Abstract
Angiogenesis is regulated by highly coordinated action of various proteins with pro- and anti-angiogenic functions. Among the many cytoplasmic signaling proteins that are activated by VEGFR-2, activation of PLCγ1 is considered to play a pivotal role in angiogenic signaling. In previous study we have identified c-Cbl as a negative regulator of PLCγ1 in endothelial cells, the biochemical and biological significance of c-Cbl, however, in angiogenesis in vivo and molecular mechanisms involved were remained elusive. Here we report that genetic inactivation of c-Cbl in mice results in enhanced tumor angiogenesis and retinal neovascularization. Endothelial cells derived from c-Cbl null mice displayed elevated cell proliferation and tube formation in response to VEGF stimulation. Loss of c-Cbl also resulted in robust activation of PLCγ1 and increased intracellular calcium release. c-Cbl-dependent ubiquitination selectively inhibited tyrosine phosphorylation of PLCγ1 and mostly refrain it from ubiquitin-mediated degradation. Hence, we propose c-Cbl as an angiogenic suppressor protein where upon activation it uniquely modulates PLCγ1 activation by ubiquitination and subsequently inhibits VEGF-driven angiogenesis.
Introduction
Angiogenesis, the growth of new blood vessels, is of key importance in a broad array of physiologic and pathologic conditions ranging from inflammation, and cancer to age-related macular degeneration. Regulation of angiogenesis is often viewed as a balance between pro-angiogenic and anti-angiogenic factors, and when the balance shifts in favor of pro-angiogenic factors, an angiogenic switch turns on the normally inactive endothelial cells to grow new blood vessels. Activation of VEGFR-2 is considered a pivotal signaling event that determines many aspects of endothelial cells function, including differentiation, proliferation and migration (reviewed in Olsson et al., 2006, Rahimi, 2006). While these outcomes are initially determined by the presence of the VEGF ligands, in the recent years, it has become evident that protein ubiquitination involving c-Cbl ubiquitin E3 ligase also significantly amend the angiogenic signaling events, particularly by targeting PLCγ1 (phospholipase Cγ1), the major substrate of VEGFR-2 in endothelial cells (Singh et al., 2005; Singh et al., 2007).
The Cbl family ubiquitin E3 ligase proteins consist of three closely related proteins, including c-Cbl, Cbl-b and Cbl-3. All of c-Cbl family gene products contain a highly conserved TKB (tyrosine kinase binding) domain and a RING finger domain in their N-terminal region. The C-terminus of Cbl family proteins interacts with various SH2 and SH3 domain-containing proteins (Thien et al., 2005). The c-Cbl protein primarily functions as an E3 ubiquitin ligase where its RING finger domain recruits ubiquitin-conjugating (E2) enzyme (Swaminathan and Tsygankov, 2006). The binding of c-Cbl to VEGFR-2 occurs directly via phospho-Tyr1052 and phospho-Tyr1057 of VEGFR-2, as well as indirectly through PLCγ1. Phospho-Tyr1057 along with phospho-Tyr1052 on VEGFR-2 recognizes the TKB (tyrosine kinase binding) domain of c-Cbl (Singh et al., 2007). Although c-Cbl is recruited to and phosphorylated by VEGFR-2, it is dispensable for ubiquitination and degradation VEGFR-2 (Singh et al., 2005, Singh et al., 2007). The C-terminus of c-Cbl on the other hand, binds to SH3 domain of PLCγ1 and mediates its ubiquitination (Singh et al., 2007).
Activation of PLCγ1 in endothelial cells is identified as a key downstream mediator of the angiogenic signaling of VEGFR-2. Targeted deletion of PLCγ1 in mouse and zebrafish causes in early embryonic lethality due to impairment of vasculogenesis and erythrogenesis (Liao H-J et al., 2002; Lawson et al., 2003). Also, mutation of Y1173 on VEGFR-2, a major PLCγ1 binding site on VEGFR-2 impairs the ability of VEGFR-2 to stimulate angiogenesis in vitro (Takahashi et al., 2001; Meyer et al., 2003; Rahimi, 2006). Consistent with the cell in vitro culture system, the mice homozygous for the mutant VEGFR-2Y1173F knock-in allele dies with sever defect in vasculogenesis (Sakurai et al., 2005), further supporting the hypothesis that PLCγ1 activation plays central role in angiogenesis.
To date, the in vivo function of c-Cbl in angiogenesis, in particular in relation to PLCγ1 has not been fully established. In this study we aimed to determine the functional consequences of c-Cbl in angiogenesis and its role in PLCγ1 activation. Our present data demonstrate that genetic inactivation of c-Cbl in mice results in an increased in phosphorylation of PLCγ1 leading to endothelial cell proliferation and angiogenesis. Taken together, our data identifies c-Cbl as an angiogenic suppressor protein, acting as an endogenous PLCγ1 inhibitor.
Methods
Cell culture and cell lines
Primary mouse dermal microvascular endothelial cells (MVE cells) were grown in HUVEC medium plus growth factor supplements and penicillin/ streptomycin (Enzo, Inc). HEK-293 and Porcine aortic endothelial (PAE) cells were grown in 10% FBS. PAE cells lack endogenous expression of VEGFR-2, expression of VEGFR-2 in these cells was established by retroviral system (Rahimi et al., 2000).
Plasmids, Growth factors and Antibodies
c-Cbl and 70Z-Cbl constructs were described previously (Singh et al., 2007). Chimeric VEGFR-2 (CKR) and its expression in PAE cells is described previously (Rahimi et al., 2000). Anti-PLCγ1 antibody and anti-phospho-783-PLCγ1 were purchased from Biosource/Invirogene), Anti-Ubiquitin (FK2) antibody was from Biomol (Plymouth Meeting, PA), anti-CD31 antibody was from Abcam. VEGF-A was purchased from R&D.
Immunoprecipitation and Western blot Analysis
Equal numbers of cells from the indicated cells were grown until 80–90% confluent. After serum starvation, cells were left either resting or stimulated with VEGF-A at 37°C as indicated in the figure legends. Cells were prepared and lysed and subjected to Western blot analysis as described (Singh et al., 2007; Meyer et al., 2008). In some occasions Western blot analyzes were quantified using NIH image J software.
Isolation of dermal microvascular endothelial cells
Mouse dermal microvascular endothelial cells (MVE) were isolated from the skin of 4 weeks-old mice and further purified consecutively by anti-CD31, a maker for endothelial marker and hematopoietic cells and anti-CD146 (Millipore/Chemicon), a marker for endothelial cells using MACS LS separation column (Miltenyi Biotec Inc.).
