Abstract
We present a traumatic fatality of a 19-year-old man who had ingested blotter paper containing 25INBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine]. Postmortem specimens were analyzed by high performance liquid chromatography with tandem mass spectrometry (HPLC/MS/MS). Toxicology findings for fluids based upon blood or urine calibrators were as follows: peripheral blood, 405 pg/mL; heart blood, 410 pg/mL; urine, 2.86 ng/mL; and vitreous humor, 99 pg/mL. While findings based upon the method of standard additions were: gastric contents, 7.1 μg total; bile, 10.9 ng/g; brain, 2.54 ng/g and liver, 7.2 ng/g. To our knowledge the presented case is the first postmortem case of 25I-NBOMe intoxication documented by toxicological analysis of tissues and body fluids.
Keywords: Designer drugs, 25I-NBOMe, HPLC/MS/MS, Bath salts
1. Introduction
At the beginning of the present millennium, a large number of new, initially non-controlled designer drugs appeared on the internet market sold as “Bath Salts”, plant food or fertilizer, insect repellent, pond cleaner or vacuum freshener with the disclaimer, “Not For Human Consumption” [1–9]. Many of these new, synthetic drugs belong to the class of beta-keto derivatives of amphetamine: methcathinone, mephedrone, ethcathinone and pentedrone, as well as, the methylene dioxy ring derivatives similar to methylenedioxymethamphetamine (MDMA, “Ecstasy”): methylone, ethylone, butylone, pentylone and methylenedioxypyrovalerone (MDPV) [1–6]. Numerous cases of severe poisonings and fatal intoxications in young adults who have ingested or smoked these “Bath Salt” designer drugs have been reported in Europe and during the past four years in the United States and Japan [9–17]. These drugs are now controlled substances in Europe and the United States [7,18].
Recently, a class of “2C” designer drugs, dimethoxyphenyl-N-[(2-methoxyphenyl)methyl]ethanamine) derivatives have become easily obtainable over the internet, particularly 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe) (Fig. 1). Blotter papers containing 25I-NBOMe appeared on the designer drug market in 2011 [19]. It is sold under various names including; 25I, INB-MeO, N-bomb, Smiles, Solaris and Cimbi-5. The terminology “2Cs” is an acronym invented by Alexander Shulgin for the 2 carbon atoms between the benzene ring and the amino group on the perceptional distorting and/or hallucinogenic phenylethylamine derivatives he synthesized [20–22]. These N-benzyl phenylethylamine derivatives are potent serotonin 5-HT2A receptor agonists [23–28]. Recent reports from “personal drug experience websites” and in the popular press indicate these drugs are the latest in a series of designer “Bath Salt” drugs of abuse. The 5-HT2A receptor has been closely linked to complex behaviors including working memory and cognitive processes. It is also implicated in the pathophysiology of affective disorders such as depression and schizophrenia. Stimulation of 5-HT2A receptors is responsible for the hallucinogenic effects of recreational drugs such as lysergic acid diethylamide (LSD) and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) [29] whereas the therapeutic effects of atypical antipsychotics are due to antagonism at the 5-HT2A receptor [30]. 25I-NBOMe was first synthesized by Ralf Heim at the Free University of Berlin as one of a series of pharmacological tools to study the 5-HT2A receptor [23–25]. Recently, positron emission tomography (PET) imaging of cerebral 5-HT2A receptors with carbon-11 labeled 25INBOMe demonstrated that 25I-NBOMe is a particularly potent agonist [31,32].
Fig. 1.
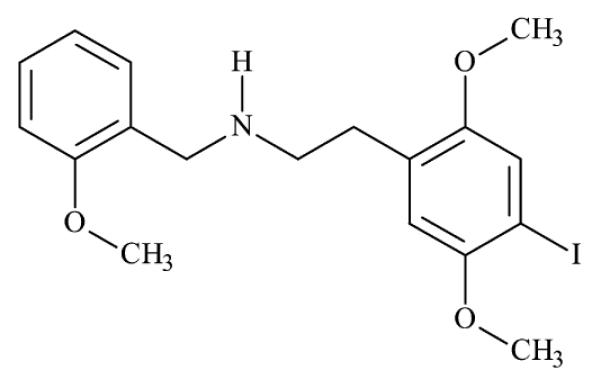
Chemical structure of 25I-NBOMe.
