Abstract
Behavioral type–environment correlations occur when specific behavioral types of individuals are more common in certain environments. Behavioral type–environment correlations can be generated by several different mechanisms that are probably very common such as niche construction and phenotypic plasticity. Moreover, behavioral type–environment correlations have important ecological and evolutionary implications. However, few studies have examined behavioral type–environment correlations in natural populations. In this study, we asked whether some behavioral types of three-spined stickleback were more likely to occur in certain social environments (alone or in a shoal with other stickleback) or in certain microhabitats in a river (in the open or under cover). We found that individuals that were in shoals with other stickleback at the time of collection from the field emerged from a refuge more quickly compared to individuals that were found alone. In addition, fish that were alone in an open microhabitat explored more of a pool compared to fish that were alone in cover, but this difference did not occur among fish that were in shoals at the time of collection. Subsequent analyses of gut contents suggested that differences in microhabitat use were consistent over time. Our study provides some of the first evidence for behavioral type–environment correlations in a natural population of non-human animals.
Keywords: Personality, Social environment, Habitat selection, Niche, Refuge, Exploratory behavior
Introduction
Populations of animals are often comprised of individuals with different behavioral types (Bell et al. 2009). That is, individuals behave consistently across time and differently from one another (Sih and Bell 2008). In this study, we adopt a statistical definition of behavioral type, namely that an individual’s behavioral type is represented by their mean behavior and behavioral types exist when a statistically significant proportion of the total variance in a behavior can be attributed to differences among individuals (individual variance; Dingemanse et al. 2010). There is accumulating evidence that certain behavioral types are more likely to disperse (Dingemanse et al. 2003; Cote and Clobert 2007; Duckworth and Badyaev 2007; Chapman et al. 2011) or utilize a larger area of the habitat (Boon et al. 2008; Kobler et al. 2009). However, we know surprisingly little about whether certain behavioral types use particular niches within natural populations of non-human animals. A simple expectation, for example, is that timid individuals are more likely to occur in relatively safe habitats where predation risk is lower. Some authors have also suggested that certain behavioral types might occur more often in certain social environments (Bergmüller and Taborsky 2010).
When particular behavioral types occur more frequently in certain environments, there is a behavioral type–environment correlation (aka individual by environment correlation; Dingemanse et al. 2010; Stamps and Groothuis 2010b). Behavioral type–environment correlations can occur via numerous mechanisms, some of which are very common (Plomin et al. 1977; Stamps and Groothuis 2010a). Between-individual differences in behavior can lead to behavioral type–environment correlations. For example, individuals of a particular behavioral type might actively seek out certain environments (niche picking; Stamps and Groothuis 2010a, b), potentially leading to an increase in fitness (Bateson 1988; Via 1999; Edelaar et al. 2008). Alternatively, an individual might influence its environment via niche construction (Odling-Smee et al. 1996; Donohue 2005). At the same time, within-individual plasticity might be a factor if the environment influences an individual’s behavior. For example, being in a safe environment can encourage individuals to be bolder (Tuttle and Ryan 1982; Sharpe and Van Horne 1998; López et al. 2005; Webster et al. 2007; Peluc et al. 2008). Habitat-specific mortality can also lead to behavioral type–environment correlations if different behavioral types are more likely to survive in different environments (Jaenike and Holt 1991). Pleiotropic genetic variation for habitat choice and behavioral type could generate a behavioral type–environment correlation. The different mechanisms that can produce nonrandom associations between behavioral type and the environment are nonexclusive and might interact with each other. For example, an individual might select a certain environment according to its behavioral type and that environment might in turn influence the individual’s behavior, potentially leading to positive feedback that reinforces the strength of the behavioral type–environment correlation (Stamps and Groothuis 2010a).
Behavioral type–environment correlations have important ecological and evolutionary implications. For example, if different behavioral types experience different environments, then the strength of selection will be unequal among members of the population. If individuals select environments for which they are particularly well suited (matching habitat choice; Edelaar et al. 2008; phenotype-matching habitat selection; Holt and Barfield 2008), then selection will be relatively weak compared to a situation where behavioral types are randomly distributed in the environment. Behavioral type–environment correlations generated by adaptive matching habitat choice provide a mechanism by which variation in behavioral type could be maintained within a population (Ravigné et al. 2003, 2009). Moreover, models of indirect genetic effects suggest that correlations between behavioral type and the social environment that have a heritable basis can lead to complex patterns of selection (Wolf et al. 1999; McGlothlin et al. 2010; Saltz and Foley 2011). Finally, behavioral type–environment correlations could potentially lead to divergent selection and ultimately reproductive isolation if certain behavioral types consistently select and experience selection in different environments (Rice 1987; Via 1999).
Although there are good examples of personality–environment correlations in humans (Rutter et al. 1997) and growing interest in behavioral types in animals (Réale et al. 2010), we are just beginning to learn about behavioral type–environment correlations in natural populations of non-human animals (Hensley et al. 2012). Perhaps the best example is a study showing that different behavioral types of pumpkinseed sunfish inhabited different parts of a lake, consumed different prey, and were afflicted by different parasite communities (Wilson et al. 1993). This study suggested that an individual’s behavioral type was part of a much larger, ecologically relevant package of characteristics that were related to habitat use. A related literature is showing that intraspecific niche variation is widespread (Skulason and Smith 1995; Bolnick et al. 2003), and differences in niche use are often accompanied by differences in morphology, life history, and/or foraging tactics that form an integrated suite of traits that are adaptive (Bentzen and McPhail 1984; Ehlinger 1990; Schluter and McPhail 1992; Skúlason et al. 1993). Individuals that differ in morphology often preferentially assort into different social environments (Ranta and Lindström 1990; Griffiths and Magurran 1999; Brown and Brown 2000; Ward et al. 2002, 2005), but grouping according to behavioral type has received less attention (but see Sih and Watters 2005). Recently, laboratory studies on Drosophila melanogaster (Saltz 2011; Saltz and Foley 2011) provided empirical evidence for a heritable basis to a behavior–social environment correlation. Altogether, these studies suggest that behavioral type–environment correlations might be common, but they have rarely been explicitly examined in non-human populations in nature.
