Abstract
A sensitive, specific, and rapid high‐pressure liquid chromatography/mass spectrometry/mass spectrometry method was developed for the quantitation of 11 tricyclic antidepressants and/or their metabolites; fluoxetine and norfluoxetine; cyclobenzaprine; and trazodone in urine. Samples were alkalinized with 0.2 N NaOH and extracted into 2 ml of hexane: ethyl acetate (1:1), evaporated to dryness, and reconstituted with 100 μl of 20 mM ammonium formate: methanol (20:80). The chromatographic separation was performed using an Allure Biphenyl 100 × 3.2 mm, 5‐μ column with a mobile phase consisting of 20 mM ammonium formate: methanol (20:80 v/v) at a flow rate of 0.5 ml/min. The detection was accomplished by multiple‐reaction monitoring via electrospray ionization source operating in the positive ionization mode. The calibration curve was linear over the investigated concentration range, 25–2,000 ng/ml, for each analyte using 1.0 ml of urine. The lower limit of quantitation for each analyte was 25 ng/ml. The intra‐ and inter‐day precisions had coefficient of variation less than 15% and the accuracy was within the range from 88% to 109%. The method proved adequate for the tricyclic antidepressants analysis of urine for emergency clinical toxicology and pain management compliance testing. J. Clin. Lab. Anal. 26:286‐294, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: tricyclic antidepressants, pain management, urine drug testing, HPLC/MS/MS
INTRODUCTION
Tricyclic antidepressants (TCAs) are commonly used to treat depression, anxiety reactions, and recently neuropathic pain 1, 2, 3, 4. Despite the risk of severe side effects, even in patients taking therapeutic doses, TCAs are still prescribed due to their effectiveness in treating these disorders compared to other antidepressants. Monitoring serum concentrations of TCAs can improve therapeutic management of depression in patients with questions of compliance, suspected toxicity, and/or drug‐drug interactions. Such therapeutic drug monitoring (TDM) is recommended for amitriptyline, nortriptyline, doxepin, imipramine, and desipramine 5, 6, 7, 8. High‐pressure liquid chromatography (HPLC) with ultraviolet (UV) spectrophotometric detection has been the recommended and is most prevalent method for TDM of TCA for over 30 years 9, 10, 11, 12, 13, 14. Recently, several HPLC/mass spectrometry/mass spectrometry (MS/MS) methods have been developed for TCA TDM 15, 16, 17. However, most clinical laboratories do not have the ability to perform serum TCA analysis. The 2010 College of American Pathologists TDM special survey indicates that only 22 laboratories including reference laboratories perform the quantification of TCAs in serum 18.
Deliberate self‐poisoning with TCAs has been a major medical problem for over 30 years. Taken in overdose, TCAs produce profound central nervous system depression, cardiotoxicity, and anticholinergic effects 19, 20. Along with drugs of abuse, prescription and over the counter analgesics, and benzodiazepines, TCA drugs have been and remain among the most commonly encountered drugs associated with emergency department visits 21. Many TCA overdosed patients arrive in the emergency department unconscious or semi‐comatose with increased pulse, hypotension, and small pupils secondary to alpha adenergic blockade; unfortunately, this is a clinical picture consistent with poisoning from a variety of drugs. Other patients present with a history of drug ingestion, although the amount or identity of the drug or drugs can seldom be obtained from the family or patient. If TCA overdose is suspected, an electrocardiography with a QRS complex broadened to greater than 100 msec has been proposed to be indicative that a serious or potentially fatal dose has been ingested 22, 23. In cases of suspected overdose, most hospitals rely on point‐of‐care urine immunoassays for the detection of TCAs in confirming the diagnosis and in selecting appropriate treatment. Such assays indicate only the presence of a TCA and typically respond to the total concentration of parent TCA and major metabolite(s) 24, 25, 26. These assays have cut‐off values of 300 or 1,000 ng/ml, which are sufficient to detect total TCAs in instances of drug overdose.
Presently, urine analysis for TCAs is performed not only for emergency toxicology screening, but also for pain management compliance testing (PMCT). In PMCT, TCA immunoassays have several limitations including the inability to detect and quantify specific TCAs, possible false‐positive results, and poor sensitivity 27, 28. These issues are resolved by the use of HPLC with UV or MS detection. We present a reliable HPLC/MS/MS method for the quantitation of 11 TCA and/or their metabolites; fluoxetine and norfluoxetine; cyclobenzaprine; and trazodone in urine. For the purposes of this report, all these analytes will be referred to as TCAs. The assay has sufficient sensitivity, reproducibility, and accuracy for zero tolerance screening of TCAs in PMCT.
MATERIALS AND METHODS
Reagents
TCA primary reference materials for amitriptyline, clomipramine, cyclobenzaprine, desipramine, doxepin, fluoxetine, imipramine, maprotiline, mirtazapine, norclomipramine, nordoxepin, norfluoxetine, nortriptyline, trimpramine, and trazodone were purchased (Cerilliant Corporation, Round Rock, TX) as 1.0 mg/ml standards. Amoxapine internal standard (ISTD) as a 1.0 mg/ml methanolic solution was also purchased (Cerilliant). These TCA methanolic stock standard solutions were stored at −20°C. Formic acid (99%) and ammonium formate were purchased (Aldrich Chemicals, Milwaukee, WI). Ethyl acetate, hexane, and methanol were purchased from B&J Brand High Purity Solvents (Burdick and Jackson, Muskegon, MI). Deionized water (DI H2O) was produced by a NANOpure DiamondTM water purification system (Barnstead International, Dubuque, IA).
