Abstract
Estradiol was found previously to have an antidepressant-like effect and to block the ability of selective serotonin reuptake inhibitors (SSRIs) to have an antidepressant-like effect. The antidepressant-like effect of estradiol was due to estrogen receptor β (ERβ) and/or GPR30 activation whereas estradiol’s blockade of the effect of an SSRI was mediated by ERα. This study focuses on investigating signaling pathways as well as interacting receptors associated with these two effects of estradiol.
In vivo chronoamperometry was used to measure serotonin transporter (SERT) function. The effect of local application of estradiol or selective agonists for ERα (PPT) or ERβ (DPN) into the CA3 region of the hippocampus of ovariectomized (OVX) rats on 5-hydroxytryptamine (5-HT, serotonin) clearance as well as on the ability of fluvoxamine to slow 5-HT clearance was examined after selective blockade of signaling pathways or that of interacting receptors.
Estradiol- or DPN-induced slowing of 5-HT clearance mediated by ERβ was blocked after inhibition of MAPK/ERK1/2 but not of PI3K/Akt signaling pathways. This effect also involved interactions with TrkB, and IGF-1 receptors. Estradiol’s or PPT’s inhibition of the fluvoxamine-induced slowing of 5-HT clearance mediated by ERα, was blocked after inhibition of either MAPK/ERK1/2 or PI3K/Akt signaling pathways. This effect involved interactions with the IGF-1 receptor and with the metabotropic glutamate receptor 1 but not with TrkB.
This study illustrates some of the signaling pathways required for the effects of estradiol on SERT function and particularly shows that ER subtypes elicit different as well as common signaling pathways for their actions.
Keywords: estradiol, ER agonist, signaling pathways, serotonin transporter, SSRI
INTRODUCTION
The mechanisms of estradiol (E2) signaling in the brain are complex, tissue specific and could include independent as well as co-dependent effects through estrogen receptor α (ERα), ERβ and GPR30 as well as interactions between ER and other membrane receptors (see Bourque et al., 2012). Two major signaling pathways have been associated with the effects of E2 in the brain; the mitogen activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK1/2) and the phosphatidylinositol 3-kinase (PI3K)/Akt pathways (Raz et al., 2008). A direct interaction between ERα and PI3K was discovered (Simoncini et al., 2000) and numerous kinase and phosphatases have been implicated in the actions of ERα and ERβ (Levin, 1999), likely mediated through an ER-signaling complex. Receptor specific ligands for ERα or ERβ can activate ERK and Akt in hippocampal and cortical neurons (Wade and Dorsa, 2003, Dominguez et al., 2007, Brinton, 2008).
The plasma membrane localization of ERs in caveolae, microdomains at the plasma membrane that assemble signaling proteins, allows their interaction with PI3K/Akt and MAPK/ERK1/2 pathways. Many ER-scaffold proteins and signaling molecules that are associated with membrane ERs may serve to facilitate activation of kinases by E2. Caveolin proteins, metabotropic glutamate receptors (mGluRs), G proteins, Src, p85, a regulatory subunit of PI3K, Shc and receptor tyrosine kinases, i.e. insulin-like growth factor-1 receptor (IGF-1R) as well as the BDNF receptor (TrkB) have all been reported to serve as components of complexes of interacting proteins with ERs (Etgen et al., 2001; Quesada and Micevych, 2004; Boulware et al., 2005; Marino et al., 2006). Thus, membrane ERs may use other receptors to initiate cell signaling, including TrkB, IGF-1R and mGluRs.