Therefore, in this study we asked whether certain behavioral types were more likely to occur in certain microhabitats and in certain social environments in three-spined stickleback (Gasterosteus aculeatus). Three-spined stickleback (stickleback hereafter) are small teleost fish that are especially well suited to the study of behavioral type–environment correlations because they exhibit pronounced within-population variation in niche use (Bentzen and McPhail 1984) and behavioral type (Bakker 1986). Moreover, juvenile stickleback are of particular interest because differences in the early environment can have strong effects on phenotypic development (Peuhkuri et al. 1995; Day and McPhail 1996; Bell et al. 2011) and therefore fitness later in life (Wootton 1973). We collected juvenile stickleback in different microhabitats and social environments in the field and measured their behavior in a standardized behavioral assay in the lab. In this behavioral assay, we recorded two behaviors, latency to emerge from a refuge (latency to emerge) and the number of sections of the test pool an individual explored (number of sections). Our rationale for focusing on these variables was that we expected that (1) remaining in a refuge or (2) restricting movement to new sections of the environment are two separate strategies that can decrease the likelihood of encountering predators (Sih 1987). We also recorded the number of transitions from one section of the pool to another as a measure of general activity so that we could avoid confounding differences in refuge use or exploration of the environment with differences in general activity. We tested a subset of these individuals repeatedly in the behavioral assay so that we could test for the statistical signature of behavioral types, repeatability, in both latency to emerge and number of sections. We then used these data to ask whether particular behavioral types (i.e., individuals that emerged more quickly or explored more sections) were more common in certain microhabitats or certain social environments in the field. We also analyzed the diet of a sample of these individuals in order to assess the stability of microhabitat use in nature and to determine if latency to emerge or number of sections were correlated with gut fullness or prey type.
Methods
Use of environment in the field
Juvenile three-spined stickleback from the Navarro River, CA were the focus of this study (less than 2 months post-hatch, standard length=21.2±4.2 SDmm, N=58). During preliminary snorkeling surveys of an approximately 100-m section of the river, we noticed that juvenile stickleback occurred in different social environments: some individuals moved through the habitat close to conspecifics in groups known as shoals (within four body lengths of at least two conspecifics, <12 cm; Pitcher and Parrish 1993), while others were alone, i.e., no other stickleback within 50 cm. We also observed differences in microhabitat use. Much of the Navarro River is moderately shallow (<1 m deep) and can be classified into either open microhabitat (shallow, gravel substrate, free of vegetation) or cover microhabitat (dense cover from grasses and submerged tree branches). While there were a variety of other microhabitats in the river (e.g., riffles and deep pools), juvenile three-spined stickleback rarely occurred outside of open or cover microhabitats. Therefore, after preliminary observations of juveniles in the field, we decided to collect individuals from four different categories: open alone (n=14), open shoal (n=13), cover alone (n=14), and cover shoal (n=17). Animals were collected with a trout landing net while snorkeling. Specifically, a focal individual was observed from a distance of approximately 2 m for at least 30 s to assign its social environment and microhabitat. We assume that the fish were not reacting to the observer during field observations because they maintained a constant level of foraging and did not orient toward or move away from the observer. One fish that began alone moved to a shoal during this observation period and was therefore excluded from the study (<2 % of the sample). No fish that was initially in a shoal moved away from the shoal during field observations. Collections alternated between open and cover microhabitats which were positioned on opposite banks of the river throughout a 100-m stretch (<2-m separation between cover and open at any point), and the same location was not sampled more than once per day to avoid collecting multiple individuals from one shoal. Sampling was not biased towards more “catchable” individuals as no focal individual escaped after observation (Biro and Dingemanse 2009).
Behavioral type
To quantify behavioral types of individuals, we first moved the fish to standardized holding chambers to minimize differences in the environment that could affect behavior. We transported the fish 14 km from the river by car to an outdoor area where observations would take place. Fish were visually isolated from each other in separate 0.5-L holding chambers within a larger 50×80-cm pool located outside and exposed to natural fluctuations in ambient light. We did not feed the fish in order to standardize hunger levels. Analysis of gut contents of a subset of these individuals showed that total number of prey in the gut at the time of collection was not related to behavioral type, microhabitat, or social environment (see “Diet analysis” section). The first behavioral assay trial began at least 1 h after the individual was transferred to the holding chamber, and was carried out in the afternoon. We euthanized a subset of these individuals after trial one to allow for analysis of gut contents (open alone n=6, open shoal n=7, cover alone n=7, cover shoal n=6; see “Diet analysis” section). The remaining individuals were run through the behavioral assay two additional times so we could calculate the repeatability of these behaviors. Trials two and three were carried out on the following day 15–20 and 18–26 h after trial one, respectively, and were separated by at least 1 h. All trials were completed within 28 h of collection from the river.
We employed a standardized behavioral assay to quantify the behavior of individuals. This behavioral assay combines elements of the open field test (Walsh and Cummins 1976; modified in Verbeek et al. 1994) as well as a refuge emergence test, which is frequently used in studies of animal personality (Hedrick 2000; Brown et al. 2005; Wilson et al. 2010; Cote et al. 2011). An individual was gently poured into an opaque cylindrical refuge (10 cm diameter, 10 cm height) where it was allowed to settle for 3 min. The refuge was in the center of a circular, plastic pool (150 cm diameter, 10 cm water depth) marked into nine equally sized sections (one circular section in the middle surrounded by eight identical sections). Each of the outer sections contained a small pile of rocks the fish could explore but to move between any two sections the fish had to cross an area with no substrate where it was highly conspicuous. Data were recorded via direct observation by one researcher (SP) who was hidden behind a blind with a small opening. After 3 min, we opened the side of the refuge remotely and recorded the time it took the individual to emerge completely (latency to emerge), which we interpret as willingness to trade the safety of the refuge for the opportunity to locate resources. If a fish did not emerge within 10 min (n=1 individual), then the individual was gently poured out of the shelter into the pool and assigned the maximum latency to emerge value of 10 min. For 3 min after the fish emerged from the refuge, we recorded the total number of sections visited (number of sections) as a measure of how thoroughly the individual explored the environment. To control for differences in general activity, we also recorded the number of times the fish transitioned from one section to another regardless of whether they had been there before (transitions). Activity has been considered as an important axis of behavioral variation in studies of animal personality, but interpreting both number of transitions (activity) and number of sections (exploratory behavior) would be inappropriate as they were measured simultaneously (Réale et al. 2007). We recorded the time of day at the beginning of each trial and length from tip of the mouth to the base of the caudal fin (standard length) after the last trial. Fish from different environments did not differ in size (standard length in millimeters±SE: alone=21.5±0.7, shoal=20.9±0.9, cover=21.3±0.8, open=21.1±0.8). The order of testing of individuals was haphazard. Approximately equal numbers of fish from each environment category were observed each day. All field observations and behavioral assays were carried out between July 10 and 19, 2010.