Stock Solution Preparation and Dilutions
A series of working standard solutions of 10 and 100 μg/ml were prepared by appropriate dilution with methanol of the stock standard solution of TCAs. ISTD working solution of 10 μg/ml amoxapine was prepared by appropriately diluting the internal standard stock solution with methanol and stored at −20°C.
Preparation of Calibrators and Quality Control Specimens
In‐house drug‐free urine provided the matrix for all prepared calibrators, quality control (QC), and other study specimens. Pooled drug‐free urine was obtained from laboratory personnel who were nonsmokers and did not use prescription, illicit, or over‐the‐counter drugs. These urine specimens were analyzed by gas chromatography—mass spectrometry and found not to contain common drugs of abuse, TCAs of interest in this study, and/or their metabolites.
Appropriate volumes of the working solutions of each of the 15 TCAs were added to urine to obtain a seven‐point calibration curve of 25, 50, 100, 250, 500, 1,000, and 2,000 ng/ml. Calibrators were prepared fresh before each analysis of each batch of samples. The following quality control urine specimens for each of the 15 TCAs were prepared and analyzed with each batch of test specimens: limit of quantification quality control (LOQC), target concentration of 25 ng/ml; low control (LQC), target concentration of 75 ng/ml; medium control (MQC), target concentration of 300 ng/ml; and high control (HQC), target concentration of 1,500 ng/ml. A drug‐free control (negative control) that contains no TCAs with ISTD added, and a double negative control containing no TCAs or ISTD, were also run with each test batch. All QC samples were stored at −20°C until analysis.
Specimen Extraction
To a 1.0 ml aliquot of urine, 50 μl of ISTD consisting of 10 μg/ml (500 ng total) of amoxapine in methanol was added with mixing. Then 2.0 ml of 2 N sodium hydroxide was added followed by 2 ml of hexane: ethyl acetate (1:1). The samples were then mixed for 5 min and then centrifuged for 10 min at 3,000 rpm. The upper organic layer was transferred to a clean test tube and evaporated to dryness under a gentle stream of nitrogen in a 40°C dry bath. The samples were reconstituted with 100 μl of mobile phase and placed in auto‐sample (HPLC/MS/MS) vials for analysis.
Instrumental Analysis
The HPLC/MS/MS system used was an A Quattro II Triple Quadrupole mass spectrometer using an electrospray ionization source (ESI) attached to Water's Alliance 2695 HPLC controlled by MassLynx version 3.5 software (Waters Corporation, Milford, MA). The chromatographic separation was performed using an Allure Biphenyl 100 × 3.2 mm, 5‐μ column (Restek Corporation, Bellefonte, PA). The mobile phase contained 20 mM ammonium formate; methanol (20:80 v/v) and was delivered at a flow rate of 0.5 ml/min. The desolvation temperature was set at 250°C and ESI nebulizing gas flow rate of 15 ml/min with the drying gases flow rates of 250 ml/min. The capillary voltage was 3.2 V, the cone was 35 V, and the extractor was 5 V. The acquisition mode used was multiple reaction monitoring (MRM). The transition ions monitored and the collision energies (CE) used for each of the TCAs and the internal standard, amoxapine, are presented in Table 1. The total chromatographic separation time for each extract injection was 12 min (Fig. 1).
Table 1.
TCA Retention Times, Multiple Reaction Monitoring (MRM) Transition Ions, and Corresponding Collision Energies (CE)
| TCA | RT (min) | Quant ion (m/z) | CE (eV) | Qual ion (m/z) | CE (eV) |
|---|---|---|---|---|---|
| Amitriptyline | 6.2 | 278 > 233 | 17 | 278 > 91 | 25 |
| Amoxapine (ISTD) | 5.6 | 314 > 271 | 25 | ||
| Clomipramine | 7.7 | 315 > 86 | 17 | 315 > 58 | 38 |
| Cyclobenzaprine | 5.7 | 276 > 231 | 18 | 276 > 216 | 24 |
| Desipramine | 3.8 | 267 > 72 | 15 | 267 > 236 | 15 |
| Doxepin | 4.4 | 280 > 235 | 15 | 280 > 107 | 23 |
| Fluoxetine | 2.2 | 310 > 148 | 8 | 310 > 44 | 5 |
| Imipramine | 5.7 | 281 > 86 | 16 | 281 > 281 | 5 |
| Maprotiline | 3.8 | 278 > 250 | 20 | 278 > 219 | 24 |
| Mirtazapine | 7.2 | 266 > 195 | 25 | 266 > 72 | 20 |
| Norclomipramine | 4.7 | 301 > 72 | 6 | 301 > 301 | 18 |
| Nordoxepin | 2.9 | 266 > 235 | 15 | 266 > 107 | 23 |
| Norfluoxetine | 2.0 | 296 > 30 | 8 | 296 > 134 | 15 |
| Nortriptyline | 4.1 | 264 > 233 | 14 | 264 > 91 | 19 |
| Trazodone | 10.3 | 374 > 176 | 25 | 374 > 148 | 34 |
| Trimipramine | 6.2 | 295 > 100 | 19 | 295 > 58 | 35 |
Figure 1.
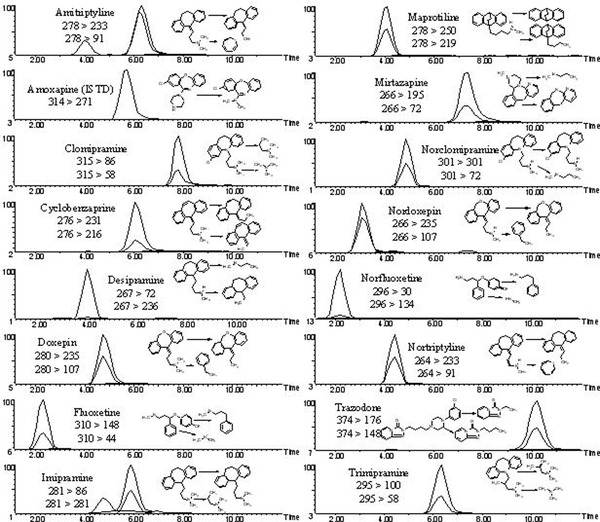
Chromatographic separation of 11 tricyclic antidepressants and/or their metabolites; fluoxetine and norfluoxetine; cyclobenzaprine; trazodone and amoxapine (ISTD) in urine.