Endothelial cell tube formation assay
Endothelial cells were seeded on Matrigel with endothelial cell growth medium (Clonetics Co.) in the absence or presence of VEGF and photographed after 16 h. Experiments were repeated three times.
Cell proliferation assay
Cell proliferation of Primary endothelial cells was evaluated by direct cell counting as described (Meyer et al., 2009). Briefly, endothelial cells were seeded at a density of 2×104 cells/well in 24-well plates and cultured overnight; the cells were then incubated in serum-free medium for 12 hours. Cells were stimulated with recombinant human VEGF-A at different concentrations as indicated in figure legends, and after48 hours they were washed with PBS, harvested by mild trypsinization, and counted with a hematocytometer. Experiments were performed in quadruplicate and values were presented as means of ±SD. Proliferation of PAE cells expressing c-Cbl and 70Z-Cbl was measured by 3H-thymidine incorporation assay as described before (Meyer et al., 2003). Comparison of the different parameters for the each group was determined by repeated measures analysis of variance (ANOVA). Significant differences were assigned using Kruskal-Wallis post hoc test. The criterion for significance for all the tests was set at p, 0.05. Analysis was done in GraphPadPrism v4.0b (GraphPad Software, San Diego, USA).
Tumor Angiogenesis
Mice (4 animals for each experiment) were injected with Matrigel (10 mg/ml), plus B16 melanoma cells (1×107) or VEGF-A. Before injection, the animals were sedated with Avertin (0.3 mL/20g mouse). Using 25-gage needle 0.3 ml matrigel mixture was injected sub-dermally into mice. After 12 days (or as outlined in the figure legends) animals were euthanized and the tumor injected tissues were removed and further analysis. In some experiments the growth of B16 melanoma cells were measured over the time of experiment. Tumor size measured using caliper as described (Woodman et al., 2003). All the statistical analyzes were done in GraphPadPrism v4.0b (GraphPad Software, San Diego, USA).
Calcium flux Assay
The assay is performed as described (Meyer et al., 2004). Briefly, cells were grown on 25 mm round glass coverslips and serum-starved for 12–18 h. Cells were incubated in an HEPES-buffered saline solution with 4 µM fluo-3 AM, supplemented with 0.02% pluronic acid for 30 minutes at 37°C. The live cells were placed in an open chamber (Molecular Probes, Inc., Eugene, OR) and positioned on the stage of a Zeiss LSM 510 Axiovert confocal laser scanning microscope equipped with an Argon laser. For each experiment, cells were scanned for at least five to 10 seconds before the addition of VEGF-A to establish a base line fluorescence reading. All the readings were made while continuously scanning the cells every 789 milliseconds.
Laser-induced choroidal neovascularization (CNV) and Fluorescein Angiography
CNV was induced using the laser photocoagulation method as described (Funakoshi et al., 2006). Green light at 532nm, 0.05 sec exposure, 200 mW power and 50 micron spot size was used from a coherent dye laser, CA. Both eyes of each mouse were treated and four spots were placed in the peripapillary area about 1–2 disc diameter from the optic nerve. Those spots that showed hemorrhagic complication were excluded from further evaluation in the follow-up study. Fourteen lesions were created in the knockout mice and eighteen lesions were created in the wild mice. Fluorescein angiography was performed on the TRC 50VT camera and Imagenet system, Topcon, Paramus, NJ. A standard 20 D lens was placed in contact with the fundus camera lens to capture the mouse fundus photographs and angiograms. Intraperitoneal injection of 0.2 ml of 1% sodium fluorescein (Akorn, Decatur, IL) was in the mouse. Angiograms were done at 1,2,3 and 4 weeks after the laser induction. Two masked retina specialists graded all the angiograms. The angiograms were graded based on previously described grading scheme: 0 (no leakage), 1 (questionable leakage), 2A (hyperfluorescence increasing in intensity but not in size) 2B (hyperfluorescence increasing in intensity and size).
Results
Loss of c-Cbl in endothelial cells promotes enhanced cell proliferation and tubulogenesis in vitro
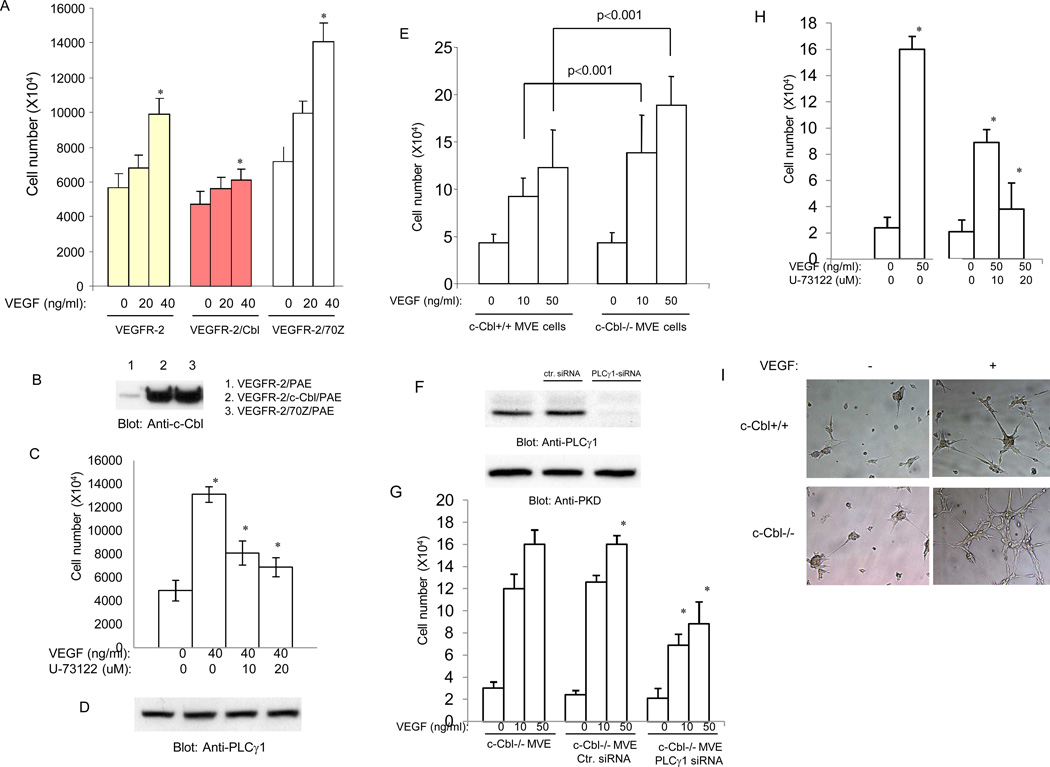
In previous study we have shown that interfering with c-Cbl activity in endothelial cells significantly increases VEGF-induced PLCγ1 phosphorylation and with it sprouting of endothelial cells in vitro (Singh et al., 2005; Singh et al., 2007). To further investigate the functional importance of c-Cbl in angiogenic signaling of VEGFR-2 we analyzed proliferation of PAE (porcine aortic endothelial cells) where we co-expressed wild type c-Cbl or inactive mutant form of c-Cbl with VEGFR-2. Over-expression of wild type c-Cbl significantly inhibited VEGF-mediated cell proliferation. Conversely, inhibition of endogenous c-Cbl activity by over-expressing a dominant negatively acting c-Cbl (70Z-c-Cbl) notably increased proliferation of PAE cells in response to VEGF stimulation (Figure 1A). The increased proliferation of PAE cells over-expressing c-Cbl-70Z was suppressed by treatment of cells with U-73122, a potent PLCγ1 inhibitor (Figure 1C). Moreover, silencing the expression of c-Cbl in PAE cells, also significantly enhanced VEGF-dependent proliferation (data not shown). Altogether, the data suggest that c-Cbl activity in endothelial cells negatively controls VEGF-dependent cell proliferation.