We calculated the repeatability of latency to emerge and number of sections to test whether a significant amount of the variation in these variables could be attributed to differences between individuals. Repeatability is a dimensionless statistic that compares between-individual variance to total variance and therefore shows the amount of overlap in the behavior of different behavioral types. We calculated the repeatability of latency to emerge and number of sections from restricted maximum likelihood mixed models with individual as a random effect and the population intercept as a fixed effect. We used parametric bootstrapping (1,000 bootstraps) to estimate 95 % confidence intervals (Nakagawa and Schielzeth 2010). All N=58 individuals including those that had only one trial of data were used in this calculation to improve the power of our repeatability estimate (Martin et al. 2011).
We tested whether latency to emerge or number of sections differed between individuals that occurred in different social environments or microhabitats using linear mixed models. We examined the normality of the data through visual inspection of the residuals. Latency to emerge data were right skewed and thus were +1 log transformed to improve normality before analysis. Number of sections were normally distributed. We used repeated measures models with individual as a random effect. We included microhabitat, social environment, and their interaction as fixed effects. Standard length was included as a covariate. We included mean number of transitions of each individual and the deviation from the individual’s mean of each trial as covariates to control for between- and within-individual differences in activity, respectively (within-subject centering; van de Pol and Wright 2009; Dingemanse and Dochtermann 2013). Degrees of freedom were determined by Satterthwaite approximation.
Diet analysis
Stability of microhabitat use
We analyzed the gut contents of a subset of our sample to gain insight into the consistency of microhabitat use (open alone n=6, open shoal n=7, cover alone n=7, cover shoal n=6). Our rationale for measuring gut contents is that we expected that fish foraging in different habitats were eating different prey types. Therefore, if there was a difference between the whole gut contents of fish from open vs. cover microhabitat that would suggest that microhabitat use is at least as enduring as the time to evacuate the gut (at least 6 h; Svanbäck and Bolnick 2007). Furthermore, Bolnick et al. (2008) found that within-population differences in gut contents in stickleback were related to differences in stable isotopes and therefore reflect long-term differences in habitat use.
As stated above, 26 individuals were euthanized after the first behavioral assay within 2 h of collection to cease the digestion of gut contents. Standard length was taken immediately following euthanasia. We identified gut contents to the lowest feasible taxonomic level (Chironomidae, Ephemeroptera, Trichoptera, Plecoptera, Arachnida, Bivalvia). We focused our analyses on two taxa, Chironomidae and Ephemeroptera, which comprised 95 % of the 446 prey items identified (SP, unpublished data).
We examined the normality of gut contents data through visual inspection of the residuals. Counts of Chironomidae were square root transformed to improve normality. Counts of Ephemeroptera and total number of prey items were normally distributed. We tested for differences in total number of prey items, number of Chironomidae, and number of Ephemeroptera between fish collected in open and cover microhabitats using general linear models that included microhabitat as a fixed effect and standard length as a covariate.
We conducted an invertebrate survey on the last day of the study to determine the composition of prey types in each microhabitat. Invertebrate samples were taken with a kicknet and a 10-cm diameter stovepipe sampler. Kicknet samples were taken by disturbing the bottom substrate 1 m upstream of the net. The stovepipe sampler was used to take a 10-cm diameter column of the substrate that was transferred to a tray for invertebrate sorting. Six samples were taken with each sampling method in each microhabitat.
Diet of behavioral types
We tested whether differences in behavior type were related to diet or hunger (Godin and Crossman 1994) by calculating Kendall’s tau-b rank correlations between number of Chironomidae, number of Ephemeroptera, total number of prey items and behavior (latency to emerge, number of sections). This correlation coefficient was used to correct for frequent rank ties. Correlations were analyzed separately for each microhabitat type to control for differences in prey availability between microhabitats.
Calculations of repeatability and bootstrapping were performed with the rptR package in R (Nakagawa and Schielzeth 2010). All other statistical analyses were conducted using SPSS v19.0.0.1. Procedures were carried out in accordance with Institutional Animal Care and Use Committee at the University of Illinois, IACUC protocol #09204.
Results
Behavioral type
We found consistent individual differences in behavior in a standardized assay. While some individuals emerged from the refuge within 4 s, others took up to 10 min (mean latency to emerge= 65.5±13.0 SEs). After emergence, some individuals moved through all nine sections of the pool, while others explored only two (mean number of sections=5.9±0.2 SE). The variation among individuals in their behavior was consistent over time. The repeatability of latency to emerge was R=0.38 [95 % confidence interval (CI), 0.14–0.58, p<0.001], and the repeatability of number of sections was R=0.26 (95 % CI, 0.02–0.46, p=0.019).
Behavioral type–environment correlations
Fish found in shoals with other stickleback emerged from the refuge faster compared to fish that were captured while alone (social environment, F1, 43.2=10.3, p=0.003, Table 1, Fig. 1). Smaller fish emerged faster compared to larger fish (Table 1). Between- and within-individual variations in number of transitions were both related to latency to emerge in the negative direction (Table 1). Latency to emerge did not differ between fish from different microhabitats.
Table 1.
