functionalizion and also slows the material degradation rate. As one of the most effective, site-specific reactions that are tolerant to water, oxygen, and a wide range of functionalities, azide-alkyne cycloaddition (AAC, click chemistry), [14, 16–25] especially copper-free click chemistry, has been a promising method for functionalizing bio-related systems. [14, 22, 23] It was also reported that the triazole rings resulting from click chemistry could imitate amide bonds serving as mechanical strength improving moieties. [20, 26] Herein, click chemistry was introduced into CABEs to serve a dual role and create a novel material chemistry design strategy to simultaneously improve the bulk material mechanical strength and enable easy surface site-specific biofunctionalization, which can also be broadly applied to other functional biodegradable polymer design
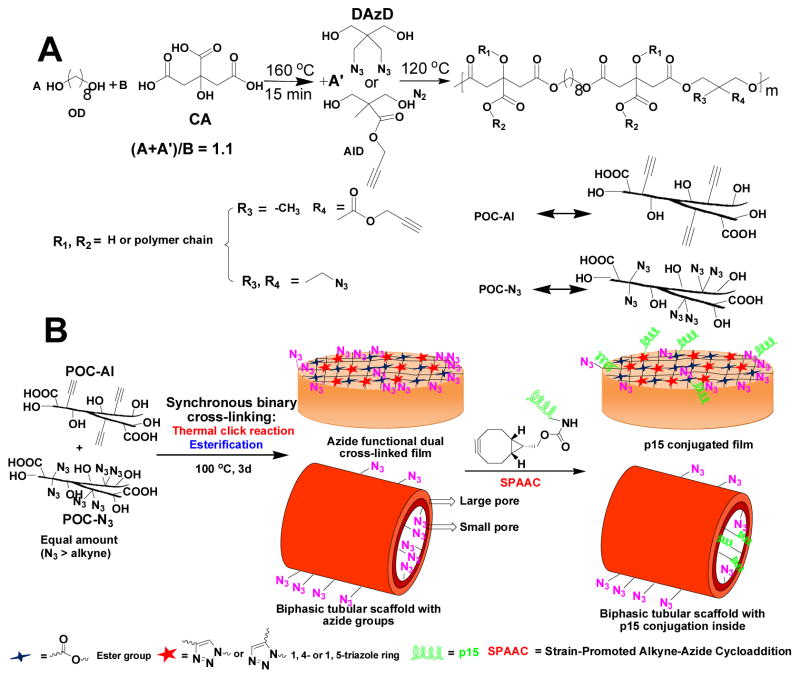
By introducing azide and alkyne functional diols, azide (pre-POC-N3) and alkyne (pre-POC-Al) functionalized POC pre-polymers were synthesized (Scheme 1A). Without the use of any toxic copper-catalysts, pre-POC-N3 and pre-POC-Al were mixed and crosslinked via a thermal synchronous binary (TSB) cross-linking mechanism. In the TSB cross-linking, thermal click reaction between azide and alkyne groups and esterification between –COOH and –OH groups [27, 28] took place simultaneously to form TSB crosslinked POC-click elastomers (Scheme 1B). POC-click elastomers possessed much improved mechanical strength (up to 40 MPa of tensile stress). The uniquely introduced extra azide groups on POC-click polymers also enabled the easy conjugation of heat-labile biomolecule such as peptides or proteins via another copper-free click reaction, strain-promoted alkyne-azide cycloaddition (SPAAC) in aqueous environment at room temperature or 37 °C. Collagen mimetic peptide p15, which can effectively promote the adhesion and proliferation of endothelial cells (ECs), [12] was exemplarily clicked onto POC-click films and scaffolds (Scheme 1B and S1) through SPAAC.
Scheme 1.
(A) Synthesis of functional POC pre-polymers: POC-N3 and POC-Al; (B) Synchronous binary cross-linked POC-click films, porous tubular biphasic scaffold preparation, and p15 conjugation by strain-promoted alkyne-azide cycloaddition (SPAAC).