Figure 1. Loss of c-Cbl increases endothelial cell proliferation and tube formation.
Equal number of serum-starved PAE cells, PAE cells co-expressing VEGFR-2 with c-Cbl, PAE cells co-expressing VEGFR-2 with 70Z-c-Cbl were stimulated with different concentrations of VEGF and 3H-thymidine incorporation was measured. Cells were grown in 24-well plates in quadruplicate (A). Expression of c-Cbl and c-Cbl-70Z is shown (B). PAE cells expressing c-Cbl-70Z were subjected to proliferation assay with the increasing concentration of PLCγ1 inhibitor, U73122 (C). Cell lysates derived from the identical cells was subjected for Western blot analysis using anti-PLCγ1 antibody (D). Serum-starved c- c-Cbl null microvascular endothelial (MVE) cells and wild type microvascular endothelial cells at density of 2×104 cells/well were stimulated with recombinant human VEGF-A at different concentrations as indicated in the figure legend, and after 48 hours they were washed with PBS, harvested by mild trypsinization, and counted with a hematocytometer. Experiments were performed in quadruplicate and 3 separate experiments were performed and values were presented as means of ± SD. *P<0.01 versus c-Cbl+/+ cells (E). c-Cbl null microvascular endothelial (MVE) cells were either transfected with control siRNA or PLCγ1 and after 48 hours expression of PLCγ1 was evaluated (F). Expression of PKD (protein kinase D) was analyzed as a loading control. c-Cbl null microvascular endothelial (MVE) cells were either transfected with control siRNA or PLCγ1 siRNA were subjected to proliferation assay as described above (G). c-Cbl null cells were subjected to proliferation assay as above but cells were also treated with increasing concentration of U73122 (H). c-Cbl null cells and wild type MVE cells were prepared for tube formation/in vitro angiogenesis as described in Materials and Method. Cells were either unstimulated (−) or stimulated with VEGF (100ng/ml) and pictures were taken after 16 hours (I). *Statistically significant at P < 0.05, by ANOVA.
To further link c-Cbl activity to VEGF-dependent endothelial cell proliferation, dermal microvascular endothelial (MVE) cells were isolated from wild type and c-Cbl knockout mice and analyzed for their ability to undergo VEGF-dependent cell proliferation and tube formation. Our analysis showed that VEGF-induced cell proliferation in c-Cbl null cells was higher compared to endothelial cells derived from wild type mice (Figure 1E). To directly demonstrate whether the increased proliferation of endothelial cells derived from c-Cbl null mice is linked to increased PLCγ1 activation we silenced expression of PLCγ1 by siRNA and then measured their proliferation in response to VEGF. The PLCγ1 siRNA was specifically reduced expression of PLCγ1 (Figure 1F). Silencing the expression of PLCγ1 also significantly reduced the VEGF-dependent proliferation of c-Cbl null cells (Figure 1G). Also, treatment of c-Cbl null cells with PLCγ1 inhibitor, U-73122, inhibited proliferation of these in response to VEGF (Figure 1H), further suggesting that increased proliferation of c-Cbl null cells is linked to elevated activation of PLCγ1 in these cells. VEGF-dependent activation of PLCγ1 is known to regulate tube formation of endothelial cells (Meyer et al., 2003; Husain et al., 2010). To test the tube formation potential of c-Cbl null cells we subjected these cells to Matrigel-based tube formation assay. The result showed that loss of c-Cbl increases the capillary tube formation of endothelial cells (Figure 1I). Taken together, the data demonstrates that c-Cbl activity negatively regulates VEGF-dependent angiogenic signaling by targeting PLCγ1.
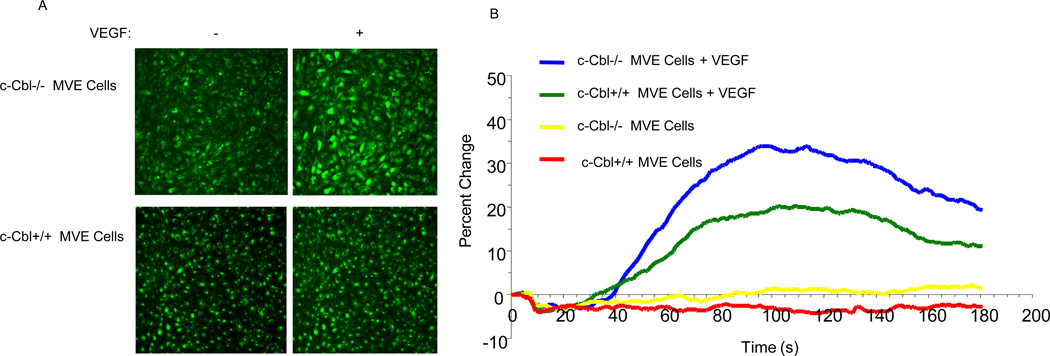
Activation of PLCγ1 by growth factors leads to the opening of calcium channels of the endoplasmic reticulum leading to the release of calcium into the cell (Rebecchi and Pentyala, 2000). To establish whether loss of c-Cbl is associated with PLCγ1-dependent intracellular calcium release, we measured intracellular calcium release in endothelial cells derived from c-Cbl null mice versus wild type cells. Our analysis demonstrates that loss of c-Cbl in endothelial cells results in elevated VEGF-dependent intracellular calcium release compared to the wild-type endothelial cells (Figure 2). Taken together, the data suggest that c-Cbl exerts its effect through PLCγ1 to inhibit VEGF-dependent angiogenic events in endothelial.