We report a case of fatal behavioral intoxication due to ingestion of 25I-NBOMe with laboratory confirmation in postmortem specimens by high performance liquid chromatography with tandem mass spectrometry (HPLC/MS/MS). The assay is a modification of a serum procedure previously developed in response to an outbreak of N-benzyl-phenethylamine derivative abuse and non-fatal overdose cases in Virginia during early 2012 [33]. To the best of our knowledge the presented case is the first and at present, only postmortem case of 25I-NBOMe intoxication documented by toxicological analysis of tissues and body fluids.
2. Case history
The decedent was a healthy 19-year-old man with no prior history of alcohol, tobacco or drug abuse. The decedent was reportedly “trip-sitting” for his friends who were using “acid” on the evening of his death. “Trip sitting” refers to someone who remains drug free for the purpose of monitoring the safety of those under the influence of a drug. At some point that evening, the decedent either knowingly or unknowingly ingested blotter paper infused with “acid”. Subsequently, the friends noticed that the decedent began to display strange and paranoid behavior, and they all agreed it would be a good idea to leave their gathering place and go for a walk. Reportedly, the decedent’s behavior became increasingly more bizarre and he abruptly walked away from his friends. His apparent bizarre behavior may be related to the fact this was the first time he had ingested a hallucinogenic drug. He was normally the trip sitter. The other individuals that took the same drug that night did not display similar side-effects but all of them had used the drug in the past. The friends went to his apartment in search of him, and found him prone and unresponsive on the pavement near the apartment complex swimming pool. 911 was activated, and shortly thereafter he was pronounced dead at the scene. According to police and paramedics, the decedent apparently had either jumped or had fallen accidentally from his apartment balcony, which was located multiple stories above the pool deck. Upon questioning, the friends informed police that the decedent had not been behaving normally that evening.
3. Autopsy findings
An autopsy was performed approximately 7 h after the decedent’s body had been found and he was pronounced dead. The autopsy revealed a well-developed and nourished 19-year-old man of normal weight with multiple blunt impact injuries, including lacerations of the heart, aorta, liver and spleen. The skull had multiple fractures of the calvarium and base, with subdural and subarachnoid hemorrhages, cortical contusions and traumatic axonal injury. No non-traumatic abnormalities or lesions were identified. The stomach contained 200 mL of tan-brown liquid and partially digested food fragments. Straining of the stomach contents revealed a rectangular piece of white fibrous paper measuring 1.4 cm × 0.6 cm × 0.1 cm. The paper was submitted to the toxicology laboratory for analysis. Postmortem specimens submitted for toxicology included heart and peripheral blood, ocular fluid, urine, bile, gastric contents and tissue samples from liver and brain. Histopathologic study of samples of lung, liver, brain and heart revealed no non-traumatic abnormalities.
4. Initial toxicological analysis
Initial toxicological screens and analysis were performed on heart blood and ocular fluid that included volatiles by gas chromatography mass spectrometry (GC/MS), immunoassay for 12 classes of drugs (acetaminophen, barbiturates, benzodiazepines, cannabinoids, carisoprodol/meprobamate, cocaine metabolite, fentanyl, methadone, methamphetamine/MDMA, opiates, oxycodone and salicylates) and a screen for alkaline extractable drugs by GC/MS. Based on case history, a targeted analysis for LSD by immunoassay and HPLC/MS/MS was also performed by a reference laboratory. No volatiles, drugs or LSD were detected. Subsequently, the blotter paper from the stomach contents was analyzed by GC/MS but only bile salts were detected. Based on negative findings and case history, the lead detective investigating the death was contacted and was able to obtain another piece of non-ingested blotter paper for analysis. Analysis of the non-ingested blotter paper by GC/MS identified the presence of 25I-NBOMe. A targeted analysis of 25I-NBOMe was then performed by HPLC/MS/MS in by the Virginia Commonwealth University Department of Pharmacology and Toxicology Mass Spectroscopy Laboratory.