Linear mixed models for latency to emerge and number of sections
| Behavior | Factor | Estimate | Estimate SE | df | Statistic | p value |
|---|---|---|---|---|---|---|
| Latency to emerge | Microhabitat | 0.09 | 0.10 | 1, 44.2 | 1.6 | 0.207 |
| Social environment | 0.23 | 0.11 | 1, 43.2 | 10.3 | 0.003* | |
| Microhabitat×social environment | 0.02 | 0.15 | 1, 42.4 | 0.0 | 0.902 | |
| Transitions (between-individual) | −0.04 | 0.01 | 1, 48.4 | 33.3 | <0.001* | |
| Transitions (within-individual) | −0.02 | 0.01 | 1, 67.7 | 4.7 | 0.034 | |
| Standard length | 0.04 | 0.01 | 1, 39.9 | 18.8 | <0.001* | |
| Individual (between-individual) | 0.02 | 0.02 | NA | 1.2 | 0.227 | |
| Individual (within-individual) | 0.11 | 0.02 | NA | 5.8 | <0.001* | |
| Number of sections | Microhabitat | 0.14 | 0.30 | 1, 50.4 | 5.8 | 0.019* |
| Social environment | 0.69 | 0.32 | 1, 49.4 | 0.0 | 0.902 | |
| Microhabitat×social environment | −1.32 | 0.43 | 1, 48.5 | 9.4 | 0.003* | |
| Transitions (between-individual) | 0.28 | 0.02 | 1, 55.1 | 177.2 | <0.001* | |
| Transitions (within-individual) | 0.27 | 0.02 | 1, 76.7 | 139.4 | <0.001* | |
| Standard length | −0.05 | 0.03 | 1, 45.8 | 3.4 | 0.072 | |
| Individual (between-individual) | 0.09 | 0.13 | NA | 0.7 | 0.458 | |
| Individual (within-individual) | 1.11 | 0.18 | NA | 6.2 | <0.001* |
Models were run with microhabitat and social environment as fixed effects, transitions and standard length as covariates, and individual as a random effect. The statistic listed for the random effect of individual is a Wald Z test. F ratios are listed for all other factors. Asterisks indicate significant factors. N=3 tests of 32 individuals. N=1 test of 26 individuals
Fig. 1.
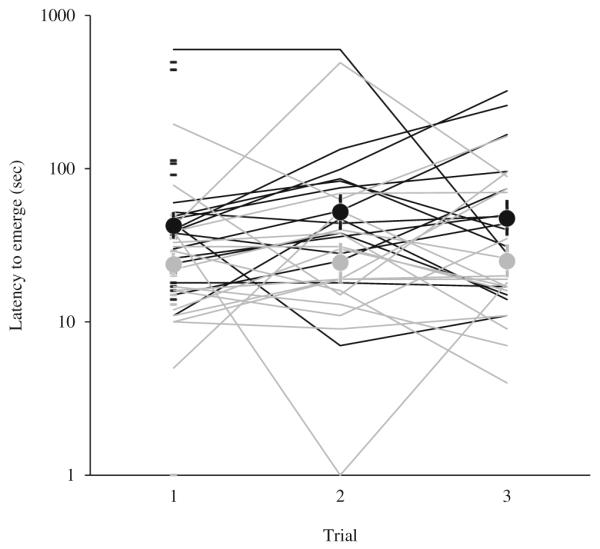
Latency to emerge. Fish that were in a shoal at the time of collection emerged from the refuge faster than fish that were collected while alone. Latency to emerge is plotted on the Y-axis on a logarithmic scale as analysis was on +1 log transformed data. Black symbols represent fish that were alone at the time of collection, gray symbols represent fish that were in shoals at the time of collection. Dashes represent individuals that were assayed once. Lines show individuals that were assayed three times. Means±one standard error are represented by circles with vertical bars. Means and standard errors were calculated with log transformed data and back transformed to the raw data scale. N=1 test of 26 individuals, 13 shoal, 13 alone. N=3 tests of 32 individuals, 17 shoal, 15 alone
In general, fish from open microhabitats explored more sections than fish from cover microhabitats (microhabitat, F1, 50.4= 5.8, p=0.019, Table 1). This effect was driven by an interaction between microhabitat and social environment (microhabitat×social environment, F1, 48.5=9.4, p=0.003, Table 1, Fig. 2). Examination of the estimated marginal means showed that, among fish that were alone, those from an open microhabitat explored more sections of the pool compared to fish from a cover microhabitat, while there was no difference across microhabitats among fish that occurred in shoals (estimated marginal means 95 % CI, open alone 6.0–6.8, cover alone 4.8–5.7, open shoal 5.3–6.2, cover shoal 5.5–6.3). Number of sections was positively related to between- and within-individual variation in number of transitions (Table 1). Size was not a significant factor (Table 1).
Fig. 2.
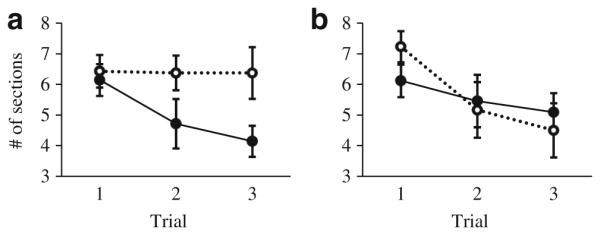
Number of sections. Among fish that were collected while alone, those in an open microhabitat explored more sections of the pool compared to fish found alone in a cover microhabitat. Data are separated into a fish that occurred alone in the field (open alone n=14, cover alone n=14) and b fish that occurred in shoals (open shoal n=13, cover shoal n=17). Dotted lines with open circles represent means for fish from open microhabitats. Solid lines with filled circles indicate individuals from cover microhabitats. Error bars are ±one standard error
Diet analysis
Stability of microhabitat use
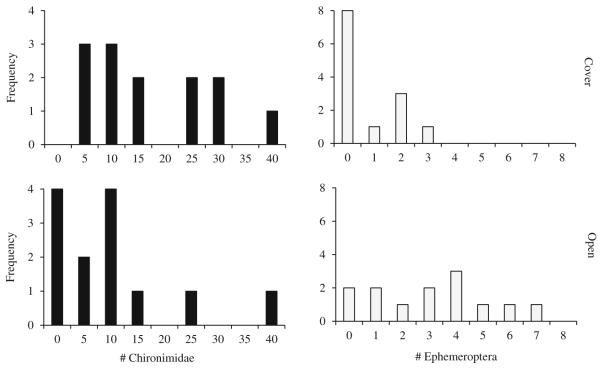
Diet was related to microhabitat use: gut contents of individuals from a cover microhabitat contained fewer Ephemeroptera (F1,23=13.6, p=0.001) and trended towards a greater number of Chironomidae (F1,23= 4.1, p=0.053, N.S.) compared to individuals from open microhabitats (Fig. 3). These data were consistent with the relative abundance of prey found via invertebrate sampling in each microhabitat (number of Chironomidae in open=51, cover=84; number of Ephemeroptera in open=40, cover=4). We did not detect a difference in the average number of total prey items between fish from the cover vs. open microhabitats (p=0.28; cover=18.1±3.0 SE; open=13±3.1 SE). Diet was not related to standard length (all p>0.18).