Assay Performance
The evaluation of the assay was conducted over five separate days. The samples batches were analyzed as recommended for biomedical assay validation 29, 30. Validation sample batches contained calibrators in duplicate, drug‐free samples with internal standard added, drug‐free samples without internal standard, and replicates of the prepared LOQC, LQC, MQC, and HQC samples.
Linearity and LOQ
A seven‐point calibration of 25, 50, 100, 250, 500, 1,000, and 2,000 ng/ml in duplicate of the TCAs was prepared in drug‐free urine for the determination of TCA concentrations. The calibration curve was constructed by a linear regression plot of the ratio of the peak area of the abundance quantification ion of TCAs to the peak area abundance quantification ion of amoxapine ISTD versus the concentration in urine.
Accuracy, Absolute Recovery, and Precision
Accuracy/bias, recovery, and precision of the method were determined from the analysis of five different batches of the prepared QC samples. The percent accuracy/bias of the method was calculated as the ratio of the mean TCA concentration of six aliquots of each QC sample analyzed in the same batch of samples, to the target concentration of the QC samples times 100. The criteria for acceptable assay accuracy/bias were quantified TCA results within ± 20% of the target value of the prepared QC samples. The percent recovery was determined by, first extracting and preparing the residues of drug‐free urine. These residues were then reconstituted with HPLC mobile phase to prepare test samples containing the target concentrations of the QC samples. This nullified any matrix effects from interfering with the recovery studies. The absolute recovery of the assay was then determined by the ratio of the quantified results for six aliquots of each TCA QC sample compared to the quantified results of the QC samples prepared in the HPLC mobile phase times 100. The intra‐day precision of the method was determined by the quantified results of replicate analysis of six aliquots of the four different prepared QC samples. The inter‐day precision was determined from quantified results of the six intra‐day aliquots and triplicate analysis of the four prepared controls on four different days.
ASSAY VALIDATION
For each of five calibration curves, each calibrator concentration of the duplicate curves was determined to be within ± 15% of the expected value. The linear regression correlation coefficients (r 2) for the calibration curves of the 15 TCAs in the first three batches yielded a mean r 2 of 0.995 ± 0.002 with a range of 0.990–1.000, n = 45. The LOQ and the lower limit of detection (LOD) for each TCA were administratively set at a concentration of 25 ng/ml. The LOD of each TCA had a response at least five times the signal‐to‐noise ratio of the response to drug‐free urine.
The accuracy/bias of the assay for the 15 TCAs at the LOQ (25 ng/ml) ranged from 88% to 106%, at the LQC (75 ng/ml) from 92% to 109%, at the MQC (300 ng/ml) from 90% to 108%, and at the HQC (1,500 ng/ml) from 93% to 107%, Table 2. Overall accuracy over the linear range of the assay varied from a low of 88% exhibited by cyclobenzaprine at a concentration of 25 ng/ml to 109% for desipramine at a concentration of 75 ng/ml.
Table 2.
Percent Accuracy/Bias
| % Accuracy/Bais (SD); n = 6 | ||||
|---|---|---|---|---|
| LOQ (25 ng/ml) | LQC (75 ng/ml) | MQC (300 ng/ml) | HQC (1,500 ng/ml) | |
| Amitriptyline | 104 (12) | 105 (5) | 102 (11) | 99 (11) |
| Clomipramine | 95 (14) | 103 (9) | 102 (9) | 107 (9) |
| Cyclobenzaprine | 88 (4) | 98 (8) | 101 (7) | 101 (12) |
| Desipramine | 106 (16) | 109 (6) | 107 (2) | 104 (8) |
| Doxepin | 95 (15) | 92 (5) | 104 (7) | 102 (8) |
| Fluoxetine | 94 (4) | 105 (4) | 108 (7) | 102 (15) |
| Imipramine | 97 (13) | 105 (5) | 102 (11) | 106 (7) |
| Maprotiline | 97 (11) | 98 (8) | 96 (11) | 95 (14) |
| Mirtazapine | 98 (7) | 98 (4) | 98 (4) | 99 (9) |
| Norclomipramine | 94 (10) | 105 (5) | 105 (5) | 107 (11) |
| Nordoxepin | 105 (10) | 99 (10) | 108 (8) | 103 (10) |
| Norfluoxetine | 102 (7) | 105 (5) | 96 (10) | 97 (8) |
| Nortriptyline | 100 (8) | 93 (6) | 90 (4) | 93 (7) |
| Trazodone | 91 (16) | 93 (3) | 102 (8) | 104 (9) |
| Trimipramine | 103 (11) | 98 (4) | 98 (4) | 103 (13) |
The absolute recovery of the assay for the 15 TCAs at the LQC (75 ng/ml) ranged from 88% to 126%, at the MQC (300 ng/ml) from 73% to 122%, and at the HQC (1,500 ng/ml) from 81% to 125%, Table 3. Overall absolute recovery over the linear range of the assay varied from a low of 88% exhibited by trazodone at a concentration of 300 ng/ml to 125% trimipramine at a concentration of 1,500 ng/ml. The absolute recovery of internal standard amoxapine was 98% ± 18%, CV = 19%, n = 18.
Table 3.