Most preclinical studies that predict the efficacy or examine the mechanism(s) of action of existing or prospective new antidepressants (ADs) are done using male experimental animals. Blockade of serotonin transporter (SERT) function by selective serotonin reuptake inhibitors (SSRIs) is the initial event that triggers a still not completely understood process that results in clinical improvement in depression. The exploration of the mechanisms involved in the modulation of the SERT by ovarian hormones could lead to a better understanding of the interaction between ovarian steroids and the SERT which could have implications for the treatment of depression in women. Previously, it was reported that local application of 17-β estradiol (E2) directly into the CA3 region of the hippocampus of ovariectomized (OVX) rats had two effects: (1) slowing of 5-HT clearance; (2) and inhibition of the ability of fluvoxamine to slow the clearance of 5-HT. Both of these effects were seen originally with systemic administration of estradiol benzoate (EB) (Benmansour et al., 2009). Perhaps most importantly, different types of ERs were involved in the two effects of estradiol. Its antidepressant-like effect (i.e., slowing of 5-HT clearance) was due to activation of ERβ and/or GPR30, as shown by effects of the selective agonists DPN for ERβ and G1 for GPR30. By contrast, its blockade of fluvoxamine’s inhibitory effect on 5-HT clearance was mediated by ERα, as shown by the effect of the ERα agonist, PPT (Benmansour et al., 2012).
Two major signaling pathways have been implicated in responses underlying antidepressant effects; the PI3K/Akt/Glycogen synthase kinase-3 signaling pathway and the MAPK/ERK signaling pathway (see Tanis and Duman, 2007; Polter and Li, 2011). Numerous studies have implicated these two pathways in the etiology and treatment of mood disorders. Post-mortem studies indicate that brains of individuals who committed suicide have reduced ERK abundance and activity (Dwivedi et al., 2001, Karege et al., 2007). The MAPK/ERK signaling pathway is another central regulator of behavior in rodent models of depression. The ERK signaling cascade is activated by ADs including fluoxetine (Mercier et al., 2004). Pharmacological manipulations of ERK signaling also affect behavior in models of depression and antidepressant response. Systemic blockade of the MAPK pathway in mice with the MEK inhibitor PD184161 produced depressive-like behavior in the forced swim test, tail suspension test, and learned helplessness paradigms, and blocked the effects of the selective norepinephrine reuptake inhibitor desipramine and fluoxetine in the forced swim test (Duman et al., 2007). These pathways have become major pharmacological targets for putative neuropsychiatric treatments. The interacting receptors investigated in this study, TrkB, IGF-1R and mGluR have not only been shown to be implicated in the effects of estradiol but have also been shown to be involved in the mechanism(s) of action of ADs (Schmidt and Duman, 2007, Duric and Duman, 2013, Artigas, 2013).
The objective of this study was to investigate the involvement of two signaling pathways: MAPK/ERK1/2 and PI3K/Akt as well as possible interacting membrane receptors (TrkB, IGF-1R and mGluRs) in effects linked to activation of ERα or ERβ. Using in vivo chronoamperometry, specific inhibitors of these signaling pathways and antagonists of interacting receptors were applied locally into the CA3 region of the hippocampus to evaluate their effect on E2’s or DPN’s (ERβ agonist) ability to block the SERT as well as the ability of E2 and that of PPT (ERα agonist) to interfere with the inhibitory effect of SSRIs on the SERT. We hypothesized that there would be specificity/selectivity in these signaling pathways and/or interacting receptors linked to either ERα and ERβ so as to produce the two distinct effects of estradiol on SERT function. It was found that the MAPK/ERK1/2 pathway and IGF-1R are important in both ERα and ERβ signaling whereas the PI3K/Akt pathway and mGluR1 interaction are only important in ERα signaling but not in ERβ signaling.
METHODS
Animals
Ovariectomized (OVX) rats (Sprague-Dawley; 250-350g, Harlan, Indianapolis, IN) were housed on a 12:12h light/dark cycle with lights on at 0700 and with food and water provided ad libitum. All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used, or stress and discomfort to the animals during the experimental procedure. OVX rats were allowed 2-3 weeks recovery after surgery before the start of the experiment.
In vivo chronoamperometry
This was carried out as described previously (Benmansour et al., 2012)
Animal preparation
Sprague-Dawley OVX rats (250-350g) were anesthetized with chloralose (70/mg/Kg)/urethane (700mg/kg) administered intraperitoneally (ip), after tracheal intubation and placed into a stereotaxic apparatus (David Kopf Instruments). The body temperature of the rat was maintained between 37-38°C using a water-circulating heating pad. Rectal temperature was continuously monitored with a probe attached to an YSI temperature meter. The scalp was incised and reflected and a hole drilled in the skull at the desired coordinates. A small burr hole was drilled over the posterior cortex for placement of Ag/AgCl reference electrode.
