Abstract
We have developed a modification of bioorthogonal click chemistry to assay the palmitoylation of cellular proteins. This assay utilizes 15-hexadecynoic acid (15-HDYA) as a chemical probe in combination with protein immunoprecipitation using magnetic beads in order to detect S-palmitoylation of proteins of interest. Here we demonstrate the utility of this approach for the mu-opioid receptor (MOR), a GPCR responsible for mediating the analgesic and addictive properties of most clinically relevant opioid agonist drugs. This technique provides a rapid, non-isotopic, and efficient method to assay the palmitoylation status of a variety of cellular proteins including most GPCRs.
Keywords: Palmitoylation, click chemistry, GPCRs, mu-opioid receptor, immunoprecipitation, Western blotting
Protein palmitoylation is a posttranslational modification that typically involves covalent attachment of palmitic acid to proteins, often resulting in lipid raft localization of membrane proteins [1,2,6]. Palmitate is attached to proteins via an enzymatic reaction that is catalyzed by a family of protein acyltransferases (PATs). Palmitoylation enhances the hydrophobicity of proteins, thereby contributing to their membrane association, subcellular trafficking between membrane compartments, and modulation of protein-protein interactions [1,3,4,5,6]. S-palmitoylation is a specific type of lipid modification that involves addition of a C16 acyl chain to cytosolic cysteines via thioester bonds, and is unique amongst lipid modifications in that it is reversible [3,4,6].
Classically, determining the palmitoylation status of a protein has relied upon metabolic labeling with [3H] palmitate, followed by autoradiographic detection of the labeled-protein on Western blots. However, due to the low specific activity of [3H] palmitate, this type of analysis can require the use of large quantities of labeled palmitate, and detection may require weeks or even months-long exposure times. Recently, a number of non-isotopic labeling methods, including bioorthogonal click chemistry, have been developed which can be used to detect and quantitate protein palmitoylation. In addition to offering significantly greater sensitivity and more rapid detection times than metabolic labeling with radioactive palmitate, these assays can also be used to determine which PATs are responsible for the palmitoylation of specific target proteins.
Bioorthogonal click chemistry (BCC) is a non-isotopic labeling technique that often uses 17-octadecynoic acid (17-ODYA) as a chemical probe. This C18 lipid probe is taken up by living cells and incorporated into proteins via PATs. Following uptake of the lipid probe, proteins are harvested from cells and reacted with a bioorthogonal azide-labeled fluorescent chromaphore via click chemistry [7]. One limitation of this technique is that some PATs are less efficient at attaching fatty acid chains that are larger than 16 carbons (i.e., 17-ODYA), to a target protein [8]. In this report, we investigated the use of 15-hexadecynoic acid (15-HDYA) as the chemical probe. The structure of 15-HDYA is identical to palmitate with the exception that it contains an ω-terminal alkyne necessary for the click reaction. Here we demonstrate the efficacy of using BCC with 15-HDYA to interrogate the palmitoylation status of the mu-opioid receptor (MOR), a G-protein coupled receptor (GPCR) responsible for mediating the analgesic and addictive properties of opioid agonist drugs. The MOR has previously been reported to be palmitoylated via conventional metabolic labeling with [3H] palmitate and another non-isotopic labeling method, acyl-biotin exchange chemistry [9,10]. Further, BCC in conjunction with magnetic bead immunoprecipitation should significantly reduce both sample loss and the time required for protein purification, thereby improving the sensitivity of the subsequent click chemistry reaction.