Absolute Recovery of Tricyclic Antidepressants by HPLC/MS/MSAssay
| % Recovery (SD); n = 6 | |||
|---|---|---|---|
| LQC | MQC | HQC | |
| (75 ng/ml) | (300 ng/ml) | (1,500 ng/ml) | |
| Amitriptyline | 117 (12) | 106 (11) | 122 (16) |
| Clomipramine | 108 (14) | 118 (13) | 94 (10) |
| Cyclobenzaprine | 118 (13) | 110 (17) | 113 (18) |
| Desipramine | 97 (12) | 122 (13) | 95 (13) |
| Doxepin | 118 (13) | 87 (21) | 120 (10) |
| Fluoxetine | 119 (13) | 81 (21) | 101 (16) |
| Imipramine | 126 (6) | 105 (14) | 120 (17) |
| Maprotiline | 111 (16) | 118 (18) | 81 (17) |
| Mirtazapine | 104 (19) | 81 (19) | 99 (14) |
| Norclomipramine | 97 (16) | 119 (13) | 87 (18) |
| Nordoxepin | 118 (12) | 87 (21) | 99 (21) |
| Norfluoxetine | 88 (16) | 93 (16) | 108 (20) |
| Nortriptyline | 105 (15) | 98 (18) | 117 (13) |
| Trazodone | 107 (12) | 73 (14) | 93 (4) |
| Trimipramine | 116 (8) | 108 (10) | 125 (24) |
The intra‐day precision for the TCAs at the four different QC concentrations ranged between 2% to 15%, with the exception of mirtazapine (13%), which displayed a CV of 18% for the 25 ng/ml LOQ, Table 4. The inter‐day precision for the TCAs at the four different QC concentrations ranged between 8 and 12% with the exception of desipramine and trazodone, which yielded CVs of 13% and 16% at the 25 ng/ml LOQ, respectively, and nordoxepin, which yielded a CV of 13% at the 1,500 ng/ml HQC (Table 5).
Table 4.
Intra‐run Precision of the Tricyclic Antidepressant HPLC/MS/MSAssay
| Mean ± SD ng/ml (%CV); n = 6 | ||||
|---|---|---|---|---|
| LOQ (25 ng/ml) | LQC (75 ng/ml) | MQC (300 ng/ml) | HQC (1,500 ng/ml) | |
| Amitriptyline | 27 ± 2.2 (8) | 83 ± 6.0 (7) | 344 ± 21 (6) | 1,420 ± 170 (12) |
| Clomipramine | 25 ± 2.3 (9) | 82 ± 5.7 (7) | 310 ± 12 (4) | 1,620 ± 130 (8) |
| Cyclobenzaprine | 26 ± 2.6 (10) | 85 ± 4.2 (5) | 276 ± 27 (10) | 1,600 ± 64 (4) |
| Desipramine | 25 ± 3.7 (15) | 78 ± 2.3 (3) | 331 ± 26 (8) | 1,400 ± 182 (13) |
| Doxepin | 23 ± 1.4 (6) | 85 ± 4.3 (5) | 276 ± 27 (10) | 1,594 ± 48 (3) |
| Fluoxetine | 27 ± 2.7 (10) | 79 ± 8.7 (11) | 297 ± 33 (11) | 1,440 ± 173 (12) |
| Imipramine | 26 ± 1.8 (7) | 80 ± 11 (14) | 312 ± 25 (8) | 1,995 ± 180 (9) |
| Maprotiline | 26 ± 1.3 (5) | 83 ± 5.0 (6) | 267 ± 16 (6) | 1,327 ± 106 (8) |
| Mirtazapine | 25 ± 4.5 (18) | 80 + 8.0 (10) | 305 ± 21 (7) | 1,648 ± 132 (8) |
| Norclomipramine | 28 ± 0.8 (3) | 82 ± 5.0 (8) | 307 ± 12 (4) | 1,581 ± 237 (15) |
| Nordoxepin | 26 ± 3.9 (15) | 72 ± 10 (15) | 283 ± 42 (15) | 1,251 ± 25 (2) |
| Norfluoxetine | 25 ± 3.0 (12) | 73 ± 8.0 (11) | 310 ± 37 (12) | 1,503 ± 150 (10) |
| Nortriptyline | 27 ± 1.9 (7) | 85 ± 1.7 (2) | 325 ± 23 (7) | 1,532 ± 153 (10) |
| Trazodone | 23 ± 2.1 (9) | 67 + 4.0 (6) | 238 ± 33 (14) | 1,681 ± 168 (10) |
| Trimipramine | 26 ± 2.8 (11) | 86 ± 4.3 (5) | 294 ± 24 (8) | 1,398 ± 154 (11) |
Table 5.