Figure 2. Loss of c-Cbl augments VEGF-induced intracellular calcium release.
Serum-starved c-Cbl null microvascular endothelial cells and wild type microvascular endothelial cells either treated with VEGF-A or left unstimulated and intracellular calcium was measured with confocal microscopy using Fluo-3AM probe as described in the Materials and Methods section (A, B). Statistically significant (measurements at 80–160 seconds) at P< 0.05 by ANOVA.
Loss of c-Cbl promotes tumor growth and choroidal neovascularization
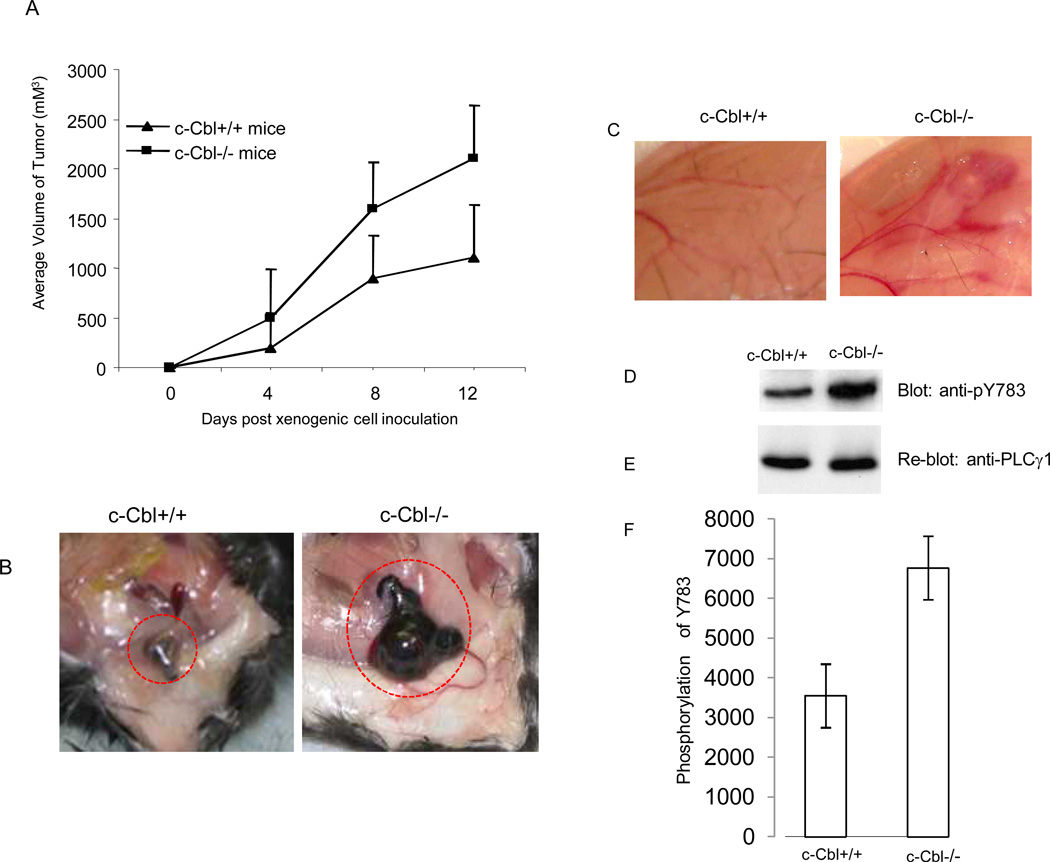
Angiogenesis is considered the hallmark of tumor growth and metastasis in vivo (Folkman 2006). We hypothesized that loss of c-Cbl might augment tumor growth by increasing angiogenesis. To test this hypothesis we measured the growth of murine B16F melanoma cells (B16F cells are originated from C57B1/6 mice and thus are immunologically compatible with the C57BL/6 mice where the c-Cbl null mice was generated) in c-Cbl knockout versus wild type mice. The result showed that the growth of B16F melanoma cells was significantly higher than the control, wild type mice (Figure 3A and 3B). Indeed, the overgrowth of tumor cells in c-Cbl knockout mice was also extensively accompanied with induction of angiogenesis as was apparent with the formation of large mother blood vessels (Figure 3B). To further establish the biological importance of c-Cbl in VEGF-mediated angiogenesis, we compared the VEGF-induced angiogenic responses in c-Cbl knockout versus the wild type mice using in vivo Matrigel plug angiogenesis assay. Our data showed that like tumor-induced angiogenesis, VEGF-induced angiogenesis was also elevated in c-Cbl knockout mice compared to wild type mice (Figure 3D). Moreover, protein sample derived from the same tissue showed that phosphorylation of PLCγ1 was significantly increased in c-Cbl knockout mice where VEGF was injected (Figure 3D, 3E).
Figure 3. Loss of c-Cbl augments tumor-induced angiogenesis.
B16F melanoma cells (1 ×107/mice) were mixed with Matrigel and injected subcutaneously into c-Cbl−/− and c-Cbl+/+ mice (n=4). The growth of tumor cells was measured every four days. Error bars represent mean ± SEM (n=4) (A). Matrigel plug containing tumor cells was removed and pictures were taken (B). VEGF mixed with Matrigel and injected subcutaneously into c-Cbl−/− and c-Cbl+/+ mice and pictures was taken after six days (C). The tissue from panel C were removed, homogenized and equal amount of proteins were loaded and subjected to Western blot analysis using anti-pY783-PLCγ1 antibody(D). The same membrane was re-probed with anti-PLCγ1 (E). Western blot analysis of phosphorylation of PLCγ1 from of cell lysates derived from three mice that were subjected to VEGF-induced angiogenesis was quantified and presented (F).