5. 25I-NBOMe analysis
5.1. Reagents
The primary reference materials 25H-NBOMe (the internal standard (ISTD)) and 25I-NBOMe were purchased from Cayman Chemical Company (Ann Arbor, MI) as hydrochloride salts. Acetic acid, ammonium acetate, dichloromethane, ethanol, formic acid, isopropanol, methanol and deionized (DI) water were purchased from Fisher Scientific (Hanover Park, IL). Ammonia was purchased from Macron Chemicals (Charlotte, NC). Hydrochloric acid and sodium phosphate dibasic were purchased from J.T. Baker (Phillipsburg, NJ). Sodium phosphate monohydrate was purchased from Sigma–Aldrich (St. Louis, MO). All reagents were ACS grade or better. Medical grade nitrogen was purchased from National Welders Supply Company (Richmond, VA) and the Clean Screen ZSDUA020 solid phase extraction (SPE) columns were purchased from UCT (Horsham, PA).
5.2. Sample preparation
No sample preparation was needed for the whole blood specimen or the vitreous humor. Bile, gastric contents and urine were diluted 1:10 with DI water. Samples were mixed using a vortex mixer and allowed to stand for 1 h. Two-gram aliquots of brain and liver tissue were diluted with 6.0 g of DI water and homogenized using an IKA®-Labortechnik Ultra-Turrax T25 homogenizer (Wilmington, NC).
5.3. Sample extraction
Quantitative analysis of 25I-NBOMe was based upon a previously described serum method [33]. Briefly, 50 μL of ISTD consisting of 10 ng/mL (500 pg total) of 25H-NBOMe was added to 1.0 mL or 1.0 g aliquots of calibrators, quality control (QC) specimens, samples or sample homogenates followed by the addition of 1 mL of 100 mM phosphate buffer (pH 6). The samples were then mixed for 5 min and centrifuged for 10 min at 3000 rpm. The Clean Screen ZSDUA020 solid phase (SPE) columns were conditioned with 3 mL of methanol, 3 mL of DI water and 1 mL of 100 mM phosphate buffer (pH 6). The samples were added to the columns and aspirated, followed by 3 mL of DI water, 1 mL 100 mM acetic acid and 3 mL of methanol. Columns were dried under vacuum. 25I-NBOMe and ISTD were then eluted with 3 mL of 78:20:2 dichloromethane/isopropanol/ammonia (v:v:v). One hundred microliters of 1% HCl in methanol (v:v) and 200 μL of DI water were added to the eluate. The samples were evaporated under nitrogen leaving approximately 200 μL of DI water. This solution was transferred to auto-sampler vials for HPLC/MS/MS analysis.
5.4. Instrumental analysis
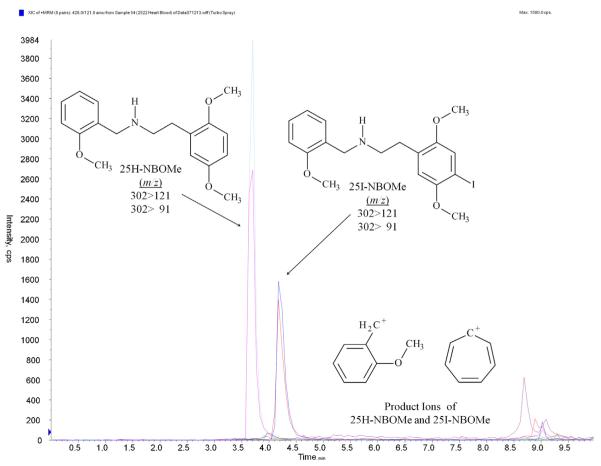
The HPLC/MS/MS analysis was performed with an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation, Fig. 2, was performed on a Luna 3μ C8(2)100 Å 100 mm × 2.0 mm column (Phenomenex, Torrance, CA). The mobile phase consisted of (A) DI water with 10 mM ammonium acetate and 0.1% formic acid and (B) methanol. The following gradient was used: 0.00–1.10 min at 20% B, a linear gradient to 40% B for 6.00 min, hold for 2 min, then return to 20% B at 8.00 min. The source temperature was set at 650 °C and had a curtain gas flow rate of 30 mL/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 at flow rates of 25 mL/min. The acquisition mode used was multiple reaction monitoring (MRM). The retention times were: 25I-NBOMe, 4.8 min; and 25H-NBOMe, 3.8 min. 25I-NBOMe had a declustering potential of 38 eV and 25H-NBOMe had a declustering potential of 19 eV. The following transition ions (m/z) were monitored in MRM mode with their corresponding collision energies (eV) in parentheses: 25I-NBOMe: 428 > 121 (26) and 428 > 91 (50); and 25H-NBOMe: 302 > 121 (28) and 302 > 91 (70). The total run time for the analytical method was 10 min.