Inter‐run Precision of the Tricyclic Antidepressant HPLC/MS/MSAssay
| Mean ± SD ng/ml (%CV); n = 18 | ||||
|---|---|---|---|---|
| LOQ (25 ng/ml) | LQC (75 ng/ml) | MQC (300 ng/ml) | HQC (1,500 ng/ml) | |
| Amitriptyline | 26 ± 2.8 (11) | 76 ± 6.8 (9) | 313 ± 34 (11) | 1,467 ± 132 (9) |
| Clomipramine | 24 ± 2.8 (12) | 74 ± 8.1 (11) | 305 ± 28 (8) | 1,540 ± 138 (9) |
| Cyclobenzaprine | 23 ± 2.7 (12) | 72 ± 7.9 (11) | 282 ± 22 (8) | 1,527 ± 122 (8) |
| Desipramine | 25 ± 4.0 (16) | 74 ± 10 (14) | 306 ± 36 (12) | 1,471 ± 176 (12) |
| Doxepin | 24 ± 2.8 (12) | 67 ± 4.0 (6) | 288 ± 31 (11) | 1,507 ± 165 (11) |
| Fluoxetine | 24 ± 2.4 (10) | 76 ± 7.6 (10) | 298 ± 39 (10) | 1,547 ± 170 (11) |
| Imipramine | 24 ± 2.6 (11) | 75 ± 8.2 (11) | 295 ± 35 (12) | 1,487 ± 133 (9) |
| Maprotiline | 26 ± 2.3 (9) | 79 ± 6.3 (8) | 311 ± 31 (10) | 1,423 ± 156 (11) |
| Mirtazapine | 25 ± 2.7 (11) | 76 ± 6.8 (9) | 294 ± 35 (12) | 1,547 ± 139 (9) |
| Norclomipramine | 26 ± 3.1 (12) | 77 ± 6.9 (9) | 306 ± 27 (9) | 1,584 ± 174 (11) |
| Nordoxepin | 25 ± 2.7 (11) | 75 ± 6.7 (9) | 302 ± 36 (12) | 1,477 ± 192 (13) |
| Norfluoxetine | 25 ± 2.2 (9) | 75 ± 6.7 (9) | 297 ± 32 (11) | 1,503 ± 150 (10) |
| Nortriptyline | 25 ± 2.2 (9) | 76 ± 7.6 (10) | 291 ± 29 (10) | 1,496 ± 134 (9) |
| Trazodone | 26 + 3.4 (13) | 69 ± 6.9 (10) | 277 ± 27 (10) | 1,545 ± 154 (10) |
| Trimipramine | 25 ± 3.0 (12) | 73 ± 8.0 (11) | 280 ± 22 (8) | 1,497 ± 149 (10) |
The selectivity of the assay was determined using six different lots of drug‐free urine. Each individual lot was analyzed with and without internal standard. No peaks were detected that co‐eluted with the targeted 15 TCAs or with the internal standard from the six lots of drug‐free urine. This ensured that endogenous urine components did not interfere with the assay. Possible matrix effects from urine were determined by preparing six different lots of drug‐free urine fortified with 75 ng/ml of each of the TCAs and analyzing each lot in triplicate. The results were determined to deviate less than ± 20% of the prepared concentration in each of the six urines for all the TCAs except nordoxepin, doxepin, norclomipramine, and fluoxetine. These TCAs yielded results that deviated less than ± 20% of the prepared concentration in five of the six lots of drug‐free urines.
Sample carryover was evaluated in each of the five validation batches using two different procedures. For the carryover study, an upper limit of quantification control (ULOQC) containing 2,000 ng/ml of each TCA was prepared in drug‐free urine. First, immediately following the injection of an extracted ULOQC, an extract of a negative control was injected. The rejection criterion for carryover was set at the detection of a TCA at a concentration less than 20% of the LOQC. Following injection of 2,000 ng/ml of each of the TCAs, the drugs were not detected in the injected negative control sample. An additional procedure to evaluate possible analyte carryover during batch analysis was by injecting an extracted HQC (1,500 ng/ml) immediately followed by an LQC (25 ng/ml). This analysis was repeated consecutively six times. The rejection criterion for carryover was set at a TCA concentration with a bias of less than 20% of the target value of the LQC. Lack of carryover was confirmed as none the TCA LQC samples demonstrated a significant quantifiedbias.
Stability of the TCAs in urine was determined under several specific conditions and time intervals. Several experiments were performed using three of the TCA control specimens: LQC (75 ng/ml), MQC (300 ng/ml), and HQC (1,500 ng/ml). All studies included six replicate analyses of each QC specimen. Since urine specimens are often stored frozen and thawed for reanalysis, the stability of TCAs in urine was determined after three freeze‐thaw cycles. The QC specimens were stored at −20°C, then twice removed and allowed to thaw. Once thawed, they were refrozen for 24 hr. The specimens were removed a third time, allowed to thaw and analyzed. The freeze‐thaw QC samples were extracted and quantified against freshly prepared calibrators. To evaluate the possible effects of specimen transportation and processing in the laboratory, the “bench‐top” stability of TCAs in urine at room temperature was assessed by having the QC specimens sit at room temperature for 72 hr. Batch analysis was performed with an auto‐sampler connected to the HPLC/MS/MS. To evaluate the “post‐preparative” stability of the TCAs, the extracts were allowed to sit in the auto‐sampler. A batch of the extracted LQC, MQC, and HQC was quantified against a freshly prepared calibration. The extracted controls were then allowed to sit in the auto‐sampler for 72 hr at room temperature after which, they were reinjected and quantified from the initial calibration. The results of the initial analysis were compared to those of the reinjected samples. In freeze‐thaw and bench‐top studies, TCAs were considered stable if the concentrations of the QC samples were within ± 20% of the target concentration samples. In the post‐preparative study, TCAs were considered stable if the concentrations of the reinjected QC samples were within ± 20% of their concentration determined by their initial injection. Under the tested conditions, TCAs were stable in frozen or room temperature urine, as well as, in urine extracts in the auto‐sampler, Table 6. The sole exception was imipramine, which had a ± 25 and ± 23% from deviation from the target concentrations of the LQC and MQC specimens at room temperature, respectively.
Table 6.