Pre-POC-N3 and pre-POC-Al were synthesized separately by polycondensation of citric acid (CA), 1, 8-octanediol (OD), and azide or alkyne functional diols (diazido-diol [DAzD] or alkyne-diol [AlD] as shown in Scheme 1A via a one-pot synthesis process. [1, 4, 7] The successful introduction of azide or alkyne groups into the pre-polymers was verified by FTIR and NMR (Figure S1 and S2), as indicated by the appearance of the characteristic infrared absorption peak of azide group (2100 cm−1 in FTIR) or peak of the protons -CH2-C≡CH in 1H-NMR (around 4.5 ppm), respectively. The intensities of both peaks increased with an increase of feeding ratios of the functional diols to OD, indicating the increasing contents of azide or alkyne groups in the corresponding pre-POC-N3-x or pre-POC-Al-x (x = 1, 2 or 3). Here, “x” represents the molar ratio of DAzD or AID to CA/10.
The optimized TSB cross-linking temperature of POC-click polymers was determined to be 100°C (Figure S3), which is suitable for the thermal click reaction between azide and alkyne groups without affecting the reactivity of residual azide groups. The cross-linking density can be controlled by varying cross-linking times and the clickable pre-polymer ratios (pre-POC-N3-x/pre-POC-Al-y, x, y = 1, 2 or 3). Some azide groups were preserved after cross-linking (Figure S4) since the specially chosen DAzD molecule contains two azide groups, while the AlD molecule contains only one alkyne group (Scheme 1A).
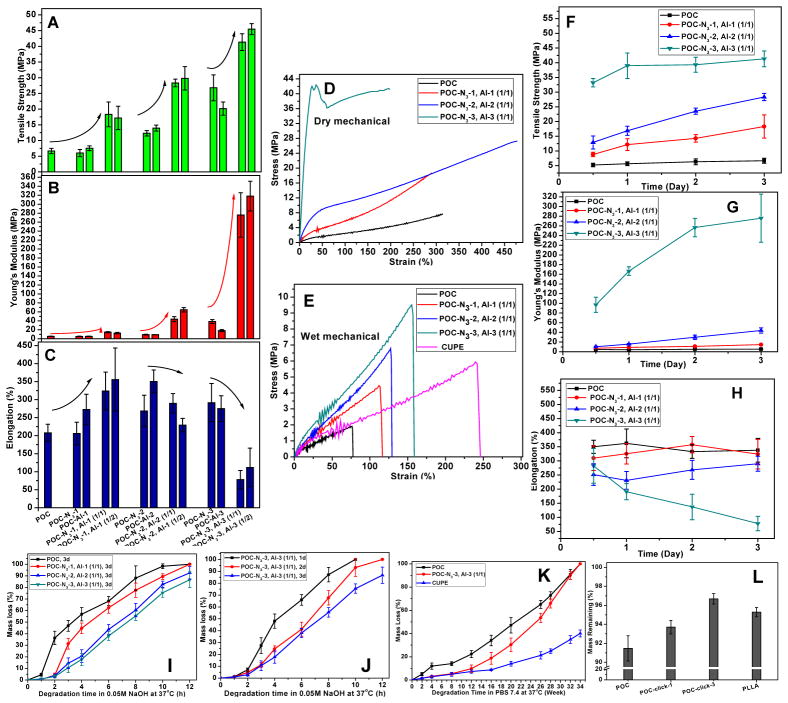
The mechanical properties of POC and POC-click polymers are shown in Figure 1A-H. The maximum tensile stress of both POC-click-x (POC-N3-x, Al-x (w/w = 1/1)) and POC-N3-x, Al-x (1/2) (x=1, 2 or 3) polymer films are 10–40 MPa higher than that of POC (5 MPa), and 10–20 MPa higher than that of the corresponding POC-N3-x and POC-Al-x films (Figure 1A and Table S1). These results suggested that although the replacement of relatively long-chain OD by short-chain DAzD or AlD diol monomers (Scheme 1) increases the tensile stress, undoubtedly, the introduction of thermal click reaction plays a predominant role. A more significant increase could be seen in the case of Young’s modulus (Figure 1B), which relates directly to the cross-linking density (Table S1) of the film. The Young’s modulus of POC-clik-3 and POC-N3-3, Al-3 (1/2) films were as high as 300 MPa, which is nearly 60 times higher than that of POC (Table S1). The difference in the tensile stress or Young’s modulus between POC-click-x and POC-N3-x, Al-x (1/2) was insignificant due to the steric hindrance limiting the further progression of thermal click reaction. A similar phenomena could also be seen in the cases of POC-click polymers made from mixtures of POC-N3-x and POC-Al-y (x, y=1, 2 or 3 and x ≠ y) with different weight ratios (Figure S5). The elongation at break of the films showed an overall inverse correlation to cross-linking density, and displayed values all around 200–300% except