Fig. 2.
Chromatographic separation of 25I-NBOMe and 25H-NBOMe.
5.5. Quantification of 25I-NBOMe
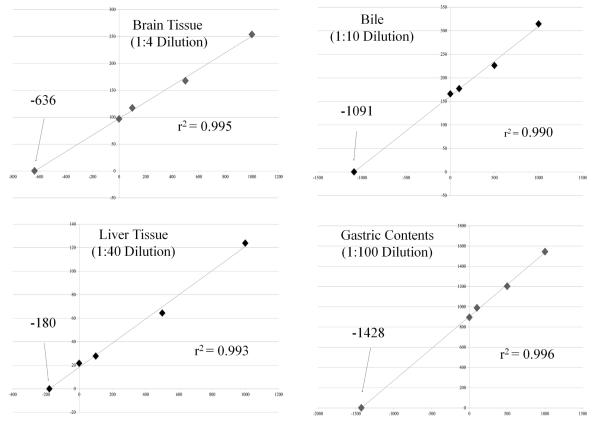
Calibrators prepared in drug-free whole blood were used to quantify 25I-NBOMe in blood, bile, gastric contents, vitreous humor and brain and liver homogenates. The 25I-NBOMe urine concentration was calculated using calibrators prepared in drug-free urine. Additional testing by the method of standard additions [34] was used to quantify 25I-NBOMe in the diluted fluids and tissues to account for possible significant matrix effects. The concentration of 25I-NBOMe used in the standard addition sample was determined by the linear regression equation for each specimen and yielded r2 values of 0.990 or better, Fig. 3. 25I-NBOMe was added in amounts of 0, 100, 500 and 1000 pg to 1.0 g aliquots of brain and liver homogenates and dilutions of bile and gastric contents. The aliquots were vortex mixed, allowed to stand for 1 h, and then were extracted by the method described above.
Fig. 3.
Standard addition curves.
5.6. Method validation of whole blood
The evaluation of the assay in whole blood was conducted over three separate days using the following QC whole blood specimens for 25I-NBOMe: limit of quantification quality control (LOQC), target concentration of 25 pg/mL; low control (LQC), target concentration of 75 pg/mL; medium control (MQC), target concentration of 400 pg/mL; high control (HQC), target concentration of 1500 pg/mL. A drug free control (negative control) with ISTD added and a double negative control containing neither 25I-NBOMe nor ISTD were also analyzed with each test batch. In order to account for the possible mistakes in the preparation of stock solutions two different lots of primary reference material from Cayman Chemicals were used. One lot was used to prepare the controls and the other lot was used to prepare the working standard used in the preparation of the calibrators. All QC samples and stock standards were stored at −20 °C until testing.