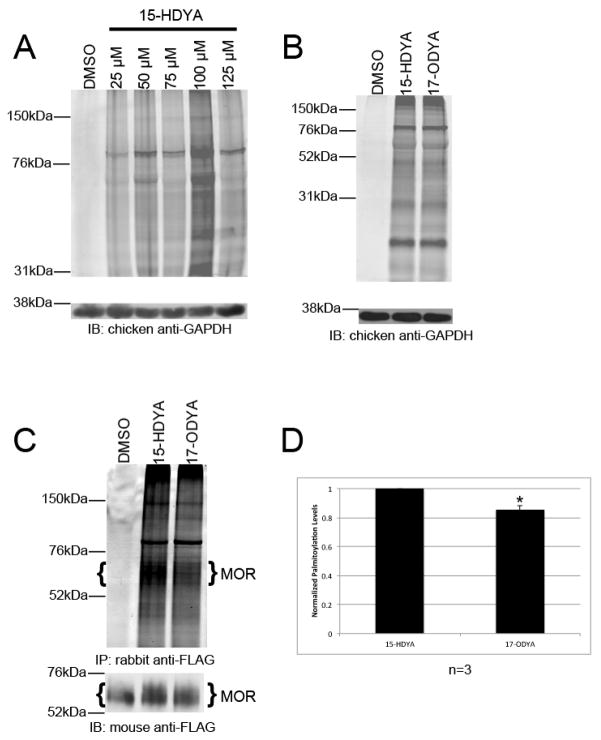
To determine whether 15-HDYA can be effectively utilized as a chemical probe in the BCC assay, HEK-293 cells were incubated for 24 hours with varying doses of 15-HDYA. Cell lysates were then prepared using a sodium phosphate-based lysis buffer. It is important to note that Tris based lysis buffers will not work with BCC as Tris can act as an inhibitory ligand for the Cu(I) species used in the click chemistry reaction [11]. In this and subsequent experiments, cells treated with DMSO alone (at the indicated concentrations) served as control. Click chemistry was performed as previously described [7,12,13] with the exception that we used TAMRA azide (Lumniprobe) as the probe instead of alkyl-TAMRA (Supplementary Information). Cell lysates (50 μg/well) were subjected to SDS-PAGE and the gel imaged using a Typhoon 9410 fluorescent imager (GE Amersham). Proteins were then transferred to a PVDF membrane and analyzed via Western blotting with a chicken anti-GAPDH antibody (1:10,000; Millipore). As shown in Figure 1A, 15-HDYA was incorporated into a similar pattern of cellular proteins at all concentrations tested, while optimal incorporation of the lipid probe was obtained at a dose of 100 μM. It is important to note that 125 μM 15-HDYA was cytotoxic to the cells while 100 μM 15-HDYA did not appear to appreciably affect cellular viability. These results are in agreement with previously published reports [13]. We next compared the ability of 15-HDYA and 17-ODYA to label cellular proteins in HEK-293 cells. HEK-293 cells were treated for 24 hours with 100 μM of either 15-HDYA or 17-ODYA. Lysates were prepared, labeled with TAMRA azide, and imaged as described above. Separated proteins were transferred to a PVDF membrane and analyzed via Western blotting with the chicken anti-GAPDH antibody. As shown in Figure 1B, 15-HDYA and 17-ODYA were incorporated to similar extents into free palmitoylation sites in HEK-293 cells.
Fig. 1.
Palmitoylation of HEK-293 cell proteins analyzed by bioorthogonal click chemistry (A) Dose response using 15-HDYA as the lipid probe on palmitoylation of endogenous HEK-293 cell proteins. Control cells were treated with DMSO (0.2%). (B) Palmitoylation of endogenous HEK-293 cell proteins with 100 μM 15-HDYA or 17-ODYA. Control cells were treated with DMSO (0.2%). In Panels A and B, GAPDH served as a loading control. (C) Palmitoylation of MOR in HEK-MOR cells treated with 100 μM 15-HDYA or 17-ODYA. MOR was immunoprecipitated using rabbit-anti FLAG antibody. After fluorescent imaging (top), proteins were analyzed by Western blotting with a mouse anti-FLAG antibody (bottom). Brackets indicate bands corresponding to MOR that were used for quantitation. (D) Levels of palmitoylated MOR were normalized to the amount of immunoprecipitated receptor. Bar graph represents the average pixel density (as determined by ImageJ) from 3 experiments. Data was analyzed using a two-sided paired Student’s t-test (expressed as ± SEM; n=3, * (p<0.05)).
To test the feasibility of using 15-HDYA as the lipid probe for detecting MOR palmitoylation, HEK-MOR cells were metabolically labeled for 24 hours with 100 μM of either 15-HDYA or 17-ODYA. HEK-MOR cells are HEK-293 cells stably expressing FLAG-tagged MORs [14]. Cell lysates were prepared, added to Protein G MAG Sepharose (GE Healthcare) magnetic beads coated in rabbit anti-FLAG antibody (Sigma), and allowed to immunoprecipitate for one hour. Magnetic beads were collected and washed. Bead-associated proteins were then subjected to the click chemical reaction (Supplementary Information). Labeled immunocomplexes were eluted from the magnetic beads using 4x protein loading dye and analyzed via SDS-PAGE followed by in-gel fluorescence imaging. Separated proteins were then transferred to a PVDF membrane and analyzed by Western blotting with a mouse anti-FLAG antibody (1:5,000; Sigma). As shown in Fig. 1C, we detected a band, corresponding in size to the MOR, in cells labeled with either 15-HDYA or 17-ODYA migrating between 55 and 70 kDa in both the in-gel Typhoon scan (upper panel) and on the Western blot (lower panel). This immunoreactive band is also present in lysates prepared from control HEK-MOR cells and is consistent in size with our previous analysis of MOR [15]. Normalization of the amount of palmitoylated MOR to the amount of immunoprecipitated MOR revealed a significant decrease (15%) in the amount of 17-ODYA incorporated into MOR compared to 15-HDYA (Fig 1D). Taken together, our results suggest that BCC using 15-HDYA or 17-ODYA as the lipid probe, coupled with magnetic bead immunoprecipitation is an effective method to detect palmitoylated MOR, and that 15-HDYA is more efficiently incorporated into MOR than 17-ODYA.