for POC-click-3 and POC-N3-3, Al-3 (1/2), which were all lower than 100% (Figure 1C and Table S1). POC-click-1 and POC-click-2 all showed elastomeric properties similar to POC (Figure 1D). Although the stress-strain curve of POC-click-3 had a yield point (Figure 1D) that is characteristic of plastic polymers, the same polymer became elastic after being immersed in PBS for about 24 hrs (Figure 1E), indicating that POC-click-3 can still serve as an elastomeric material in vivo (wet conditions). POC-click wet mechanical strengths were stronger than that of another citrate-based mechanically strong elastomer, CUPE. [5] When the cross-linking time was increased from 0.5 day to 3 days, the tensile stress and Young’s modulus of POC-click polymers showed a remarkable and continuous increase, especially for POC-click-1 and POC-click-2, while POC only showed very limited improvement during the same time period (Figure 1F and 1G). These results further demonstrate the impact of thermal click reactions on mechanical properties. The elongation of the polymer films showed no significant change when the cross-linking time increased (Figure 1H), except in the case of POC-click-3. The above investigations suggested that the introduction of click chemistry into POC could significantly improve the mechanical strengths of the TSB crosslinked polymers. To further expand click chemistry-based elastomers, clickable biodegradable photoluminescent polymer (BPLP-Ser[6]) and urethane-doped polyester (UPE[5]) were also synthesized. The TSB crosslinked polymers (CBPLP-Ser-click, CUPE-click) also showed significantly enhanced mechanical strengths compared to normal BPLP and CUPE (Figure S6).
Figure 1.
Mechanical properties of polymer films: Tensile strength (A), Young’s modulus (B) and Elongation at break (C) of cross-linked films (all 100°C, 3d) of POC, POC-N3 series, POC-Al series, and TSB cross-linked POC-click series (1/1 and 1/2 between the parentheses represents the weight ratio between pre-POC-N3-x and pre-POC-Al-x, x=1, 2, or 3). (D) Tensile stress-strain curves of POC-click series and POC cross-linked films (100°C, 3d) under dry condition. (E) Wet mechanical properties: Tensile stress-strain curves of POC-click series, POC and CUPE cross-linked films (100°C, 3d) after being immersed in PBS (pH 7.4) for around 24 hrs. (F, G and H) The change of mechanical properties of POC-click and POC films after heating at 100°C for different times: Tensile strength (F), Young’s modulus (G) and Elongation at break (H). Degradation study of POC-click polymer series and controls (POC or/and CUPE/PLLA) in vitro, in 0.05M NaOH solution (I, J), PBS (pH 7.4) (K), and 20 weeks in vivo (L). All the polymer films used were cross-linked at 100°C for 3 days except where specified otherwise. Films with thicknesses of 0.15–0.30 mm and 0.75–0.95 mm were used in in vitro degradations in 0.05M NaOH or PBS solutions (I, j and K) and in vivo (L) degradation, separately.