The samples were analyzed in batches as recommended for biomedical assay validation [35] for linearity, LOQ, accuracy/bias, precision, selectivity and carryover. The assay was also analyzed for absolute recovery and ion suppression. Linearity was verified from seven point calibration curves with concentrations of 25, 50, 100, 200, 500, 1000 and 2000 pg/mL prepared in drug-free in-house certified expired whole blood. A linear regression of the ratio of the peak area counts of 25I-NBOMe and 25H-NBOMe (the ISTD) versus concentration was used to construct the calibration curves. The linear regression correlation coefficients (r2) for the 25I-NBOMe calibration curves in the three batches yielded a mean r2 of 0.995 ± 0.003. The lower limit of quantification (LOQ) was administratively set at 25 pg/mL. LOQC samples were used to verify that the LOQ was within ±20% of the target value and had a response at least five times greater than the signal to noise ratio of drug-free whole blood. Accuracy/bias and precision were determined from the prepared QC blood samples. Both intra- and inter-assay accuracy/bias were determined to have a range 89–113% for all QC samples and did not exceed a 15% coefficient of variation (CV), Table 1. One-way of Variation (ANOVA) Approach was used to calculate combined intra- and inter-day precision. The CV for the Inter-day at the LOQ was 16%, while the other control specimens yielded intra-day and inter-day CVs not exceeding 15%, Table 2. The selectivity of the assay was determined using six different lots of drug-free blood. Each individual lot was analyzed with and without internal standard and no peaks were detected that co-eluted with 25I-NBOMe or with the internal standard. No interferences were observed from drug free whole blood with 2000 pg/mL of the following nine NBOMe analytes added: 4-bromo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe); 2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (2CC-NBOMe); 2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe); N-(benzo[d][1,3]dioxol-4-ylmethyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (25I-NBMD); N-(2-fluorobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (25I-NBF); (E)-2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzylidene)ethanamine (25I-NBOMe imine analog); (E)-2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzylidene)ethanamine (25H-NBOMe imine analog); 2-(2,5-dimethoxy-3,4-dimethylphenyl)-N-(2-methoxybenzyl)ethan-1-amine (25G-NBOMe); and 2-(2,5-dimethoxy-4-(methylthio)phenyl)-N-(2-methoxybenzyl)ethan-1-amine (2CT-NBOMe). Sample carryover was assessed in each of the three validation batches using two different procedures. First, immediately following the injection of the 2000 pg/mL 25I-NBOMe calibrator, a negative control was injected. No carryover was detected in the negative control. Second, an injection of the HQC (1500 pg/mL) sample was immediately followed by injection of the LQC (75 pg/mL) sample. This procedure was routinely applied each time HQC and LQC samples were analyzed. Lack of carryover was confirmed as none of the 25I-NBOMe LQC samples demonstrated a significant quantified bias. The absolute recovery and ion suppression of the assay for 25I-NBOMe at 400 pg/mL (n = 6) was 84 ± 8% and 4 ± 10%, respectively and for 25H-NBOMe the ISTD at 500 pg/mL (n = 6) was 104 ± 10% and 10 ± 5%, respectively.
Table 1.
Accuracy/bias, intra- and inter-day precision for 25I-NBOMe in whole blood.
| 25I-NBOMe | Control | Mean conc. (pg/mL) | %CV (%) | Accuracy/bias |
|---|---|---|---|---|
| Accuracy/bias and precision | ||||
| Intra-day (n = 6) | LOQ (25 pg/mL) | 28 | 6 | 111 |
| LQC (75 pg/mL) | 85 | 4 | 113 | |
| MQC (400 pg/mL) | 335 | 14 | 89 | |
| HQC (1500 pg/mL) | 1530 | 5 | 102 | |
| Inter-day (n = 12) 3-days | LOQ (25 pg/mL) | 26 | 12 | 106 |
| LQC (75 pg/mL) | 82 | 6 | 109 | |
| MQC (400 pg/mL) | 380 | 13 | 95 | |
| HQC (1500 pg/mL) | 1534 | 9 | 102 |
Table 2.
One-way analysis of variation (ANOVA) combined intra- and inter-day precision.
| LOQ (25 pg/mL) | LQC (75 pg/mL) | MQC (400 pg/mL) | HQC (1500 pg/mL) | |
|---|---|---|---|---|
| Combined intra- and inter-day precision | ||||
| Intra-day (%CV) | 8 | 6 | 11 | 5 |
| Inter-day (%CV) | 16 | 7 | 14 | 9 |
6. Results
The results of the toxicological analysis of 25I-NBOMe by both direct analysis using whole blood or urine calibrators, as well as by the method of standard addition are presented in Table 3.