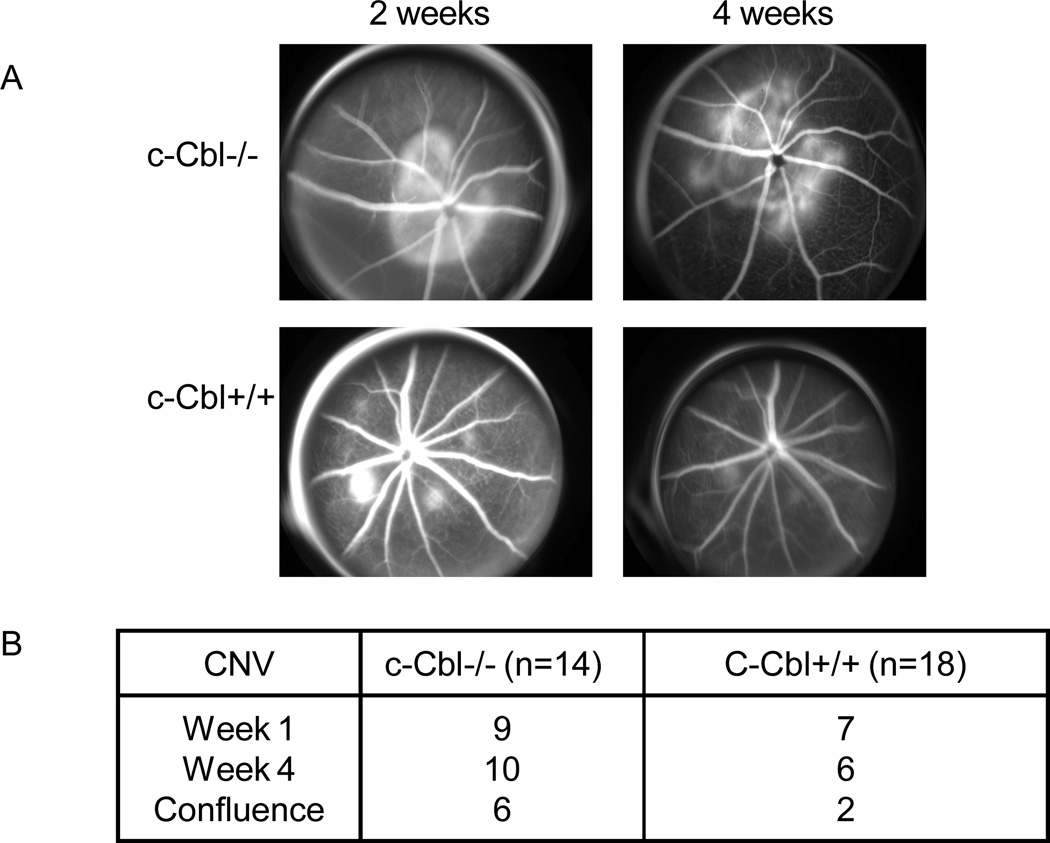
Choroidal neovascularization (CNV)/retinal angiogenesis is considered the major pathological characteristic of age-related macular degeneration and VEGF system is identified as a paramount player of its formation. To investigate role of c-Cbl in CNV formation we subjected c-Cbl knockout and wild type mice to laser-induced CNV formation. CNV was created using the laser photocoagulation method (Funakoshi et al., 2006). Lesions were induced and followed up to four weeks. Fundus photography and angiography was done to document size and leakage from the CNV in these lesions at each visit as describe in the methods and Materials. The follow-up fluorescein angiography of the lesions showed that they were lesions were progressively larger in size at the 4-week as compared to the earlier time points in the c-Cbl knockout mice (Figure 4A). Our analysis showed that leaking CNV were found in 10/14 (71%) lesions in the c-Cbl knockout mice as compared to 6/18 lesions (33%) in the wild type mice (Figure 4B). Also, the confluences of lesions were significantly higher in c-Cbl knockout mice compared to the wild type mice. Indeed, the confluence was found in 6/14 (42%) lesions in c-Cbl knockout and 2/18 (11%) lesions in wild type mice. Altogether, the data strongly suggest that c-Cbl acts as a negative regulator of tumor-induced angiogenesis and Choroidal neovascularization.
Figure 4. Enhanced choroidal neovascularization (CNV) in c-Cbl null mice.
CNV was induced using the laser photocoagulation as described in Materials and Method section. Both eyes of each mouse were treated and four spots were placed in the peripapillary area about 1–2 disc diameter from the optic nerve. Angiograms were performed every week after the laser induction. The angiograms from week two and four is shown (A). The confluence of CNV in c-Cbl null mice versus wild type mice is shown. In the c-Cbl null mice CNV were found to be confluent in 6 of the 14 (42%) lesions, as compared to 2 lesions becoming confluent out of the 18 (11%) lesions in the wild mice (B).
Tyrosine phosphorylation of PLCγ1 is elevated in c-Cbl deficient microvascular endothelial cells
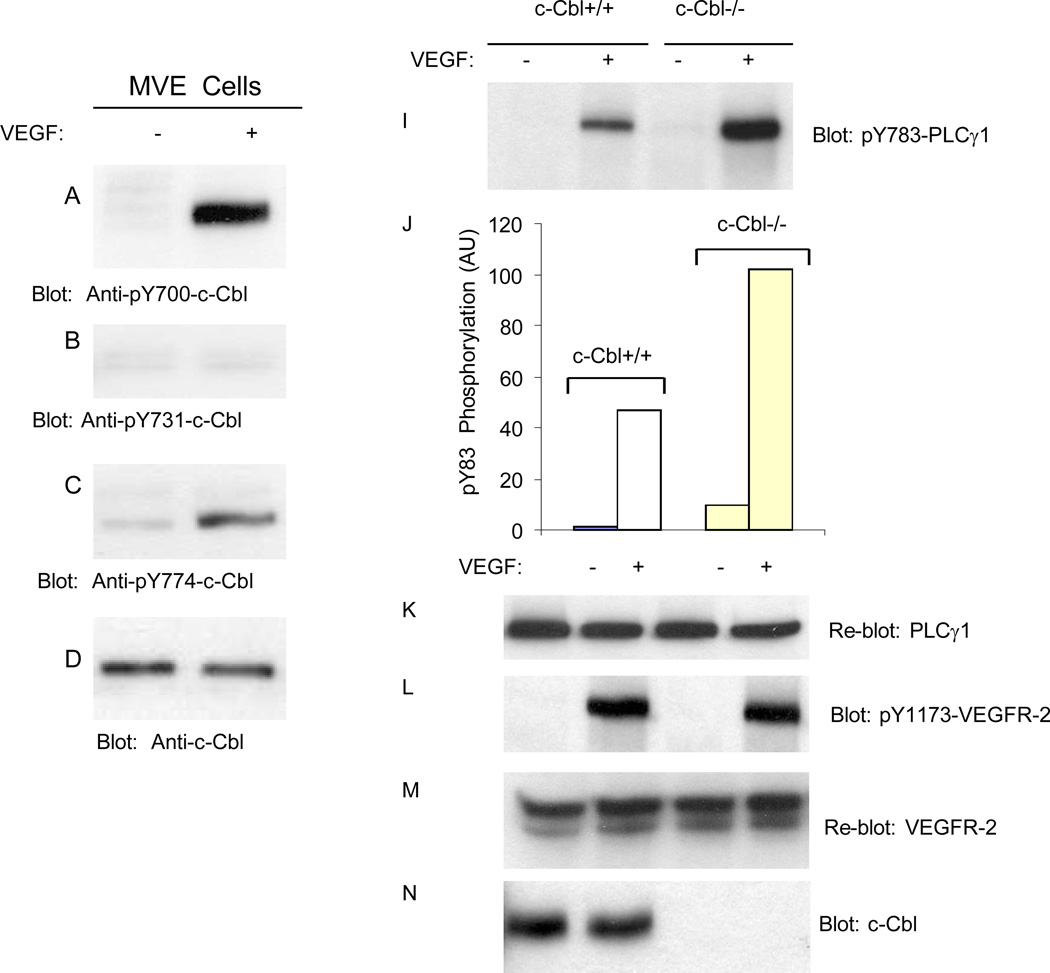
Activation of VEGFR-2 in porcine aortic endothelial (PAE) cells stimulates phosphorylation of c-Cbl at Y774 (Singh et al., 2007). To delineate activation of c-Cbl by VEGFR-2 in the context of primary endothelial cells, we initially analyzed phosphorylation of c-Cbl at Y700, Y731 and Y774 in response to VEGF stimulation in primary dermal microvascular endothelial cells. The data demonstrate that VEGF stimulates c-Cbl tyrosine phosphorylation at Y700, a site involved in the recruitment of VAV, GEF specific for Rho-family GTPases (Miura-Shimura et al., 2003) and Y774, a site along with Y700, involved in the Crk binding to c-Cbl (Feshchenko et al..1998) (Figure 5A, 5C). Interestingly, Y731, a site involved in the recruitment of PI3-kinase to c-Cbl (Miyazaki et al., 2004;Teckchandani et al., 2005) is not phosphorylated in these primary endothelial cells in response to VEGF stimulation (Figure 5B). This suggests that Y700 and Y774 on c-Cbl but not Y731 are engaged in c-Cbl-dependent biological functions in endothelial cells.