Electrode preparation
Carbon fiber electrodes (30 m tip diameter, 95-175 m in length) were coated with Nafion to improve their selectivity, then tested for sensitivity to 5-hydroxyindole acetic acid (5-HIAA, 250 M) and calibrated in vitro with 5-HT. Only electrodes displaying a selectivity ratio for 5-HT over 5-HIAA > 1000:1 and a linear response (r2 > 0.977) to 5-HT (0.5-3.0 M) were used.
Micropipette preparation
The carbon fiber electrode was positioned adjacent a 7 barrels micropipette using a micromanipulator and the tip separation determined using a dissecting microscope and was between 250-350 m. The electrode and the multibarrel micropipette were then attached using sticky wax. Micropipette barrels were filled with 5-HT (200 M, Sigma-Aldrich, St Louis, MO), fluvoxamine (400 M, Sigma-Aldrich, St Louis, MO) [fluvoxamine was always used at 4x the amount of 5-HT applied], and the other hormones or drugs to be tested. All drugs were prepared in 0.1M phosphate buffered saline (PBS) and supplemented with 100 M ascorbic acid. The pH of all solutions was 7.4. All drugs were delivered by pressure ejection in a volume of 20-100nl, using a PLI-100 reproducible pico-injector. The volume of the fluid was determined using a dissection microscope (Nikon SMZ-1) fitted with a reticule eye piece.
Electrochemical recordings
The electrode-pipette assembly was lowered into the CA3 region of hippocampus (stereotaxic coordinates (mm) anterior-posterior (AP), −4.10 from bregma; medio-lateral (ML), +3.30 from midline; dorsal-ventral (DV), −3.60 from dura; Paxinos and Watson (1986)). Chronoamperometric recordings were started 20-30min after the lowering of the assembly to allow the baseline electrochemical signal to stabilize. High-speed chronoamperometric recordings were made using the Fast-16 system (Quanteon). Oxidation potentials consist of 100msec pulses of +0.55 V versus Ag/AgCl were delivered one per second; the electrode was held at the resting potential of 0.0 V between measurements. Oxidation and reduction currents were digitally integrated during the last 80msec of each 100msec voltage pulse.
The electrode placement was confirmed at the conclusion of the electrochemical recordings. Only rats having recordings made in the CA3 region of the hippocampus (>95%) were included in the analysis.
Experimental design
Both basal 5-HT clearance and the ability of fluvoxamine to block 5-HT clearance were measured 10min after local administration of PBS, E2 or ER agonists (as shown in the schematic in Figure 1). Since the ERα agonist, PPT, had no effect on 5-HT clearance but blocked the ability of fluvoxamine to slow clearance, PPT was only tested for the latter effect.
Figure 1.
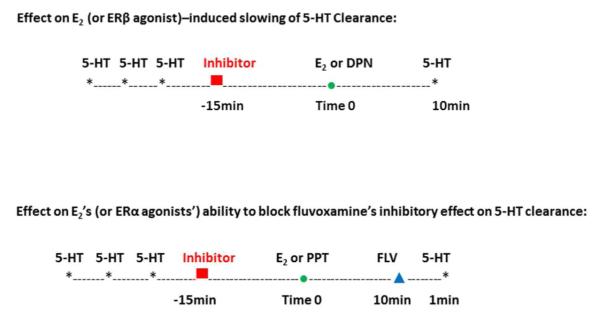
Schematic showing the experimental design used for local administration of estradiol, DPN or PPT and an inhibitor to study effects on 5-HT clearance or effects on fluvoxamine ability to alter 5-HT clearance.