Tricyclic Antidepressant Stability under Various Conditions of Storage and Analysis
| TCA | Mean + SD ng/ml (%CV); n = 6 | |||
|---|---|---|---|---|
| Stability test | LQC (75 ng/ml) | MQC (300 ng/ml) | HQC (1,500 ng/ml) | |
| Amitriptyline | Freeze/Thaw | 72 ± 5.0 (7) | 307 ± 34 (11) | 1,465 ± 176 (12) |
| Bench‐top | 73 ± 7.3 (10) | 265 ± 24 (9) | 1,519 ± 122 (8) | |
| Post preparative | 77 ± 7.7 (10) | 340 ± 17 (5) | 1,556 ± 62 (4) | |
| Clomipramine | Freeze/Thaw | 73 ± 9.4 (13) | 315 ± 22 (7) | 1,618 ± 81 (5) |
| Bench‐top | 81 ± 4.9 (6) | 298 ± 44 (15) | 1,728 ± 69 (4) | |
| Postpreparative | 75 ± 1.5 (2) | 289 ± 31 (11) | 1,546 ± 170 (11) | |
| Cyclobenzaprine | Freeze/Thaw | 71 ± 11 (16) | 297 ± 26 (9) | 1,531 ± 92 (6) |
| Bench‐top | 77 ± 8.4 (11) | 276 ± 30 (11) | 1,622 ± 113 (7) | |
| Postpreparative | 76 ± 5.3 (7) | 265 ± 13 (5) | 1,678 ± 84 (5) | |
| Desipramine | Freeze/Thaw | 68 ± 8 (12) | 301 ± 36 (12) | 1,559 ± 125 (8) |
| Bench‐top | 73 ± 10 (14) | 280 ± 36 (13) | 1,494 ± 149 (10) | |
| Postpreparative | 73 ± 5 (6) | 290 ± 32 (11) | 1,690 ± 152 (9) | |
| Doxepin | Freeze/Thaw | 68 ± 5.4 (8) | 278 ± 19 (7) | 1,482 ± 89 (6) |
| Bench‐top | 75 ± 7.5 (10) | 268 ± 16 (6) | 1,394 ± 125 (9) | |
| Postpreparative | 78 ± 5.4 (7) | 288 ± 29 (10) | 1,708 ± 119 (7) | |
| Fluoxetine | Freeze/Thaw | 70 ± 2.8 (4) | 278 ± 22 (8) | 1,537 ± 169 (11) |
| Bench‐top | 77 ± 10 (13) | 259 ± 26 (10) | 1,556 ± 171 (11) | |
| Postpreparative | 76 ± 7.6 (10) | 280 ± 8.4 (3) | 1,690 ± 202 (12) | |
| Imipramine | Freeze/Thaw | 70 ± 9.8 (14) | 268 ± 8.4 (3) | 1,464 ± 176 (12) |
| Bench‐top | 56 ± 2.2 (4) | 230 ± 21 (9) | 1,331 ± 106 (8) | |
| Postpreparative | 80 ± 2.4 (3) | 312 ± 47 (15) | 1,579 ± 268 (17) | |
| Maprotiline | Freeze/Thaw | 73 ± 8.7 (12) | 314 ± 35 (11) | 1,603 ± 208 (13) |
| Bench‐top | 80 ± 6.4 (8) | 306 ± 55 (18) | 1,621 ± 65 (4) | |
| Post preparative | 78 ± 5.5 (7) | 321 ± 19 (6) | 1,651 ± 182 (11) | |
| Mirtazapine | Freeze/Thaw | 80 ± 5.6 (7) | 306 ± 54 (18) | 1,620 ± 70 (4) |
| Bench‐top | 73 ± 9.0 (12) | 314 ± 34 (11) | 1,603 ± 212 (13) | |
| Postpreparative | 78 ± 5.5 (7) | 320 ± 19 (6) | 1,652 ± 182 (11) | |
| Norclomipramine | Freeze/Thaw | 71 ± 7.1 (10) | 300 ± 36 (12) | 1,518 ± 303 (20) |
| Bench‐top | 76 ± 6.0 (8) | 318 ± 16 (5) | 1,587 ± 95 (6) | |
| Postpreparative | 77 ± 4.6 (6) | 273 ± 27 (10) | 1,694 ± 186 (11) | |
| Nordoxepin | Freeze/Thaw | 71 ± 11 (16) | 277 ± 30 (11) | 1,531 ± 168 (11) |
| Bench‐top | 77 ± 10 (13) | 272 ± 19 (7) | 1,598 ± 160 (10) | |
| Postpreparative | 84 ± 6.7 (8) | 285 ± 57 (20) | 1,679 ± 67 (4) | |
| Norfluoxetine | Freeze/Thaw | 77 ± 11 (15) | 303 ± 45 (15) | 1,422 ± 100 (7) |
| Bench‐top | 81 ± 8.1 (10) | 306 ± 21 (7) | 1,362 ± 40 (3) | |
| Postpreparative | 78 ± 5.5 (7) | 297 ± 5.9 (2) | 1,619 ± 145 (9) | |
| Nortriptyline | Freeze/Thaw | 66 ± 11 (17) | 264 ± 37 (14) | 1,518 ± 167 (11) |
| Bench‐top | 74 ± 9.6 (13) | 287 ± 25 (9) | 1,596 ± 191 (12) | |
| Postpreparative | 69 ± 4.1 (6) | 290 ± 46 (16) | 1,526 ± 91 (6) | |
| Trazodone | Freeze/Thaw | 76 ± 8.3 (11) | 285 ± 40 (14) | 1,441 ± 72 (5) |
| Bench‐top | 74 ± 8.8 (12) | 281 ± 39 (14) | 1,315 ± 170 (13) | |
| Postpreparative | 68 ± 8.1 (12) | 241 ± 14 (6) | 1,627 ± 178 (11) | |
| Trimipramine | Freeze/Thaw | 67 ± 4.0 (6) | 268 ± 13 (5) | 1,363 ± 136 (10) |
| Bench‐top | 70 ± 5.6 (8) | 267 ± 21 (8) | 1,366 ± 191 (14) | |
| Postpreparative | 81 ± 6.5 (8) | 299 ± 27 (9) | 1,545 ± 169 (11) | |
Until PMCT, few, if any, clinical laboratories quantified TCAs in urine. Therefore, test results from a similar method in another laboratory were not readily available for comparison. However, we analyzed patient urines by our previously described HPLC/UV serum TCA method and compared these finding to the described HPLC/MS/MS assay 9. This TCA HPLC/UV assay had an LOQ of 50 ng/ml in urine. In all, 23 urine specimens were tested yielding 30 TCA quantified results by both methods. TCAs detected by both methods were: amitriptyline (86–2,000 ng/ml, n = 10), nortriptyline (75–2,500 ng/ml, n = 9), cyclobenzaprine (280–554 ng/ml, n = 6), doxepin (3,500 ng/ml), nordoxepin (2,500 ng/ml), fluoxetine (2,300 ng/ml), norfluoxetine (1,290 ng/ml), and trazodone (10,000 ng/ml). Results obtained by the presented HPLC/MS/MS assay within ± 25% of the HPLC/UV values. An additional specimen contained 37 ng/ml of nortriptyline by HPLC/MS/MS, but this was below the LOQ of the HPLC/UV method. Also, four TCAs were detected at concentrations below the LOQ of the HPLC/UV assay; but yielded acceptable retention times and ion ratios for identification. This brief comparison study demonstrated the ability of the assay to detect TCAs at very low concentrations.