Figure 5. Loss of c-Cbl in endothelial cells increases tyrosine phosphorylation of PLCγ1 but has no effect on its proteolysis.
Serum-starved primary microvascular endothelial (MVE) cells either unstimulated (−) or stimulated with VEGF-A for 10 minutes and cells lysed and whole cell lysates were blotted for phospho-Y700-Cbl (A), phospho-Y731-Cbl (B), phospho-Y774-Cbl (C) and total c-Cbl (D). Serum-starved c-Cbl+/+ and c-Cbl−/− microvascular endothelial cells were either unstimulated (−) or stimulated with VEGF for 10 minutes, and whole cell lysates were blotted with anti-phospho-Y783 PLCγ1 (I), anti-PLCγ1 (B), anti-phospho-Y1173-VEGFR-2 (L), anti-VEGFR-2 (M), and anti-c-Cbl (N) antibodies. Quantification of phosphorylation of PLCγ1 from panel (I) in c-Cbl+/+ and c-Cbl−/− MVE cells is shown (J).
Our previous study has identified PLCγ1 as a substrate for c-Cbl (Singh et al., 2007). To analyze the biological significance of c-Cbl in endothelial cells, dermal microvascular endothelial cells derived from wild type and c-Cbl knockout mice were subjected to various biochemical assays in the context of PLCγ1 ubiquitination and degradation. To test the effect of loss of c-Cbl in the activation of PLCγ1 we analyzed phosphorylation status of PLCγ1. Consistent with previous observation (Singh et al., 2007), loss of c-Cbl significantly increased VEGF-dependent phosphorylation of PLCγ1 (Figure 5I). Loss of c-Cbl had no effect on the phosphorylation of VEGFR-2 or its protein levels (Figure 5L and 5M).
PLCγ1 is ubiquitinated by c-Cbl in endothelial cells
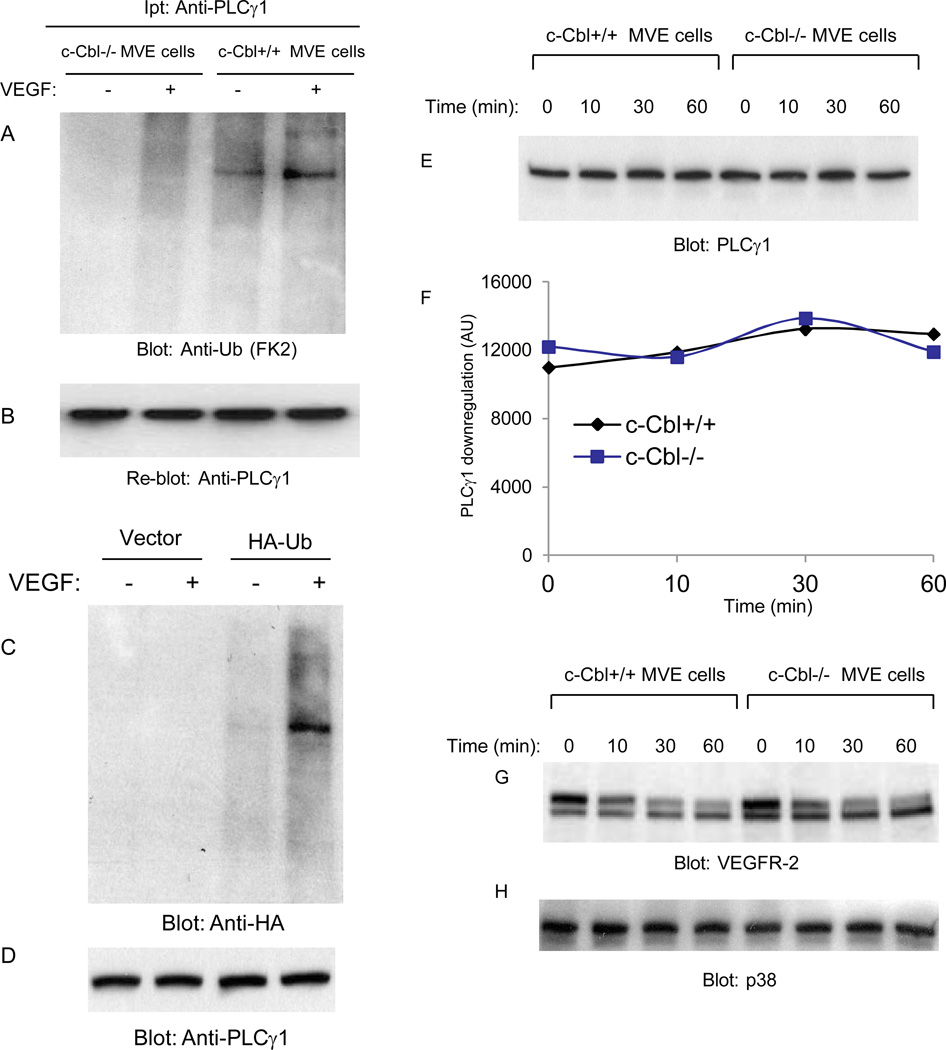
To examine the biochemical consequence of loss of c-Cbl on PLCγ1 in endothelial cells further, we measured ubiquitination of PLCγ1 in c-Cbl null endothelial cells. The data show that PLCγ1 is ubiquitinated in the wild type endothelial cells but not in the c-Cbl null cells. Indeed, it appears that PLCγ1undergoes both poly- and mono-ubiquitination based on its migration pattern as detected by anti-ubiquitin FK2 antibody which is known to recognize both polyubiquitination and monoubiquitination (Figure 6A). To further establish ubiquitination of PLCγ1, we over-expressed HA-tagged ubiquitin construct in HEK-293 cells ectopically expressing VEGFR-2 and analyzed ubiquitination of PLCγ1 where we immunoprecipitated PLCγ1 and immunoblotted with anti-HA antibody. The result shows that PLCγ1 is ubiquitinated as detected by anti-HA antibody (Figure 6C). Interestingly, PLCγ1 was detected mainly in monoubiquitinated form where it appears that only a small fraction of PLCγ1 was polyubiquitinated (Figure 6C). Altogether, the data demonstrate that PLCγ1 is ubiquinated in c-Cbl-dependent manner.