The results presented here using BCC and previously by others [9,10] indicate that the MOR is a palmitoylated GPCR. However, which PATs are responsible for palmitoylating the MOR is not known. Twenty-three mammalian PATs have been identified and substrates for many (but not all) PATs have been characterized [6,16]. We have chosen to examine two mammalian PATs, GODZ (zDHHC3) and zDHHC4, as potential candidates for MOR palmitoylation. GODZ (zDHHC3) is a PAT that is known to palmitoylate a variety of membrane proteins including the GABAAγ2 subunit and GluR1/2 [16]. Studies we have carried out suggest that GODZ and zDHHC4 are each capable of palmitoylating the D2 dopamine receptor (Ebersole et. al., unpublished work). To determine whether GODZ and/or zDHHC4 have the potential to palmitoylate the MOR, we first asked whether either of these PATs interacted with the receptor. To do this, we tested the ability of anti-FLAG antibodies to coimmunoprecipitate FLAG-tagged MOR and myc-tagged zDHHC4 or GODZ from transfected HEK-MOR (stably expressing FLAG-tagged MOR) cells. As shown in Fig. 2A, GODZ and zDHHC4 each coimmunoprecipitated with the MOR. These results suggest that these two PATS interact with the MOR, and are therefore candidate MOR palmitoylating enzymes.
Fig. 2.
Palmitoylation of the MOR. (A) zDHHC4 and GODZ interact with MOR. HEK-MOR cells were transiently transfected with myc-tagged zDHHC4 or GODZ, and MOR was immunoprecipitated using a rabbit anti-FLAG antibody. Rabbit IgG was used as a mock control (M). Blots were probed for PATs using a mouse anti-myc antibody. Lysates (L) contain 5% of the total protein compared to mock (M) and immunoprecipitation (IP) lanes. (B) zDHHC4 and GODZ can palmitoylate the MOR. HEK-MOR cells were transiently transfected with myc-tagged zDHHC4 or GODZ. MOR was immunoprecipitated with rabbit anti-FLAG antibody. Rabbit IgG was used as a control. Double headed arrow indicates bands corresponding to MOR. Single headed arrows indicate palmitoylated PATs (upper: GODZ; lower: zDHHC4). (C) Proteins from B were transferred to a PVDF membrane and Western blotting was performed using either mouse anti-FLAG (top) or mouse anti-myc (bottom) antibodies. (D) Palmitoylated levels of MOR were normalized to the amount of immunoprecipitated MOR. Bar graph shows average pixel density (as determined by ImageJ) from 3 experiments. Data was analyzed using a two-sided paired Student’s t-test (expressed as ± SEM; n=3, * (p<0.05), **(p<0.005).
To determine whether GODZ and zDHHC4 are capable of palmitoylating the MOR, we tested whether overexpression of each PAT caused an increase in the palmitoylation levels of the MOR. Overexpression of PATS in cultured cells has commonly been used to ascertain substrate specificity of a variety of different PATS [17,18,19,20]. For this experiment, GODZ and zDHHC4 were separately transfected into HEK-MOR cells and BCC was performed as described above. The MOR was immunoprecipitated using a rabbit anti-FLAG antibody and the palmitoylated receptor visualized by in-gel fluorescence imaging (Fig. 2B), and after protein transfer, on a Western blot using the mouse anti-FLAG antibody (Fig. 2C). Palmitoylated MOR levels were quantitated and normalized to the amount of immunoprecipitated MOR (Figure 2C, top panel). As shown in Fig. 2D, overexpression of zDHHC4 and GODZ produced an increase in MOR palmitoylation of 113% and 246%, respectively, compared to untransfected cells. Interestingly, both in-gel imaging (Fig. 2B) and probing of the Western blot with anti-myc antibodies (Fig. 2C) revealed that the two PATs used to palmitoylate the MOR were themselves palmitoylated in the BCC assay. These results suggest that zDHHC4 and GODZ contribute to the palmitoylation of the MOR in transfected cells. It is interesting to note that zDHHC4 coimmunoprecipitates more efficiently with the MOR than GODZ, but is less efficient than GODZ at palmitoylating the receptor. Thus GODZ may be more efficient than zDHHC4 at palmitoylating the MOR in this cell system.