Fig. 3.
Gut contents. Comparison of the distributions of numbers of Chironomidae and Ephemeroptera in the guts of individuals from the open and cover microhabitats. Note that the scale of the X and Y axes differs between prey types. Open n=13. Cover n=13
Diet of behavioral types
We tested whether diet was quantitatively related to behavioral type. Among fish from cover microhabitats, individuals that explored a greater number of sections had more Ephemeroptera in their guts (tau=0.69, p=0.007, n=13). We interpret this correlation with caution as it did not achieve significance at the Bonferroni corrected level (p≤0.004). Behavioral type was otherwise unrelated to diet (Table 2).
Table 2.
Correlations between gut contents and behavioral type
| # Chironomidae |
# Ephemeroptera |
Total # prey items |
||||
|---|---|---|---|---|---|---|
| tau | p value | tau | p value | tau | p value | |
| Open microhabitat | ||||||
| Latency to emerge | 0.08 | 0.71 | −0.03 | 0.90 | 0.09 | 0.67 |
| Number of sections | −0.29 | 0.19 | 0.08 | 0.71 | −0.35 | 0.11 |
| Cover microhabitat | ||||||
| Latency to emerge | 0.21 | 0.33 | −0.25 | 0.29 | 0.18 | 0.39 |
| Number of sections | −0.01 | 0.95 | 0.69 | 0.007 | 0.04 | 0.85 |
Correlation coefficients are Kendall’s tau-b. Significant correlation is in bold. Open n=13. Cover n=13
Discussion
This study provides evidence for behavioral type–environment correlations in a natural population. We found evidence that differences in latency to emerge from a refuge and number of sections of a pool explored were repeatable over time, which indicates that, while there is overlap in the behavior of different behavioral types, a significant proportion of variation is due to differences between individuals. Behavioral types that emerged from the refuge relatively quickly were more likely to occur in shoals in the field. In addition, fish that were alone in an open microhabitat explored more sections of the pool compared to fish that were alone in cover, but this difference did not occur among fish that were in shoals at the time of collection.
An issue with studies of individual differences in behavior in the field is that, if behavioral observations are conducted in the animal’s natural habitat, we cannot determine whether differences between individuals are due to differences in the environment, i.e., plasticity (Martin and Réale 2008). Indeed, phenotypic plasticity is widespread (West-Eberhardt 2003), and future studies should utilize the behavioral reaction norm framework to tease apart the role of the environment in generating inter-population differences in behavior (Dingemanse et al. 2010). However, in this study we sought to control for differences in the environment by standardizing the holding environment and behavioral tests of all individuals. Furthermore, differences in behavior between individuals that occurred in different environments in the river did not decrease over time and were not related to or overall number of prey items in the gut, which suggests that these differences did not reflect a carryover of environmental effects. Instead, our findings suggest that individuals with enduring differences in behavior occurred in different environments in nature.
We detected a behavioral type–environment correlation between social environment in the field and latency to emerge from a refuge: individuals that were in shoals at the time of collection were relatively fast to emerge from a refuge compared to individuals that were alone. There are mixed results in the literature about the relationship between “boldness” and social environment (Budaev 1997; Ward et al. 2004; Rödel et al. 2006; Pike et al. 2008; Cote et al. 2010, 2011). One possible explanation for the pattern observed in this study is that individuals that do not join shoals are willing to accept the predation risk of being alone (Krause and Ruxton 2002), but compensate for their increased vulnerability by relying more heavily on other anti-predator strategies, such as hiding in refuges. There are important evolutionary implications of this type of phenotypic plasticity: if individuals that occur in environments that put them at a heightened risk of predation compensate by behaving cautiously, that could influence patterns of selection because it can decrease variance in fitness between individuals (Dewitt et al. 1999; Hedrick 2000; Fowler-Finn and Hebets 2011).
Larger and less active (lower mean number of transitions) individuals remained in the refuge longer compared to smaller and more active individuals. Our results are consistent with theory, which states that individuals that maintain larger energy reserves can afford to remain in a refuge for longer to reduce their exposure to predators (Dill 1987). Ours is not the first study to support the hypothesis that refuge use is state-dependent. Krause et al. (1998) found the same positive relationship between body size and refuge use. Killen et al. (2011) recently showed that individuals with higher metabolism that depleted their energy reserves faster spent less time in a refuge compared to low-metabolism individuals. Accelerated depletion of reserves might also explain why individuals that are more active in general emerged earlier in our study. This explanation does not, however, account for the significant effect of within-individual variation in transitions on refuge use; when an individual’s latency to emerge from the refuge decreased, that individual increased its activity. Inactivity was not required for the juvenile stickleback to remain in the shelter (i.e., emergence did not appear to be inadvertent). Instead, this within-individual correlation provides evidence that an underlying mechanism links plasticity in refuge use and activity.
We detected a relationship between microhabitat and the number of sections of a pool explored among fish that were alone: individuals from open microhabitats that were alone consistently explored more of the pool compared to fish that were alone in cover at the time of collection. Fish that were in shoals at the time of collection did not show this difference across microhabitats and instead explored an intermediate number of sections. This correlation between habitat use and exploratory behavior might reflect niche picking; perhaps intermediate behavioral types move to shoals while particular extreme behavioral types preferentially move to different microhabitats. This complex behavioral type–environment association might be adaptive if individuals are moving to environments that increase their fitness. Adaptive niche picking has ecological and evolutionary implications that are just beginning to be appreciated (Edelaar et al. 2008). For example, phenotype-dependent habitat selection can maintain genetic diversity (Levene 1953; Van Valen 1965) and increase the rate of local adaptation and adaptive speciation (Via 1999; Bolnick et al. 2003). Distinguishing between different mechanisms that generate behavioral type–environment correlations is an important task for future work (Edelaar et al. 2008; Stamps and Groothuis 2010a, b).
In our interpretation of behavioral type–environment correlations, we hypothesize that the behaviors we quantified, refuge use and exploration of the environment, have fitness consequences. However, experiments that explicitly test whether these behaviors affect differences in predation risk in nature are a necessary step toward developing hypotheses about the evolutionary consequences of behavioral type–environment correlations (Adriaenssens and Johnsson 2011).