The in vitro and in vivo degradation results of different polymers are shown in Figure 1E-H. POC-click polymers degraded slower than POC in 0.05M NaOH solution. POC-click-1 and POC degraded completely, but around 80% of POC-click-3 degraded after 12 hrs incubation. The degradation rates decreased when the cross-link density increased (Figure 1I). A similar trend was found with an increase of cross-linking times from 1 day to 3 days (Figure 1J). The degradation profiles of POC-click-3, POC, and CUPE in PBS (pH 7.4) are shown in Figure 1K. During the first 12 weeks, POC-click-3 demonstrated a mass loss of no more than 5% while POC lost around 25% of its initial mass. After the 12th week, however, POC-click-3 entered into a relatively rapid degradation period and the mass loss eventually caught up with that of POC by the 32nd week. POC-click-3 and POC were completely degraded after 34 weeks, while no more than 40% of CUPE was degraded. The “first slow then fast” degradation phenomenon of POC-click-3 was considered to be related to its chemical structure (Scheme S1). Both ester bonds (green) and triazole rings (red) exist in POC-click-3. Ester bonds degraded much faster than triazole rings, [29] which is why initially more hydrophobic POC-click-3 degraded much slower than relatively hydrophilic pure polyester, POC. Once the ester bonds surrounding DAzD (in the blue circle in Scheme S2) in POC-click-3 were hydrolyzed, the DAzD crosslink points were destroyed. Along with the destruction of the DAzD cross-link points, the degradation rate of POC-click-3 became even faster than that of POC, which allowed the mass loss to catch up with that of POC. The “first slow then fast” degradation characteristic of POC-click polymers is favorable in many biomedical applications especially tissue engineering, as the preservation of mechanical strength in the initial period after implantation before tissue regeneration is preferred. After 20 weeks of subcutaneous implantation in the back of Sprague Dawley (SD) rats, the mass loss of POC-click-1, POC-click-3, POC, and PLLA were 6.28%, 3.28%, 9.54% and 5.71% respectively (Figure 1L). On another note, we have previously demonstrated that partially replacing 1,8-octanediol with N-methyldiethanolamine (MDEA) in POC synthesis could simultaneously improve the polymer mechanical strength and degradation rate. [7] This MDEA strategy may also be applied to POC-click synthesis to further increase the material mechanical strength and degradation rate.
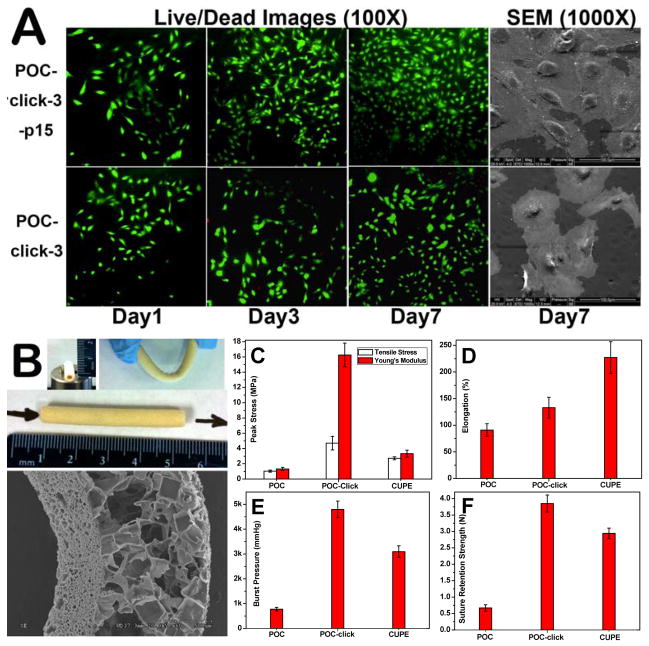
The residual azide groups on POC-click polymers (Figure S4) have paved the way for convenient bioactive molecule conjugation on the surface of POC-click films or scaffolds via SPAAC (Scheme 1B). As an example, collagen mimetic peptide p15, which can effectively promote the adhesion and proliferation of ECs, was conjugated onto the surface of POC-click-3 films by SPAAC (Scheme 1B and S1), and the viability/proliferation of human umbilical vein endothelial cells (HUVEC) on POC-click-3-p15 films were investigated. The successful conjugation of p15 onto the surface of POC-click-3 films was confirmed by the decrease of azide absorption peak (2100 cm−1) after p15 conjugation shown in the FTIR spectra (Figure S7A) as well as the appearance of the characteristic peak of guanidine groups on p-15 peptides as shown in the UV-vis spectra (Figure S7B) after Sakaguchi reactions (Figure S8). [11] The effect of p15 conjugation on HUVEC proliferation was investigated by MTT assay, Live/Dead assay, and SEM using untreated POC-click-3 films as control (Figure S7C and 2A). From the MTT resulst (Figure S7C), it could be seen that the initial HUVEC cell number (day 1) on POC-click-3-p15 films was higher than that o untreated POC-click-3 films. However, the difference between them was not significant, which could also be seen from the Live/Dead assay images (Figure 2A). After the initial cell adhesion, HUVEC proliferation on POC-click-3-p15 films was obviously faster than that on untreated POC-click-3 films, similar to the trend on p15-modified PTFE as reported previously. [11] The HUVEC cell density on POC-click-3-p15 films at day 7 nearly doubled compared to the control POC-click-3 films. The Live/Dead images