Similarly the ERβ agonist, DPN, blocked 5-HT clearance but did not alter the effects of fluvoxamine, so it was only tested for its effect on 5-HT clearance. In addition only inhibitors that altered one or both of estradiol’s effects were examined for their effects with the specific ER agonists. In other words, if inhibition of the signaling pathways or the interacting receptors did not alter the effects of estradiol mediated via either ERα or ERβ, then the effect on the inhibition on responses elicited by selective agonists for ERα or ERβ was not studied. As we found previously that the effects of E2 and ER agonists are similar at an early time point (1-10min) and later time point (40-60min) (Benmansour et al., 2012), we elected to evaluate effects only at 10min.
Effect on E2 (or ERβ agonist)–induced slowing of 5-HT Clearance
For these experiments, sufficient 5-HT was ejected to produce a peak amplitude of 0.3-0.5μM (amplitudes of this magnitude are small enough not to lose sensitivity of the recording electrode after multiple applications of 5-HT and large enough to enable the detection of decreases in the signal). After a stable signal was obtained following three applications of 5-HT, the inhibitor (inhibitors of signaling pathways or antagonists for interacting receptors) was applied 15min before the application of E2 or DPN and 5-HT was applied 10min afterwards (see schematic in Figure 1). The clearance time parameter, T80, the time it takes for the peak signal amplitude to be reduced by 80%, was analyzed from the generated 5-HT signal. Data are expressed as percent change in T80 ([T80 of signal at 10min /T80 of signal before inhibitor application] x100).
Effect on E2’s (or ERα agonists’) ability to block fluvoxamine’s inhibitory effect on 5-HT clearance
Fluvoxamine’s ability to block 5-HT clearance is determined by its ability to increase the T80 value. As shown in the schematic in Figure 1, after a stable signal was obtained, the inhibitor was applied 15min before the application of E2 or PPT and fluvoxamine’s effect tested 10min after that. Data are expressed as percent change in T80 ([T80 of signal post fluvoxamine /T80 of signal before inhibitor application] x100).
The inhibitors tested were: specific inhibitors for MAPK/ERK1/2: (1) PD98059 (Alessi et al., 1995), 10μg; (2) U0126 (Favata et al., 1998), 100ng; Specific inhibitors for PI3K/Akt: (1) wortmannin (Stephens et al., 1994), 50ng; (2) LY294,002 (Sanchez-Margálet et al., 1994), 30 g; Specific antagonists: for TrkB, k252a (Deltheil et al., 2008; Benmansour et al., 2008), 10ug, for IGF-1R, JB-1 (Pietrzkowski, 1992), 20 g; for mGluR1, LY367,385 (Eaton et al., 1993), 20 g. PD98059, U0126, wortmannin, LY294002, K252a (Sigma-Aldrich, St Louis, MO), JB-1 (Bachem, Torrance, CA) and LY367,385 (Tocris, Ellisville, MO) were all dissolved in PBS or PBS containing ethanol (less than 0.0001%).
The doses of E2 (20pmoles) and ER agonists- ERα agonist, PPT (60pmoles) and ERβ agonist, DPN (90pmoles) (Tocris, Ellisville, MO) were selected based on our previous studies (Benmansour et al., 2009, 2012). The doses of inhibitors of signaling pathways and those of interacting receptors were selected in pilot studies. In these studies, dose response curves of inhibitors were carried out and the lowest dose, devoid of any effect on the 5-HT signal by itself, and inducing changes on at least one of the effects of E2; (i.e., either E2-induced slowing of 5-HT clearance or E2’s inhibition of fluvoxamine-induced slowing of 5-HT clearance), were selected.
4-12 rats were used for each tested drug in each of the two experimental designs 1) the effects of drugs on 5-HT clearance; 2) effects of drugs on fluvoxamine’s blockade of 5-HT clearance.
Statistical analysis
Data were analyzed (SigmaStats, Systat Software Inc, San Jose, CA) by two way analysis of variance (ANOVA) followed by Dunnett’s analysis for comparisons with the control groups or with the estradiol, DPN or PPT alone groups. Only when there was a significant main effect and/or interaction effect in the ANOVA’s were post-hoc analyses carried out. Significance was determined at p < 0.05.