Table 3.
25I-NBOMe findings in decedents’ specimens as determined by direct analysis and the method of standard addition.
| Specimen | Direct analysis | Standard addition |
|---|---|---|
| 25I-NBOMe (pg/mL or pg/g) | ||
| Heart blood | 410 | ND |
| Peripheral blood | 405 | ND |
| Urine | 2860 | ND |
| Vitreous humor | 99 | ND |
| Brain | 2780 | 2540 |
| Liver | 5640 | 7200 |
| Bile | 12,100 | 10,900 |
| Gastric contents | ND | 7.1 μg (total) |
Comparison of the results from standard addition to direct analysis indicates that brain values were 9% less by the method of standard addition; while bile and liver results were 11% and 22% higher, respectively. For postmortem specimens, the results between the procedures were in good agreement and demonstrate the lack of matrix effects and ease of extraction of 25I-NBOMe and the ISTD, 25H-NBOMe from tissue matrices.
7. Discussion
Recently, several published abstracts and a few papers have described signs, symptoms and treatment of 25I-NBOMe intoxication [36–40]. These publications have addressed a total of 22 cases of 25I-NBOMe intoxication with the following demographics: the average age of the patients were 18 years with a range of 14–29 years; thirteen of these cases included the sex of the patient, all were male [36,38,39] except one [40]. We calculated incidence of clinical presentations as a percentage of the total reported cases and found: tachycardia (95%), agitation (77%), hallucinations (76%), hypertension (73%), and seizures (45%) to be present in these cases. A noticeable finding in 25I-NBOMe intoxicated patients was persistent seizure activity, with resultant rhabdomyolysis, requiring continuous administration of sedatives and skeletal muscle blocking agents, often for several days.
While in vivo binding studies indicate that the pharmacology of 25I-NBOMe would reflect 5-HT2A receptor agonist effects such as agitation and hallucinations [26,27], it is interesting to note that symptoms were consistent with those in 236 cases of “Bath Salt” intoxication reported by Murphy et al. [9]. In their study the exact intoxicating compounds were not identified in body fluids from the patients; but, indirectly identified by history and/or perceived availability of “Bath Salt” products. The data collected was primarily from intoxications from the commonly abused designer drugs: MDPV, methedrone and methylone. These compounds have been found to cause significant alternations in brain monoamine transporters such as increasing release and/or blocking reuptake of synaptic norepinephrine and dopamine [41]. Resultantly, the most common sympathomimetic effects and symptoms documented were agitation (82%), combative violent behavior (57%), hallucinations (40%), paranoia (36%), confusion (34%), and chest pain (17%). The most common signs of intoxication were tachycardia (56%), hypertension (17%), and mydriasis (13%). The most common laboratory abnormalities were elevated CPK and hypokalemia, noted in 9% and 4% of cases, respectively.
At present, there is limited data in the published literature concerning the pharmacokinetics and disposition of 25I-NBOMe in man or whole animals. Hill et al. [39] identified 25I-NBOMe in patient plasma in seven clinical cases but did not quantify it. Serum specimens from a number of 25I-NBOMe cases drawn 25–48 h after admission to the emergency department (ED) were reported to have 25I-NBOMe concentrations ranging from 250 to 2700 pg/mL [37,38]. However, the ratio of 25I-NBOMe serum to whole blood concentrations is unknown. Importantly, the peripheral whole blood concentration of 25I-NBOMe in this postmortem case was 405 pg/mL and is within the range of known serum values from intoxicated patients. The decedent’s urine was determined to contain 2.8 ng/mL of 25I-NBOMe which was within the range of concentrations, 2–36 ng/mL, reported by Kelley et al. [36] from specimens drawn from intoxicated patients 3.5–6 h after admission to the ED. These values were also comparable to the 180 pg/mL serum and 1900 pg/mL urine 25B-NBOMe, the bromide analog of 25I-NBOMe concentrations reported by Poklis et al. [42].