Figure 6. Ubiquitination and downregulation of PLCγ1 in c-Cbl null cells.
Serum-starved c-Cbl null and wild type microvascular endothelial (MVE) cells were either unstimulated (−) or stimulated with VEGF-A (+) for 10 minutes. Cell lysates were immunoprecipitated with anti- PLCγ1 antibody and blotted with anti-ubiquitin antibody (A). The same membrane was re-blotted for PLCγ1 (B). HEK-293 cells were transfected with empty vector or with wild type HA-tagged ubiquitin. Cells were stimulated with VEGF for 10 minutes, lysed and endogenous PLCγ1 was immunoprecipitated with an anti-PLCγ1 antibody and immunoblotted with an anti-HA antibody (C). The same membrane was re-blotted for PLCγ1 (D). Serum-starved c-Cbl null microvascular endothelial (MVE) cells and wild type microvascular endothelial cells were treated with VEGF-A for different time points as indicated and blotted for PLCγ1(E), and VEGFR-2 (G).The same cell lysates was blotted for p38MAPK as a loading control (F). The quantification of PLCγ1 levels also is shown (F).
Since in the absence of c-Cbl, PLCγ1 is not ubiquitinated, we decided to analyze its degradation rate in response to VEGF stimulation. The result showed that although its ubiquitination requires c-Cbl, degradation of PLCγ1 is not regulated by c-Cbl (Figure 6E). Indeed, PLCγ1 appears to be a long-lived and stable protein and stimulation of cells with VEGF up to 60 minutes had no apparent effect on its degradation (Figure 6E, 6F). As shown, VEGF stimulation of wild type and c-Cbl null cells induced time-dependent down regulation of VEGFR-2 (Figure 6G), suggesting that c-Cbl activity is not required for downregulation of VEGFR-2 as previously reported (Singh et al., 2005). Taken together, these results demonstrate that PLCγ1 undergoes c-Cbl-dependent mono-ubiquitination and ubiquitination attenuates its tyrosine phosphorylation without apparent effect on its degradation.
Discussion
The data presented in this manuscript identifies c-Cbl as an important protein whose activity critically regulates angiogenesis. Our findings demonstrate that loss of c-Cbl in mouse is associated with increased VEGF, tumor and laser-induced angiogenesis. Endothelial cells derived from c-Cbl null mice were also more sensitive to VEGF stimulated cell proliferation and their growth was elevated compared to endothelial cells derived from the wild type mice and silencing the expression of PLCγ1 or treatment of cells with PLCγ1 inhibitor reversed this effect. The data further indicates that elevated proliferation of endothelial cells in c-Cbl null cells is likely linked to the state of activation of PLCg1. In support of this possibility tyrosine phosphorylation of PLCg1 and its direct downstream cellular effector, intracellular calcium release was elevated in c-Cbl null cells. Taken together, the data presented in this manuscript strongly implicates c-Cbl as a negative regulator of angiogenesis. The observed effect of c-Cbl on PLCg1 is consistent with the conventional and well recognized function of c-Cbl as a negative regulator of receptor tyrosine kinases signaling (Thien and Langdon, 2005). However, unlike the c-Cbl-mediated degradation of RTKs, c-Cbl mediates ubiquitination of PLCγ1 but spares it from degradation.
What is the biological significance of c-Cbl in endothelial cells function and angiogenesis? The earlier studies have demonstrated that loss of c-Cbl alone is dispensable for normal embryonic development (Murphy et al., 1998) where loss of both c-Cbl and Cbl-b was embryonic lethal before E10.5 (Naramura et al., 2002). Hence, it is reasonable to speculate that c-Cbl activity is not stringently required during development and the function of Cbl family proteins may be compensatory in their ability to regulate the activity of target proteins. It has also well recognized that in pathological angiogenesis such as tumor-induced angiogenesis while tumors are known to co-opt normal physiological pathways to induce angiogenesis, tumor associated vessels however, are leaky, torturous and defective in their interaction with smooth muscle cells and pericytes (Folkman, 1992; Adams and Alitalo, 2007). Recent anti-VEGF studies also revealed that these agents increase normalization of endothelial cells (Fukumura and Jain, 2007). Consistent with distinct phenotype of pathological angiogenesis there is a growing number of mouse models in which tumor-mediated angiogenesis is defective while embryonic angiogenesis is not (Woodman et al., 2003; Zeng et al., 2006; Zhang et al., 2008), suggesting that perhaps c-Cbl selectively plays role in pathological angiogenesis.
The c-Cbl protein is tyrosine phosphorylated at multiple sites including, Y700, Y774 and Y731 in response to growth factor stimulation (Thien and Langdon, 2005). Our analysis shows that VEGF stimulation of endothelial cells selectively induces phosphorylation of c-Cbl at Y700 and Y774 but not Y731. Phosphorylation of c-Cbl at Y731 is linked to recruitment of PI3-kinase to c-Cbl, where in certain systems is suggested to provide growth stimulatory signal (Feng and Liu, 2006). Differential tyrosine phosphorylation of c-Cbl by VEGFR-2 or VEGFR-2 activated tyrosine kinases suggests that c-Cbl activity is uniquely regulated by this receptor system and it may play a distinct role in the integration of angiogenic signaling of VEGFR-2.