also supported the same growth pattern. Both Live/Dead assay images and SEM images (Figure 2A) showed the characteristic cobblestone morphology of live cells (green fluorescence in Live/Dead images). Only few red fluorescence (dead cells) positive cells were found in Live/Dead images. The number of dead HUVEC cells on POC-click-3-p15 films was much less than that on POC-click-3 films (Figure 2A). The HUVEC cell proliferation results show that the p15 conjugation on POC-click-3 surfaces could promote HUVEC cell adhesion and proliferation. Using the convenient SPAAC method, other bioactive molecules can also be easily conjugated onto POC-click films or scaffolds in an amiable condition with much less risk of destroying the bioactivities of the conjugated bioactive molecules.
Figure 2.
A: Live/Dead assay (1, 3, and 7 days) and SEM (7 day) images of HUVECs proliferation on POC-click-3-p15 films and POC-click-3 films; B: Photographs and representative SEM image of POC-click-3 tubular porous biphasic scaffold. The mechanical properties of POC, POC-click (using POC-click-3), and CUPE tubular biphasic scaffold (TBS): tensile strength and Young’s modulus (C) and elongation at break (D) in uniaxial tension experiments; vascular graft burst pressure results (E) and graft suture retention strengths (F).
To further support the potentials of POC-click polymers in tissue engineering applications, especially blood vessel tissue engineering, POC-click-3 was chosen as a representative material to be molded into anatomically correct tubular biphasic scaffolds (TBS), which consist of an inner thin porous phase (pore size of 1–20 μm) to simulate the native elastic lamina and outer macroporous phase (pore size of 150–250 μm). The mechanical properties of POC-click-3 scaffolds were tested and compared with TBSs made by POC and CUPE. Furthermore, the conjugation of p15 onto the inner surface of POC-click TBS was also conducted. It was demonstrated that a rough inner lumen surface is more favorable for the growth of ECs, and pore size of 1–20 μm is preferable for the compartmentalization of ECs and smooth muscle cells (SMCs) simulating the elastic lamina in native vessels. Pore sizes of 150–250 μm have been proven to be ideal for the growth of fibroblasts and SMCs and the formation of extracellular matrix (ECM). [3, 30] The photographs and representative SEM images of POC-click TBS shown in Figure 2B clearly imply the soft nature of the biphasic scaffold. Although molded into a porous scaffold, the tensile strength and Young’s modulus of POC-click TBS (around 5 and 17 MPa, respectively) are all much higher than that of POC TBS (~1 and 1.2 MPa respectively), and even higher than that of CUPE TBS (~3.8 and 4 MPa, respectively) (Figure 2C). The tensile strength of native radial arteries is 2.68 ± 1.81 MPa. Although the elongation of POC-click TBS is somewhat lower than that of CUPE TBS, it is higher than that of POC TBS (Figure 2D). Scaffold burst pressure is another key parameter that determines the suitability of a vascular graft for implantation. As seen from Figure 2E, the burst pressure of POC-click TBS is around 5000 mmHg, which is higher than that of POC (below 1000 mmHg) and CUPE (around 3500 mmHg) TBSs, and also higher than that of both saphenous veins and mammary arteries (1599 ± 877 and 4225 ± 1368 mmHg, respectively), which are currently used as the “gold standards” of vascular grafts. [31] The suture retention strength of POC-click-3 TBS is around 3.75 N, which is higher than both POC TBS (~0.75 N) and CUPE TBS (~3.0 N) (Figure 2F) and also significantly higher than the reported value of 1.20 ± 0.23 N required for suturing arterial vascular grafts.[3] The above results provide evidence that POC-click TBS possess sufficient mechanical strengths to create off-the-shelf vascular grafts. In addition to the superior mechanical properties of POC-click vascular grafts compared with POC and even CUPE grafts, the residual azide groups on the surface of POC-click scaffolds enabled an easy bioactive molecules conjugation through SPAAC. P15 could be easily conjugated onto the surface of the inner layer of POC-click TBS, which was confirmed by FTIR spectra (Figure S9A) and UV-vis spectra (Figure S9B) as previously described.