Using bioorthogonal click chemistry, we have confirmed previous reports that the MOR is palmitoylated [9,10]. The data presented here is consistent with the following conclusions. First, non-isotopic labeling of a GPCR with a lipid probe, in combination with immunoprecipitation of the labeled protein with magnetic beads, represents a rapid and sensitive means to characterize GPCR palmitoylation. Second, BCC can be used as an analytical tool to determine which PATs are responsible for palmitoylating a specific receptor. Our results suggest that two candidate PATs, GODZ and zDHHC4, are capable of interacting with and palmitoylating the MOR, at least within the context of transfected mammalian cells. However, the potential interaction of these (and other) PATs with the MOR in vivo is not known, and will depend in large part on whether the MOR and a particular PAT are co-expressed within the same cell or cellular compartment within the nervous system.
Supplementary Material
Acknowledgments
We thank Bernard Lüscher and Casey Kilpatrick (Penn State University, University Park) for suggesting the use of 15-HDYA. This study was supported by grants from NIDA (DA025995, W. Berrettini, PI) and a CURE grant to RL (S. Grigson, PI) from the Pennsylvania Department of Health using Tobacco Settlement Funds. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcellular Biochemistry. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA. Lipid rafts, detergent-resistant membranes and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 3.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nature Reviews. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 4.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nature Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charollais J, Van Der Goot FG. Palmitoylation of membrane proteins (Review) Molecular Membrane Biology. 2009;26:55–66. doi: 10.1080/09687680802620369. [DOI] [PubMed] [Google Scholar]
- 6.Blaskovic S, Blanc M, Van Der Goot FG. What does S-palmitoylation do to membrane proteins? FEBS Journal. 2013;280:2766–2774. doi: 10.1111/febs.12263. [DOI] [PubMed] [Google Scholar]
- 7.Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. Robust fluorescent detection of protein fatty-acylation with chemical reporters. Journal of the American Chemical Society. 2009;131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 8.Jennings BC, Linder ME. DHHC protein S-acyltransferases use similar ping-pong kinetic mechanisms but display different acyl-CoA specificities. The Journal of Biological Chemistry. 2012;287:7236–7245. doi: 10.1074/jbc.M111.337246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Shahabi V, Xu W, Liu-Chen LY. Palmitoylation of the rat μ opioid receptor. FEBS Letters. 1998;441:148–152. doi: 10.1016/s0014-5793(98)01547-6. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Pearsall EA, Hurst DP, Zhang Y, Chu J, Zhou Y, Reggio PH, Loh HH, Law PY. Palmitoylation and membrane cholesterol stabilize mu-opioid receptor homodimerization and G protein coupling. BMC Cell Biology. 2012;13:1–45. doi: 10.1186/1471-2121-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Yang X, Verhelst SHL. Comparative analysis of click chemistry mediated activity-based protein profiling in cell lysates. Molecules. 2013;18:12599–12608. doi: 10.3390/molecules181012599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya R, Barren C, Kovacs DM. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. The Journal of Neuroscience. 2013;33:11169–11183. doi: 10.1523/JNEUROSCI.4704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannoush RN. Profiling cellular myristoylation and palmitoylation using ω-alkynyl fatty acids. Methods in Molecular Biology. 2012;800:85–94. doi: 10.1007/978-1-61779-349-3_7. [DOI] [PubMed] [Google Scholar]
- 14.Jin J, Kittanakom S, Wong V, Reyes BA, Van Bockstaele EJ, Stagljar I, Berrettini W, Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neuroscience. 2010;11:33–47. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petko J, Justice-Bitner S, Jin J, Wong V, Kittanakom S, Ferraro TN, Stagljar I, Levenson R. MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0067608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends in Biomedical Sciences. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Sharma C, Yang XH, Hemler ME. DHHC2 affects palmitoylation, stability, and functions of tetraspanins CD9 and CD151. Molecular Biology of the Cell. 2008;19:3415–3425. doi: 10.1091/mbc.E07-11-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Lüscher B. GODZ-mediated palmitoylation of GABAA receptors is required for normal assembly and function of GABAergic inhibitory synapses. The Journal of Neuroscience. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Planey SL, Ceballos C, Stevens SM, Jr, Keay SK, Zacharias DA. Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Molecular & Cellular Proteomics. 2008;7:1378–1388. doi: 10.1074/mcp.M800069-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas GM, Hayashi T, Chiu S, Chen C, Huganir RL. Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking. Neuron. 2012;73:482–496. doi: 10.1016/j.neuron.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.