We provide indirect evidence that the conditions under which we collected individuals in the field reflect stable habitat use. Fish from open microhabitats had more Ephemeroptera in their guts, which is consistent with the higher abundance of Ephemeroptera in open microhabitats. Likewise, Chironomidae were more abundant in cover microhabitats and fish found in cover had consumed more Chironomidae. These data suggest that stickleback had been in their respective microhabitats at least as long as it takes to digest gut contents (at least 6 h, Svanbäck and Bolnick 2007) and potentially over much longer periods (Bolnick et al. 2008). Moreover, other populations of sticklebacks exhibit consistent intraspecific variation in diet and microhabitat use (benthic-limnetic; Larson 1976; Bentzen and McPhail 1984), lending plausibility to the claim that the variation in microhabitat use that we observed in this study is relatively long-lasting. Interestingly, we also detected evidence that individuals that explored a greater number of sections might be more likely to move between microhabitats: among the individuals collected in cover microhabitats, those that explored more sections had more Ephemeroptera in their guts, suggesting that they might recently have been in the open microhabitat where Ephemeroptera are more abundant. Systematic studies that track the microhabitat use of different behavioral types of individuals in the field are a priority for future work and may provide interesting insights into ecological effects of different behavioral types.
We assumed that our brief observations adequately characterized differences in social environment among individuals. More rigorous field studies that follow individuals over time are required to validate this assumption. However, a recent study showed that there is a heritable basis to shoaling behavior in three-spined stickleback (Wark et al. 2011). Therefore, some of the observed behavioral variation in shoaling behavior might reflect genetic variation that predisposes certain individuals to be more likely to occur in certain social environments. We leave it to future studies to determine whether particular individuals do indeed occur in certain social environments more than expected by chance.
The observed behavioral type–environment correlations represent a departure from the simple assumption that behavioral types are randomly distributed in the environment. Although this study was conducted on a short temporal scale, we do not view our results as trivial. Even a short-term deviation from a random distribution could be ecologically very important. For example, important selective events often happen instantaneously, e.g., being depredated or parasitized, and are probably more likely to occur in certain environments. Therefore, if certain behavioral types (e.g., fast emergers) occur more often in a particular environment (e.g., a shoal) because they make short forays to that environment, selection will be different for different behavioral types. For example, a stickleback that is more likely to join a shoal for any length of time might be more likely to be afflicted by a parasitic copepod that is transmitted via close contact between individuals (Poulin 1999).
In conclusion, despite the fact that the mechanisms that generate behavioral type–environment correlations are probably widespread (West-Eberhard 2003; Edelaar et al. 2008; Stamps and Groothuis 2010a) and growing evidence for consistent individual differences in behavior (Bell et al. 2009), we know little about whether particular behavioral types are more likely to occur in certain environments in nature. This study provides evidence for behavioral type–environment correlations in a snapshot of time in one location. By presenting these data, we hope to stimulate work that will improve on our study by (1) increasing sample sizes at the individual and population level, (2) tracking the use of different environments and changes in behavior across development, and (3) teasing apart the mechanisms that generate behavioral type–environment correlations to determine whether they affect ecological or evolutionary processes.
Acknowledgments
We thank RJH Pearish for assistance in the field and J Tompkins for assistance in identifying invertebrates. River’s Bend Retreat generously provided access to the Navarro River. KL Laskowski reviewed an early version of this manuscript. Comments from NJ Dingemanse and two anonymous reviewers greatly improved this manuscript.
Footnotes
Ethical standards All experiments comply with the current laws of the United States and were approved by the University of Illinois Institutional Animal Care and Use Committee (Protocol number 09024).
References
- Adriaenssens B, Johnsson JI. Shy trout grow faster: exploring links between personality and fitness-related traits in the wild. Behav Ecol. 2011;22:135–143. [Google Scholar]
- Bakker TCM. Aggressiveness in sticklebacks (Gasterosteus aculeatus L.): a behaviour–genetic study. Behaviour. 1986;98:1–144. [Google Scholar]
- Bateson P. The active role of behaviour in evolution. In: Ho MW, Fox SW, editors. Evolutionary processes and metaphors. Wiley; New York: 1988. pp. 191–207. [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL. The repeatability of behaviour: a meta-analysis. Anim Behav. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM, Dingemanse NJ, Hankison SJ, Langenhof MBW, Rollins K. Early exposure to nonlethal predation risk by size-selective predators increases somatic growth and decreases size at adulthood in threespined sticklebacks. J Evol Biol. 2011;24:943–953. doi: 10.1111/j.1420-9101.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen P, McPhail JD. Ecology and evolution of sympatric sticklebacks (Gasterosteus): specialization for alternative trophic niches in the Enos Lake species pair. Can J Zool. 1984;62:2280–2286. [Google Scholar]
- Bergmüller R, Taborsky M. Animal personality due to social niche specialisation. Trends Ecol Evol. 2010;25:504–511. doi: 10.1016/j.tree.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Biro PA, Dingemanse NJ. Sampling bias resulting from animal personality. Trends Ecol Evol. 2009;24:66–67. doi: 10.1016/j.tree.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. The ecology of individuals: incidence and implications of individual specialization. Am Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Caldera EJ, Matthews B. Evidence for asymmetric migration load in a pair of ecologically divergent stickleback populations. Biol J Linn Soc. 2008;94:273–287. [Google Scholar]
- Boon AK, Réale D, Boutin S. Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos. 2008;117:1321–1328. [Google Scholar]