Conjugation of ubiquitin to target proteins is recognized to regulate a broad range of cellular functions beyond protein degradation including, kinase activation, endocytosis and protein trafficking (Ravid and Hochstrasser, 2008; Hochstrasser, 2009; Schwartz and Ciechanover, 2009). Despite the clear importance of ubiquitination in protein degradation in other systems, ubiquitination of PLCγ1 plays no significant role in its degradation. Another interesting aspect of our finding is that PLCγ1 is mainly monoubiquitinated. This may explain why PLCγ1 is spared from degradation. Monoubiquitination is mainly linked to endocytosis where polyubiquitination is suggested to target proteins for proteasome-mediated degradation (Ravid and Hochstrasser, 2008). Instead of degradation, ubiquitination of PLCγ1 suppresses its tyrosine phosphorylation. Although, from the current data presented in this manuscript it is not clear how ubiquitination achieves suppression of tyrosine phosphorylation of PLCγ1, however, conjugating a single ubiquitin onto one or more lysines on PLCγ1, could influence dephosphorylation of Y783 by protein tyrosine phosphatases. Also conjugation of ubiquitin to PLCγ1 could alter its association with other proteins, though its ubiquitination does not alter its binding to VEGFR-2 (Singh et al., 2007).
In summary, our observation suggests that c-Cbl activity is required for angiogenesis by acting as a molecular switch to fine-tune angiogenic events during pathological conditions such as cancer and other angiogenesis-associated diseases. This work provides a better understanding of the molecular mechanism of pathological angiogenesis and key role of c-Cbl signaling in these events. Our study further suggests that c-Cbl may be a novel target for the treatment for angiogenesis-associated diseases.
Acknowledgment
This study was supported by grants from the National Institutes of Health (NIH/NEI) to NR and Massachusetts Lions Foundation grant to Department of Ophthalmology.
Footnotes
Author contribution statement:
Rosana D Meyer, Deeba Husain, and Nader Rahimi all performed experiments. Nader Rahimi wrote the manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
REFERENCES
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007 Jun;8(6):464–78. doi: 10.1038/nrm2183. Review. [DOI] [PubMed] [Google Scholar]
- Fukumura Dai, Jain Rakesh K. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 2007 Sep-Nov;74(2–3):72–84. doi: 10.1016/j.mvr.2007.05.003. Epub 2007 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Liu Y-C. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- Feshchenko EA, Langdon WY, Tsygankov AY. Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J Biol Chem. 1998 Apr 3;273(14):8323–8331. doi: 10.1074/jbc.273.14.8323. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Graham LJ, Stoica BA, Shapiro M, DeBell KE, Rellahan B, Laborda J, Bonvini E. Sequences surrounding the Src-homology 3 domain of phospholipase C gamma-1 increase the domain’s association with Cbl. Biochem Biophys. Res. Commun. 1998;249:537–541. doi: 10.1006/bbrc.1998.9177. [DOI] [PubMed] [Google Scholar]
- Graham LJ, Veri M-C, DeBell KE, Noviello C, Rawat R, Jen S, Bonvini E, Rellahan B. 70Z/3 Cbl induces PLC gamma1 activation in T lymphocytes via an alternate Lat- and Slp-76-independent signaling mechanism. Oncogene. 2003;22:2493–2503. doi: 10.1038/sj.onc.1206318. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain D, Meyer R, Mehta M, Pfeifer WM, Chou E, Navruzbekov G, Ahmed E, Rahimi N. Role of c-Cbl Dependent Regulation of Phospholipase C gamma 1 Activation in Experimental Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2010 Jun 30; doi: 10.1167/iovs.10-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q-S, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnusen MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 inmammalian growth and development. Proc. Natl. Acad. Sci. USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Mugford JW, Diamond BA, Weinstein BM. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H-J, Kume T, McKay C, Xu M-J, Ihle JN, Carpenter G. Absence of erythrogenesis and vasculogenesis in Plc gamma-deficient mice. J. Biol. Chem. 2002;277:9335–9341. doi: 10.1074/jbc.M109955200. [DOI] [PubMed] [Google Scholar]
- Lupher ML, Jr., Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- Meyer RD, Latz C, Rahimi N. Recruitment and activation of PLCγ1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J. Biol. Chem. 2003;278:16347–16355. doi: 10.1074/jbc.M300259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Lupher ML, Jr., Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor. Proc. Natl. Acad. Sci. USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Mullane-Robinson KP, Lill NL, Douillard P, Band H. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation. A critical role for Cbl tyrosine kinase-binding domain. J. Biol. Chem. 1999;274:16619–16628. doi: 10.1074/jbc.274.23.16619. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004 Apr 23;279(17):17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998 Aug;18(8):4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359–371. doi: 10.1038/nrm1911. Review. [DOI] [PubMed] [Google Scholar]
- Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front. Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J. Biol. Chem. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin–proteasome system. Nat. Rev. Mol. Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000 Oct;80(4):1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol. 2009;49:73–79. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Singh AJ, Meyer RD, Band H, Rahimi N. The carboxyl terminus of VEGFR-2 is required for PKC-mediated downregulation. Mol. Biol. Cell. 2005;16:2106–2118. doi: 10.1091/mbc.E04-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AJ, Meyer RD, Navruzbekov G, Shelke R, Duan L, Band H, Leeman SE, Rahimi N. A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5413–5418. doi: 10.1073/pnas.0700809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006 Oct;209(1):21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLCγ1 and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teckchandani AM, Panetti TS, Tsygankov AY. c-Cbl regulates migration of v-Abl-transformed NIH 3T3 fibroblasts via Rac1. Exp Cell Res. 2005 Jul 1;307(1):247–58. doi: 10.1016/j.yexcr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Thien CBF, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and negative regulation of signaling responses. Biochem. J. 2005;391:153–165. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verí MC, DeBell KE, Seminario MC, Dibaldassare A, Reischl I, Rawat R, Graham L, Noviello C, Rellahan BL, Miscia S, Wange RN, Ezio Bonvini Membrane raft-dependent regulation of phospholipase Cγ1 activation in T lymphocytes. Mol. Cell. Biol. 2001;21:6939–6950. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Ashton AW, Schubert W, Lee H, Williams TM, Medina FA, Wyckoff JB, Combs TP, Lisanti MP. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003 Jun;162(6):2059–2068. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006 Mar 20;203(3):719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, Sessa WC, Min W. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118(12):3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]