In conclusion, the introduction of click chemistry into citrate-based biodegradable elastomer (CABE), such as poly (1, 8-octanediol citrate) (POC), can vastly increase the mechanical strength of the resulting polymer and greatly facilitate biomolecule conjugation through the clickable moieties. POC-click elastomers were polymerized under a unique thermal synchronous binary (TSB) cross-linking mechanism (post-esterification between –COOH and –OH groups and thermal click reactions between newly introduced alkyne and azide groups). Furthermore, the residual azide groups on the surface of POC-click films and scaffolds enabled facile and efficient surface conjugation of bioactive molecules through strain-promoted alkyne-azide cycloaddition (SPAAC). The present work successfully combined two kinds of copper-free click reactions, thermal click reaction and SPAAC, which serve as a novel cross-linking method and efficient surface conjugation route, respectively. A dual role application of click chemistry can also be extended to the other CABEs such as PAMC, CUPE and BPLP and possibly many other biodegradable polymers such as PGS and PCL. We believe that the clickable biodegradable elastomer design will greatly expand the application of biodegradable polymers, especially for CABEs in many biomedical areas such as tissue engineering, drug delivery, orthopedic fixation devices, and other medical implants where mechanical properties and biofunctionality are key in their success. The synthesis of clickable POC represents an innovation in the design of functional biodegradable polymers and may facilitate their translation in diversified biomedical applications.
Supplementary Material
Acknowledgments
This work was supported in part by a National Institute of Biomedical Imaging and Bioengineering (NIBIB) Award EB012575, a National Cancer Institute (NCI) Award CQ182670, National Science Fundation (NSF) Awards (DMR1313553, CMMI 1266116), and a National Natural Sciences Foundation of China Award (31228007).
Footnotes
Supporting Information is available online from the Wiley Online Library or from the author.
Contributor Information
Dr. Jinshan Guo, Department of Biomedical Engineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA
Dr. Zhiwei Xie, Department of Biomedical Engineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA
Dr. Richard T. Tran, Department of Biomedical Engineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA
Denghui Xie, Academy of Orthopedics of Guangdong Province, Guangzhou, 510630, China, Department of Orthopedics, The Third Affiliated Hospital, Southern Medical University, Guangzhou, 510630, China, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, USA.
Prof. Jian Yang, Department of Biomedical Engineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA, Academy of Orthopedics of Guangdong Province, Guangzhou, 510630, China, Department of Orthopedics, The Third Affiliated Hospital, Southern Medical University, Guangzhou, 510630, China, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, USA.