RESULTS
1-Effects of inhibitors of signaling pathways on estradiol’s effects on SERT function
Two important signaling pathways, MAPK and PI3K have been shown to be associated with actions of estradiol in brain. The involvement of these signaling pathways in E2’s-induced slowing of serotonin clearance or on the ability of E2 to block fluvoxamine’s inhibition of serotonin clearance was examined.
a- Role of MAPK/ERK1/2
As described in the methods section, two selective inhibitors of MAPK/ERK1/2: PD98059 (10 g) and U0126 (100ng) were tested. The inhibitors were applied locally into the CA3 region of the hippocampus 15min prior to the application of estradiol (20pmol) to evaluate their effect on E2’s ability to block the SERT as well as their ability to interfere with the inhibitory effect of fluvoxamine on SERT function. None of the inhibitors alone had any effect on 5-HT clearance or on fluvoxamine’s ability to slow clearance (Figure 2). Local application of PD98059, or U0126 15min prior to E2, blocked E2’s ability to increase the T80 clearance time parameter as E2+PD98059 and E2+U0126 were significantly different from the E2 alone group (Figure 2a). In addition, these inhibitors restored the ability of fluvoxamine to slow 5-HT clearance, as shown by the increased T80 value in the presence of fluvoxamine (Figure 2b). Similar results were obtained when using the selective ER agonists as PD98059 blocked DPN’s-induced slowing of 5-HT clearance and PPT’s ability to inhibit the fluvoxamine-induced slowing of 5-HT clearance (Tables 1 and 2). Thus, the MAPK pathway seems to be important in these two effects of estradiol mediated by ERα and ERβ.
Figure 2.
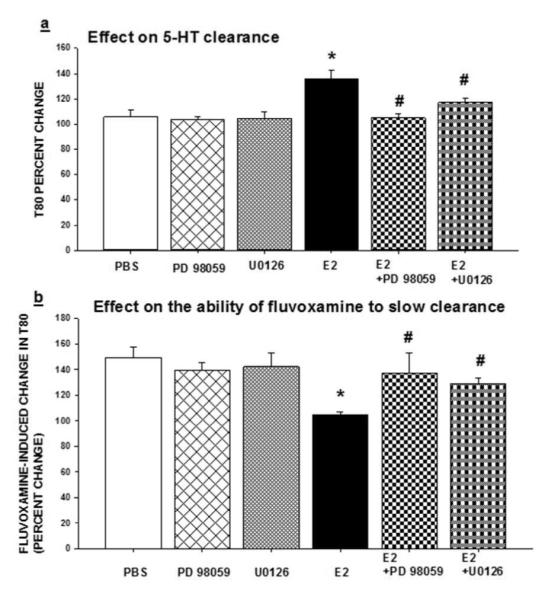
Effects of inhibitors of MAPK/ERK1/2 on estradiol’s effects on SERT function. Selective inhibitors of MEK1/2, PD98059 (10μg) or U0126 (100ng), were applied locally into the CA3 region of the hippocampus 15min prior to local application of 17-β-estradiol (E2; 20 pmol), and their effects on:
(a) The clearance time parameter T80 were measured at 10 min post E2 administration. Bar and brackets represent the T80 value as a percentage of the pretreatment value ± SEM (n = 4–8); Two way analysis of variance was carried out for the percent change in T80 values for E2 and/or the inhibitors. There was a significant main effect for inhibitor [F(2,30)=6.715, p=0.004] and for E2 [F(1,30)=14.879, p<0.001] as well as a significant interaction between inhibitor x E2 [F(2,30)=4.852, p=0.015]. Dunnett’s post hoc analysis was carried out. *p < .001, comparing E2 within PBS and #p < .01, comparing value of E2 with inhibitor to value of E2 alone.