- Brown CR, Brown MB. Heritable basis for choice of group size in a colonial bird. Proc Natl Acad Sci U S A. 2000;97:14825–14830. doi: 10.1073/pnas.97.26.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Jones F, Braithwaite V. In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim Behav. 2005;70:1003–1009. [Google Scholar]
- Budaev SV. Alternative styles in the European wrasse, Symphodus ocellatus: boldness-related schooling tendency. Environ Biol Fish. 1997;49:71–78. [Google Scholar]
- Chapman BB, Hulthén K, Blomqvist DR, Hansson LA, Nilsson JÅ, Brodersen J, Anders Nilsson P, Skov C, Brönmark C. To boldly go: individual differences in boldness influence migratory tendency. Ecol Lett. 2011;14:871–876. doi: 10.1111/j.1461-0248.2011.01648.x. [DOI] [PubMed] [Google Scholar]
- Cote J, Clobert J. Social personalities influence natal dispersal in a lizard. Proc R Soc Lond B. 2007;274:383–390. doi: 10.1098/rspb.2006.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis) Proc R Soc Lond B. 2010;277:1571–1579. doi: 10.1098/rspb.2009.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A. Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc R Soc Lond B. 2011;278:1670–1678. doi: 10.1098/rspb.2010.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T, McPhail JD. The effect of behavioural and morphological plasticity on foraging efficiency in the threespine stickleback (Gasterosteus sp.) Oecologia. 1996;108:380–388. doi: 10.1007/BF00334665. [DOI] [PubMed] [Google Scholar]
- Dewitt TJ, Sih A, Hucko JA. Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim Behav. 1999;58:397–407. doi: 10.1006/anbe.1999.1158. [DOI] [PubMed] [Google Scholar]
- Dill LM. Animal decision making and its ecological consequences: the future of aquatic ecology and behaviour. Can J Zool. 1987;65:803–811. [Google Scholar]
- Dingemanse NJ, Dochtermann NA. Quantifying individual variation in behaviour: mixed- effect modelling approaches. J Anim Ecol. 2013;82:39–54. doi: 10.1111/1365-2656.12013. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ. Natal dispersal and personalities in great tits (Parus major) Proc R Soc Lond B. 2003;270:741–747. doi: 10.1098/rspb.2002.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Kazem AJN, Réale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Donohue K, Polisetty CR, Wender NJ. Genetic basis and consequences of niche construction: plasticity-induced genetic constraints on the evolution of seed dispersal in Arabidopsis thaliana. Am Nat. 2005;165:537–550. doi: 10.1086/429162. [DOI] [PubMed] [Google Scholar]
- Duckworth RA, Badyaev AV. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci U S A. 2007;104:15017–15022. doi: 10.1073/pnas.0706174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelaar P, Siepielski AM, Clobert J. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution. 2008;62:2462–2472. doi: 10.1111/j.1558-5646.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- Ehlinger TJ. Habitat choice and phenotype-limited feeding efficiency in bluegill: individual differences and trophic polymorphism. Ecology. 1990;71:886–896. [Google Scholar]
- Fowler-Finn KD, Hebets EA. The degree of response to increased predation risk corresponds to male secondary sexual traits. Behav Ecol. 2011;22:268–275. [Google Scholar]
- Godin J-GJ, Crossman SL. Hunger-dependent predator inspection and foraging behaviours in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav Ecol Sociobiol. 1994;34:359–366. [Google Scholar]
- Griffiths SW, Magurran AE. Schooling decisions in guppies (Poecilia reticulata) are based on familiarity rather than kin recognition by phenotype matching. Behav Ecol Sociobiol. 1999;45:437–443. [Google Scholar]
- Hedrick AV. Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc R Soc Lond B. 2000;267:671–675. doi: 10.1098/rspb.2000.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley NM, Cook T, Lang M, Petelle MB, Blumstein DT. Personality and habitat segregation in giant sea anemones (Condylactis gigantea) J Exp Mar Biol Ecol. 2012;426–427:1–4. [Google Scholar]
- Holt RD, Barfield M. Habitat selection and niche conservatism. Isr J Ecol Evol. 2008;54:295–309. [Google Scholar]
- Jaenike J, Holt RD. Genetic variation for habitat preference: evidence and explanations. Am Nat. 1991;137:s67–s90. [Google Scholar]
- Killen SS, Marras S, McKenzie DJ. Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. J Anim Ecol. 2011;80:1024–1033. doi: 10.1111/j.1365-2656.2011.01844.x. [DOI] [PubMed] [Google Scholar]
- Kobler A, Klefoth T, Mehner T, Arlinghaus R. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia. 2009;161:837–847. doi: 10.1007/s00442-009-1415-9. [DOI] [PubMed] [Google Scholar]
- Krause J, Ruxton GD. Living in groups. New York, Oxford: 2002. [Google Scholar]
- Krause J, Loader SP, McDermott J, Ruxton GD. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proc R Soc Lond B. 1998;265:2373–2379. [Google Scholar]
- Larson GL. Social behavior and feeding ability of two phenotypes of Gasterosteus aculeatus in relation to their spatial and trophic segregation in a temperate lake. Can J Zool. 1976;54:107–121. [Google Scholar]
- Levene H. Genetic equilibrium when more than one ecological niche is available. Am Nat. 1953;87:331–333. [Google Scholar]
- López P, Hawlena D, Polo V, Amo L, Martín J. Sources of individual shy-bold variations in antipredator behaviour of male Iberian rock lizards. Anim Behav. 2005;69:1–9. [Google Scholar]
- Martin JGA, Réale D. Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim Behav. 2008;75:309–318. [Google Scholar]
- Martin JGA, Nussey DH, Wilson AJ, Réale D. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol Evol. 2011;2:362–374. [Google Scholar]
- McGlothlin JW, Moore AJ, Wolf JB, Brodie ED., III Interacting phenotypes and the evolutionary process III. Social evolution. Evolution. 2010;64:2558–2574. doi: 10.1111/j.1558-5646.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche construction. Am Nat. 1996;147:641–648. [Google Scholar]
- Peluc SI, Sillett TS, Rotenberry JT, Ghalambor CK. Adaptive phenotypic plasticity in an island songbird exposed to a novel predation risk. Behav Ecol. 2008;19:830–835. [Google Scholar]
- Peuhkuri N, Ranta E, Juvonen S-K, Lindström K. Schooling affects growth in the three-spined stickleback, Gasterosteus aculeatus. J Fish Biol. 1995;46:221–226. [Google Scholar]