DISCUSSION
The presented HPLC/MS/MS method demonstrated acceptable reliability and reproducibility for the detection and quantification of TCAs in urine specimens. Accuracy as well as intra‐day and inter‐day precisions were determined not to exceed CVs of ± 20% at the LOQ and were within CVs of < 15% over the dynamic range of the assay. The variance in these parameters may be reduced with the use of deuterated internal standards for each of the TCA analytes. However, this would increase the cost of testing and an additional 32 ions to process for each test would increase the time of analysis. Tables 3, 4, 5 through 6 present data from 168 different tests. The quantified results in 99% (166/168) of these tests were within the acceptable performance of ± 20% of their targeted values with the application of only amoxapine as a single nondeuterated internal standard. Amoxapine was used as the ISTD as it is not a popular drug and is seldom prescribed for depression. The drug is not subject to abuse. Additionally, the drugs prescribed to pain patients are known to the laboratory. Therefore, it would be unlikely to interfere with the assay. If amoxapine was present the sample could be analyzed without the ISTD and a qualitative finding could be reported. This performance is consistent with industry standards for urine drug testing. It certainly demonstrates the robustness of the assay.
The assay was free of significant interference from matrix effects and free from significant analyte carryover. The TCAs were shown to be stable under conditions of specimen handling in the laboratory. Presently, laboratories offering PMCT apply a 100 ng/ml as their LOQ for TCAs in urine. The presented HPLC/MS/MS method with its 25 ng/ml TCA LOQ greatly enhances the sensitivity of the assay. This decrease in LOD will detect TCAs in urine below the cut‐off values of TCA immunoassays. Pesce et al. have recently demonstrated the use of HPLC/MS/MS compared to immunoassays significantly decreased the number of false‐negative results in PMCT of drugs of abuse 31.
The primary use of the proposed method is for assuring compliance in PMCT programs. The validation of the method and the ability to quantitate each of the drugs was performed to verify the reliably of the method. At present, there is no established association between TCA urine concentrations and drug dose or efficacy in pain management. TCA PMCT is performed only to assure that the prescribed drug has been administered. Serum or whole blood rather than urine would be an appropriate specimen for the TDM of TCAs for treatment of depression.
CONCLUSION
A sensitive, rapid, accurate method for the determination of 15 TCAs and other psychoactive drugs in urine was developed and validated. The assay used a simple liquid/liquid extraction procedure prior to chromatographic analysis. The assay is particularly suited for the testing of pain management patients with zero tolerance of false‐negative results. Further, the assay may be easily applied to testing emergency room TCA overdose urine specimens.
REFERENCES
- 1. Baldessarini RJ. Current status of antidepressants: Clinical pharmacology and therapy. J Clin Psychiat 1989;50:117–126. [PubMed] [Google Scholar]
- 2. Donoghue J, Hylan TR. 2001. Antidepressant use in clinical practice: efficacy v. effectiveness. Br J Psychiat Suppl 42:9–17. [DOI] [PubMed] [Google Scholar]
- 3. Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clinic Proceed 2010;85:S3‐S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bajwa ZH, Simopoulos TT, Pal J, et al. Low and therapeutic doses of antidepressants are associated with similar response in the context of mulimodal treatment of pain. Pain Physician 2009;12:893–900. [PubMed] [Google Scholar]
- 5. Furlanut M, Benetello P, Spina E. Pharmacokinetic optimisation of tricyclic antidepressant therapy. Clin Pharmacokinet 1993;24:301–318. [DOI] [PubMed] [Google Scholar]
- 6. Preskorn SH, Jerkovich GS. Central nervous system toxicity of tricyclic antidepressants: Phenomenology, course, risk factors, and role of therapeutic drug monitoring. J Clin Psychopharmacol 1990;10:88–95. [DOI] [PubMed] [Google Scholar]
- 7. Preskorn SH, Dorey RC, Jerkovich, GS . Therapeutic drug monitoring of tricyclic antidepressants. Clin Chem 1998;34:822–828. [PubMed] [Google Scholar]
- 8. Orsulak PJ. Therapeutic monitoring of antidepressant drugs; guidelines updated. Therap drug Monitor 1998;11:497–507. [PubMed] [Google Scholar]
- 9. Poklis A, Soghoian D, Crooks CR, Saady JJ. Evaluation of the Abbott ADx total serum tricyclic immunoassay. J Toxicol Clin Toxicol 1990;28:235–248. [DOI] [PubMed] [Google Scholar]