References
- 1.Yang J, Webb AR, Ameer GA. Adv Mater. 2004;16:511. [Google Scholar]
- 2.Wu W, Allen RA, Wang Y. Nat Med. 2012;18:1148. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey J, Xu H, Nguyen KT, Yang J. J Biomed Mater Res A. 2010;95A:361. doi: 10.1002/jbm.a.32846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA. Adv Mater. 2006;18:1493. [Google Scholar]
- 5.Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L, Yang J. Biomaterials. 2008;29:4637. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L. Proc Natl Acad Sci USA. 2009;106:10086. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Biomaterials. 2006;27:1889. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 8.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Biomaterials. 2012;33:7972. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano MC, Chung EJ, Ameer GA. Adv Funct Mater. 2010;20:192. [Google Scholar]
- 10.de Mel A, Jell G, Stevens MM, Seifalian AM. Biomacromolecules. 2008;9:2969. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Lai Y, Yu L, Ding J. Biomaterials. 2010;31:7873. doi: 10.1016/j.biomaterials.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Hill A, Imran M. J Biomater Sci Polym Ed. 2005;16:875. doi: 10.1163/1568562054255754. [DOI] [PubMed] [Google Scholar]
- 13.Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang L, Yang J. Soft Matt. 2010;6:2449. doi: 10.1039/C001605E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dongen SFM, Maiuri P, Marie E, Tribet C, Piel M. Adv Mater. 2013;25:1687. doi: 10.1002/adma.201204474. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Hill PS, Siegwart DJ, Vacanti N, Lytton-Jean AKR, Cho SW, Ye A, Langer R, Anderson DG. Adv Mater. 2011;23:H95. doi: 10.1002/adma.201003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao D, Tan S, Yuan D, Lu W, Rezenom YH, Jiang H, Wang LQ, Zhou HC. Adv Mater. 2011;23:90. doi: 10.1002/adma.201003012. [DOI] [PubMed] [Google Scholar]
- 17.He C, Zhuang X, Tang Z, Tian H, Chen X. Adv Healthcare Mater. 2012;1:48. doi: 10.1002/adhm.201100008. [DOI] [PubMed] [Google Scholar]
- 18.Tian H, Tang Z, Zhuang X, Chen X, Jing X. Prog Polym Sci. 2012;37:237. [Google Scholar]
- 19.Guo J, Meng F, Jing X, Huang Y. Adv Healthcare Mater. 2013;2:784. doi: 10.1002/adhm.201200157. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Wei Y, Zhou D, Cai P, Jing X, Chen XS, Huang Y. Biomacromolecules. 2011;12:737. doi: 10.1021/bm1013662. [DOI] [PubMed] [Google Scholar]
- 21.Liang K, Such GK, Zhu Z, Yan Y, Lomas H, Caruso F. Adv Mater. 2011;23:H273. doi: 10.1002/adma.201101690. [DOI] [PubMed] [Google Scholar]
- 22.Becer CR, Hoogenboom R, Schubert US. Angew Chem Int Ed Engl. 2009;48:4900. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- 23.Manova R, van Beek TA, Zuilhof H. Angew Chem Int Ed Engl. 2011;50:5428. doi: 10.1002/anie.201100835. [DOI] [PubMed] [Google Scholar]
- 24.Heller DA, Levi Y, Pelet JM, Doloff JC, Wallas J, Pratt GW, Jiang S, Sahay G, Schroeder A, Schroeder JE, Chyan Y, Zurenko C, Querbes W, Manzano M, Kohane DS, Langer R, Anderson DG. Advanced Materials. 2013;25:1449. doi: 10.1002/adma.201202881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong T, Adzima BJ, Baker NH, Bowman CN. Advanced Materials. 2013;25:2024. doi: 10.1002/adma.201203815. [DOI] [PubMed] [Google Scholar]
- 26.Horne WS, Yadav MK, Stout CD, Ghadiri MR. J Am Chem Soc. 2004;126:15366. doi: 10.1021/ja0450408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J, Meng F, Li X, Wang M, Wu Y, Jing X, Huang Y. Macromol Biosci. 2012;12:533. doi: 10.1002/mabi.201100394. [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Luo Q, Wan X, Petrović ZS, Shah BK. Biomacromolecules. 2011;13:261. doi: 10.1021/bm201554x. [DOI] [PubMed] [Google Scholar]
- 29.Yao K, Wang J, Zhang W, Lee JS, Wang C, Chu F, He X, Tang C. Biomacromolecules. 2011;12:2171. doi: 10.1021/bm200460u. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Tran RT, Qattan IS, Tsai YT, Tang L, Liu C, Yang J. Biomaterials. 2013;34:4048. doi: 10.1016/j.biomaterials.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NAF, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.