It is reasonable to assume that the decedent in this postmortem case was suffering from hallucinations and/or delusional thoughts and/or mental confusion due to 25I-NBOMe intoxication which possibly contributed to his fall, whether intentional, i.e. impulsively attempting to fly or commit suicide, or accidental. It is also possible that another person or persons pushed him. However, the autopsy findings, circumstances of the death and the results of the police investigation render this possibility highly unlikely.
The supplier of the “acid” was arrested following the death of the decedent. He informed the police that 25I-NBOMe was purchased on line in powder form. He also stated that 1 g of 25I-NBOMe after mixing will yield 2000 “hits”. Therefore each “hit” on a section of blotter paper would contain approximately 500 μg of 25I-NBOMe. As only one piece of white blotter paper was found in the decedent’s gastric contents the suspected dose in this case was approximately 500 μg of 25I-NBOMe.”
8. Conclusion
The cause of death was attributed to skull fractures with contusions of brainstem and lacerations of heart and aorta due to blunt impact to head and torso. The manner of death was ruled undetermined. We found that direct quantification of 25I-NBOMe in brain, liver and bile using a whole blood calibration curve was comparable to the quantification of the tissue and bile by method of standard addition. The greater concentrations of 25I-NBOMe in the tissues then in the blood or vitreous humor may indicate a high apparent volume of distribution of 25I-NBOMe.
Acknowledgment
This project was supported by the National Institute on Drug Abuse Center grant P50DA005274.
References
- [1].Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- [2].Zaitsu K, Katagi M, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K. Recently abused b-keto derivatives of 3,4-methylenedioxyphenylalkylamines: a review of their metabolisms and toxicological analysis. Forensic Toxicol. 2011;29:73–84. [Google Scholar]
- [3].Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, Davey Z, Corkery J, Siemann H, Scherbaum N, Farre’ M, Torrens M, Demetrovics Z, Ghodse AH. Mephedrone, (4-Methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology (Berlin) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- [4].Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test Anal. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- [5].Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Test Anal. 2011;3:569–575. doi: 10.1002/dta.204. [DOI] [PubMed] [Google Scholar]
- [6].Coppola M, Mondola R. 3,4-Methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol. Lett. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- [7].Gibbons S. ‘Legal highs’ – novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clin. Toxicol. (Phila.) 2012;50:15–24. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- [8].Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of bath salts and legal highs (synthetic cathinones) in the United States. Clin. Toxicol. (Phila.) 2011;6:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- [9].Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. Bath salts and plant food products: the experience of one regional US poison center. J. Med. Toxicol. 2013;9:42–48. doi: 10.1007/s13181-012-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AGA. A case of extreme agitation and death after the use of mephedrone in the Netherlands. Forensic Sci. Int. 2011;206:e93–e95. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- [11].Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, 3rd, Garg U, Pietak BR. Three fatal intoxications due to methylone. J. Anal. Toxicol. 2012;36:444–451. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- [12].Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, Holler JM. Distribution of methylone in four postmortem cases. J. Anal. Toxicol. 2012;36:434–439. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- [13].Cosbey SH, Peters KL, Quinn A, Bentley A. Mephedrone (methylmethcathi-none) in toxicology casework: a Northern Ireland perspective. J. Anal. Toxicol. 2013;37:74–82. doi: 10.1093/jat/bks094. [DOI] [PubMed] [Google Scholar]
- [14].Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug bath salts containing 3,4-methylenedioxypyrovalerone (MDPV) J. Med. Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem tissue distribution of MDPV following lethal intoxication by bath salts. J. Anal. Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- [16].Namera A, Urabe S, Saito T, Torikoshi-Hatano A, Shiraishi H, Arima Y, Nagao M. A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of apyrrolidinobutiophenone and a-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol. 2013;31:338–343. [Google Scholar]