- 10. Theurillat R, Thormann W. Monitoring of tricyclic antidepressants in human serum and plasma by HPLC: Characterization of a simple, laboratory developed method via external quality assessment. J Pharm Biomed Anal 1998;18:751–760. [DOI] [PubMed] [Google Scholar]
- 11. Nyanda AM, Nunes MG, Ramesh A. A simple high‐performance liquidchromatography method for the quantitation of tricyclic antidepressant drugs in human plasma or serum. J Toxicol Clin Toxicol 2000;38:631–636. [DOI] [PubMed] [Google Scholar]
- 12. Malfara WR, Bertucci C, Costa Queiroz ME, et al. Reliable HPLCmethod for therapeutic drug monitoring of frequently prescribed tricyclic and nontricyclic antidepressants. J Pharm Biomed Anal 2007;44:955–962. [DOI] [PubMed] [Google Scholar]
- 13. Samanidou VF, Nika MK, Papadoyannis IN. Development of an HPLCmethod for the monitoring of tricyclic antidepressants in biofluids. J Sep Sci 2007;30:2391–2400. [DOI] [PubMed] [Google Scholar]
- 14. Aymard G, Livi P, Pham YT, Diquet B. Sensitive and rapid method for the simultaneous quantification of five antidepressants with their respective metabolites in plasma using high‐performance liquid chromatography with diode‐array detection. J Chromatogr B Biomed Sci Appl 1997;700:183–189. [DOI] [PubMed] [Google Scholar]
- 15. Kollroser M, Schober C. Simultaneous determination of seven tricyclic antidepressant drugs in human plasma by direct‐injection HPLC‐APCI‐MS‐MSwith an ion trap detector. Ther Drug Monit 2002;4:537–544. [DOI] [PubMed] [Google Scholar]
- 16. Sauvage FL, Gaulier JM, Lachatre G, Marquet P. A fully automated turbulent‐flow liquid chromatography‐tandem mass spectrometry technique for monitoring antidepressants in human serum. Ther Drug Monit 2007;28:123–130. [DOI] [PubMed] [Google Scholar]
- 17. Alves C, Santos‐Neto AJ, Fernandes C, Rodrigues JC, Lancas FM. Analysis of tricyclic antidepressant drugs in plasma by means of solid phase microextraction‐liquid chromatography‐mass spectrometry. J Mass Spectrom 2007;42:1342–1347. [DOI] [PubMed] [Google Scholar]
- 18. Participant Summary UDS‐11 . Urine Drug Testing (Screening) AACC/CAPSurveys 2010. College of American Pathologists; 2010. [Google Scholar]
- 19. Nicotra MB, Rivera B, Pool JL. Tricyclic antidepressant overdose: Clinical and pharmacological observations. Clin Toxicol 1981;18:599–613. [DOI] [PubMed] [Google Scholar]
- 20. Spiker DG, Weiss AN, Chang SS, Ruwitch FW, Biggs TJ. Tricyclic antidepressant overdose: Clinical presentation and plasma levels. Clin Pharmacol Ther 1975;18:539–546. [DOI] [PubMed] [Google Scholar]
- 21.“Weighted Emergency Room Estimates ” Annual Emergency Room Data 2008, Drug Abuse Warning Network, Statistical Series 1, No.12A, National Institute on Drug Abuse, Rockville, MD, 2008:32–33. [Google Scholar]
- 22. Boehnest DG, Lovejoy, Jr, FH . Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after acute overdose of tricyclic antidepressant. N England J Med 1985;313:474–479. [DOI] [PubMed] [Google Scholar]
- 23. Rose JB. Tricyclic antidepressant toxicity. Clin Toxicol 1977;11:381–402. [DOI] [PubMed] [Google Scholar]
- 24. Poklis A, Edinboro LE, Lee JS, Crooks CR. Evaluation of a colloidal metal immunoassay evice for the detection of tricyclic antidepressants in urine. J Toxicol Clin Toxicol 1997;35:77–82. [DOI] [PubMed] [Google Scholar]
- 25. Phillips JE, Bogema S, Fu Paul, et al. Signify@ ERDrug Screen Test evaluation: Comparison to Triage@ Drug of Abuse Panel plus tricyclic antidepressants. Clin Chim Acta 2003;328:31–38. [DOI] [PubMed] [Google Scholar]
- 26. Attema‐de Jonge ME, Peeters SY, Franssen EJ. Performance of three point‐of‐care urinalysis test devices for drugs of abuse and therapeutic drugs applied in the emergency department. J Emerg Med 2011; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Melanson SEF, Lewandrowski EL, Griggs DA, Flood JG. Interpreting tricyclic antidepressant measurements in urine in an emergency department setting: Comparison of two qualitative point‐of‐care urine tricyclic antidepressant drug immunoassays with quantitative serum chromatographic analysis. J Anal Toxicol 2007;31:270–275. [DOI] [PubMed] [Google Scholar]
- 28. Hendrickson RG, Morocco RG. Quetiapine cross‐reactivity among three tricyclic antidepressant immunoassays. J Toxicol Clin Toxicol 2003;41:105–108. [DOI] [PubMed] [Google Scholar]
- 29. Bioanalytical method validation: A guidance for industry. US Department of Health and Human Services; 2010;2010:1–25. [Google Scholar]
- 30. Shah VP, Midha KK, Findlay JWA, et al. Bioanalytical method validation: A revisit with a decade of process. Pharmaceutical Res 2000;17:1551–1557. [DOI] [PubMed] [Google Scholar]
- 31. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography‐tandem mass spectrometry versus immunoassay drug tesing in pain patients. Pain Phys 2010;13:273–281. [PubMed] [Google Scholar]