- Pike T, Samanta M, Lindström J, Royle NJ. Behavioural phenotype affects social interactions in an animal network. Proc R Soc Lond B. 2008;275:2515–2520. doi: 10.1098/rspb.2008.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher TJ, Parrish JK. Functions of shoaling behaviour in teleosts. In: Pitcher TJ, editor. Behaviour of teleost fishes. 2nd edn Chapman and Hall; London: 1993. pp. 363–440. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype–environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977;84:309–322. [PubMed] [Google Scholar]
- Poulin R. Parasitism and shoal size in juvenile sticklebacks: conflicting selection pressures from different ectoparasites? Ethology. 1999;105:959–968. [Google Scholar]
- Ranta E, Lindström K. Assortative schooling in three-spined sticklebacks. Ann Zool Fenn. 1990;27:67–75. [Google Scholar]
- Ravigné V, Olivieri I, Dieckmann U. Implications of habitat choice for protected polymorphisms. Evol Ecol Res. 2003;5:1–20. [Google Scholar]
- Ravigné V, Dieckmann U, Olivieri I. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. Am Nat. 2009;174:E141–E169. doi: 10.1086/605369. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Réale D, Dingemanse NJ, Kazem AJN, Wright J, editors. Evolutionary and ecological approaches to the study of personality [Theme issue] Philos T Roy Soc B. 2010;365:3937–4106. doi: 10.1098/rstb.2010.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Speciation via habitat specialization: the evolution of reproductive isolation as a correlated character. Evol Ecol. 1987;1:301–314. [Google Scholar]
- Rödel HG, Monclús R, von Holst D. Behavioral styles in European rabbits: social interactions and responses to experimental stressors. Physiol Behav. 2006;89:180–188. doi: 10.1016/j.physbeh.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Rutter M, Dunn J, Plomin R, Simonoff E, Pickles A, Maughan B, Ormel J, Meyer J, Eaves L. Integrating nature and nurture: implications of person–environment correlations and interactions for developmental psychopathology. Dev Psychopathol. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Saltz JB. Natural genetic variation in social enviornment choice: context-dependent gene–environment correlation in Drosophila melanogaster. Evolution. 2011;65:2325–2334. doi: 10.1111/j.1558-5646.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Saltz JB, Foley BR. Natural genetic variation in social niche construction: social effects of aggression drive disruptive sexual selection in Drosophila melanogaster. Am Nat. 2011;177:645–654. doi: 10.1086/659631. [DOI] [PubMed] [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. Am Nat. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Sharpe PB, van Horne B. Influence of habitat on behavior of Townsend’s ground squirrels (Spermophilus townsendii) J Mammal. 1998;79:906–918. [Google Scholar]
- Sih A. Prey refuges and predator–prey stability. Theor Popul Biol. 1987;31:1–12. [Google Scholar]
- Sih A, Bell AM. Insights for behavioral ecology from behavioral syndromes. Adv Stud Behav. 2008;38:1–56. doi: 10.1016/S0065-3454(08)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Watters J. The mix matters: behavioural types and group dynamics in water striders. Behaviour. 2005;142:1417–1431. [Google Scholar]
- Skúlason S, Smith TB. Resource polymorphisms in vertebrates. Trends Ecol Evol. 1995;10:366–370. doi: 10.1016/s0169-5347(00)89135-1. [DOI] [PubMed] [Google Scholar]
- Skúlason S, Snorrason SS, Ota D, Noakes DLG. Genetically based differences in foraging behaviour among sympatric morphs of arctic charr (Pisces: Salmonidae) Anim Behav. 1993;45:1179–1192. [Google Scholar]
- Stamps JA, Groothuis TGG. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Philos T Roy Soc B. 2010a;365:4029–4041. doi: 10.1098/rstb.2010.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps JA, Groothuis TGG. The development of animal personality: relevance, concepts and perspectives. Biol Rev. 2010b;85:301–325. doi: 10.1111/j.1469-185X.2009.00103.x. [DOI] [PubMed] [Google Scholar]
- Svanbäck R, Bolnick DI. Intraspecific competition drives increased resource use diversity within a natural population. Proc R Soc Lond B. 2007;274:839–844. doi: 10.1098/rspb.2006.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, Ryan MJ. The role of synchronized calling, ambient light, and ambient noise, in anti-bat-predator behavior of a tree-frog. Behav Ecol Sociobiol. 1982;11:125–131. [Google Scholar]
- van de Pol M, Wright J. A simple method for distinguishing within- versus between- subject effects using mixed models. Anim Behav. 2009;77:753–758. [Google Scholar]
- Van Valen L. Morphological variation and width of ecological niche. Am Nat. 1965;99:377–390. [Google Scholar]
- Verbeek MEM, Drent PJ, Wiepkema PR. Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav. 1994;48:1113–1121. [Google Scholar]
- Via S. Reproductive isolation between sympatric races of pea aphids. I. Gene flow restriction and habitat choice. Evolution. 1999;53:1446–1457. doi: 10.1111/j.1558-5646.1999.tb05409.x. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummings RA. The open-field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Ward AJW, Botham MS, Hoare DJ, James R, Broom M, Godin J-GJ, Krause J. Association patterns and shoal fidelity in the three-spined stickleback. Proc R Soc Lond B. 2002;269:2451–2455. doi: 10.1098/rspb.2002.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJW, Thomas P, Hart PJB, Krause J. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus) Behav Ecol Sociobiol. 2004;55:561–568. [Google Scholar]
- Ward AJW, Holbrook RI, Krause J, Hart PJB. Social recognition in sticklebacks: the role of direct experience and habitat cues. Behav Ecol Sociobiol. 2005;57:575–583. [Google Scholar]
- Wark AR, Greenwood AK, Taylor EM, Yoshida K, Peichel CL. Heritable differences in schooling behavior among threespine stickleback populations revealed by a novel assay. PLoS One. 2011;6:e18316. doi: 10.1371/journal.pone.0018316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MM, Ward AJW, Hart PJB. Boldness is influenced by social context in threespine sticklebacks. Behaviour. 2007;144:351–371. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; Oxford: 2003. [Google Scholar]
- Wilson DS, Coleman K, Clark AB, Biederman L. Shy-bold continuum in pumpkinseed sunfish: an ecological study of a psychological trait. J Comp Psychol. 1993;107:250–260. [Google Scholar]
- Wilson ADM, Godin J-GJ, Ward AJW. Boldness and reproductive fitness correlates in the eastern mosquitofish, Gambusia holbrooki. Ethology. 2010;116:96–104. [Google Scholar]
- Wolf JB, Brodie ED, III, Moore AJ. Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. Am Nat. 1999;153:254–266. doi: 10.1086/303168. [DOI] [PubMed] [Google Scholar]
- Wootton RJ. Fecundity of the three-spined stickleback, Gasterosteus aculeatus (L.) J Fish Biol. 1973;5:683–688. [Google Scholar]