- [17].Saito T, Namera A, Osawa M, Aoki H, Inokuchi S. SPME–GC–MS analysis of α-pyrrolidinovaleorophenone in blood in a fatal poisoning case. Forensic Toxicol. 2013;31:328–332. [Google Scholar]
- [18].Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann. Pharmacother. 2012;46:436–441. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- [19].Zuba D, Sekula K, Buczek A. 25C-NBOMe – new potent hallucinogenic substance identified on the drug market. Forensic Sci. Int. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]
- [20].Shulgin AT, Carter MF. Centrally active phenethylamines. Psychopharmacol. Commun. 1975;1:93–98. [PubMed] [Google Scholar]
- [21].Glennon RA, Kier LB, Shulgin AT. Molecular connectivity analysis of hallucinogenic mescaline analogs. J. Pharm. Sci. 1979;68:906–907. doi: 10.1002/jps.2600680733. [DOI] [PubMed] [Google Scholar]
- [22].Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- [23].Pertz HH, Heim R, Elz S. N-Benzylated phenylethanamines are highly potent partial agonists at 5-HT2A receptors. Arch. Pharm. Pharm. Med. Chem. 2000;333:30. (abstract) [Google Scholar]
- [24].Heim R, Pertz HH, Elz S. Partial 5-HT2A-receptor agonists of the phenylethanamine series: effect of a trifluoromethyl substituent. Arch. Pharm. Pharm. Med. Chem. 2000;333:45. (abstract) [Google Scholar]
- [25].Heim R. Entwicklung eines neuen Struktur-Wirkungskonzepts. Free University of Berlin; Berlin, Germany: 2003. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. [Google Scholar]
- [26].Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with super-potent N-benzyl phenethylamine agonists. Mol. Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- [27].Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg. Med. Chem. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dean BV, Stellpflug SJ, Burnett AM, Engebretsen KM. 2C or not 2C: phenethylamine designer drug review. J. Med. Toxicol. 2013;9:172–178. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [30].Abbas A, Roth BL. Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin. Pharmacother. 2008;9:3251–3259. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- [31].Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- [32].Ettrup A, Holm S, Hansen M, Wasim W, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36. Mol. Imaging Biol. 2013;15:376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- [33].Poklis JL, Charles J, Wolf C, Poklis A. High performance liquid chromatography tandem mass spectrometry method for the determination of 2CC-NBOMe and 25I-NBOMe in human serum. Biomed. Chromatogr. 2013 doi: 10.1002/bmc.2999. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Novák J, Janák J. Comments on the standard addition method used in quantitative gas chromatographic analysis. J. Chromatogr. 1967;28:392–395. doi: 10.1016/s0021-9673(01)85982-3. [DOI] [PubMed] [Google Scholar]
- [35].US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research . Rockville, MD: 2001. [Google Scholar]
- [36].Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation. Clin. Toxicol. (Phila.) 2012;50:702. (abstract) [Google Scholar]
- [37].Rose SR, Cumpston KL, Stromberg PE, Wills BK. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clin. Toxicol. (Phila.) 2012;50:707–708. [Google Scholar]
- [38].Rose RS, Poklis JL, Poklis A. A case of 25I-NBOMe (25-I) intoxication: a new potent 5HT2a agonist designer drug. Clin. Toxicol. 2013;51:174–177. doi: 10.3109/15563650.2013.772191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, Blain P, Thomas SH. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin. Toxicol. (Phila.) 2013;51:487–492. doi: 10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]
- [40].Stellpflug SJ, Kealey SE, Hegarty CB, Janis GC. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): clinical case with unique confirmatory testing. J. Med. Toxicol. 2013 doi: 10.1007/s13181-013-0314-y. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baumann MH, Partilla JS, Lehner KR. Psychoactive bath salts: not so soothing. Eur. J. Pharmacol. 2011;689:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Poklis JL, Nanco CR, Troendle MM, Wolf CE, Poklis A. Determination of 4-bromo-2, 5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe) in serum and urine by high performance liquid chromatography with tandem mass spectrometry in a case of severe intoxication. Drug. Test. Anal. 2013 doi: 10.1002/dta.1522. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]