(b) The ability of fluvoxamine to increase the T80 value was measured at 10 min post E2 administration. Bar and brackets represent the percent change in T80 value after fluvoxamine ± SEM (n = 4–8). The data were analyzed by a two-way ANOVA. The percentage change in the T80 value produced by fluvoxamine was the parameter analyzed and the effect of E2 in the absence or presence of the inhibitors was evaluated. There was no significant main effect for inhibitor [F(2,31)=2.051, p=0.146] but there was a significant main effect for E2 [F(1,31)=16.398, p<0.001] as well as a significant interaction between inhibitor x E2 [F(2,31)=6.934, p=0.003]. Dunnett’s post hoc analysis was carried out. *p < .001, comparing E2 within PBS and #p < .01, comparing value of E2 with inhibitor to value of E2 alone.
Table 1.
Effects of inhibition of signaling pathways and that of interacting receptors on the DPN-increased T80 value
| Vehicle + vehicle |
Vehicle + DPN |
PD98059 + DPN |
K252a + DPN |
JB-1+ DPN |
|
|---|---|---|---|---|---|
| T80 % change |
106.0 ±5.04 |
149.5* ±9.61 |
103.1# ±6.00 |
96.7# ±6.05 |
103.1# ±7.02 |
Mean of T80 value expressed as a percentage of pretreatment value ±SEM of 5-7 rats
Two way analysis of variance was carried out for the percent change in T80 values for DPN and/or the inhibitors. Inhibitors alone, not shown in this table did not change T80 values (as shown in Figures 2a and 4a). The data from the inhibitors alone were included in the statistical analyses. There was a significant main effect for inhibitor [F(3,41)=14.122, p<0.001] and for DPN [F(1,41)=7.522, p=0.009], as well as a significant interaction between inhibitor x DPN [F(3,41)=12.984, p<0.001]. Dunnett’s post hoc analysis was carried out.
p < .001, comparing DPN within PBS and
p < .001, comparing value of DPN with inhibitor to value of DPN alone.
Table 2.
Effects of inhibition of signaling pathways and that of interacting receptors on PPT’s blockade of the fluvoxamine-induced increase in the T80 value
| Fluvoxamine+ vehicle+ vehicle |
Fluvoxamine+ vehicle+ PPT |
Fluvoxamine+ PD98059+ PPT |
Fluvoxamine+ wortmannin+ PPT |
Fluvoxamine+ JB-1+ PPT |
Fluvoxamine+ LY367,385+ PPT |
|
|---|---|---|---|---|---|---|
| T80 % change |
149.3a ±7.80 |
102.4* ±3.66 |
130.7# ±9.04 |
132.3# ±11.06 |
133.3# ±14.01 |
141.2# ±15.00 |
Mean of percent in T80 value after fluvoxamine ±SEM of 4-7 rats
The data were analyzed by Two-way ANOVA. The percentage change in the T80 value produced by fluvoxamine was the parameter analyzed and the effect of PPT in the absence or presence of the inhibitors was evaluated. Inhibitors alone, not shown in this table, did not change the fluvoxamine-induced increased T80 value (as shown in Figures 2b, 3b and 4b). There was no significant main effect for inhibitor [F(4,50)=1.554, p=0.201] but there was a significant main effect for PPT [F(1,50)=11.321, p<0.001] and a significant interaction between inhibitor x PPT [F(4,50)=3.013, p=0.026]. Dunnett’s post hoc analysis was carried out.
p < .001, comparing PPT within PBS and
p < .05, comparing value of PPT with inhibitor to value of PPT alone.
b- Role of PI3k/Akt
Local application of selective PI3K/Akt inhibitors, wortmannin (50ng) or LY294,002 (30μg), did not alter the ability of E2 to increase the T80 value as a result of the slowing of 5-HT clearance (Figure 3a), but they blocked E2’s inhibition of fluvoxamine’s ability to increase the T80 value as shown by significant differences between E2 + PI3K/Akt inhibitor and the E2 alone group (Figure 3b). Consistent with this, wortmannin also blocked PPT’s ability to inhibit the effect of fluvoxamine, as shown by the increased T80 after fluvoxamine in the presence of both wortmannin and PPT (Table 2). These data indicate that the PI3K pathway might be important in the effect of E2 mediated only via ERα activation. None of the inhibitors alone had any effect on 5-HT clearance or on fluvoxamine’s ability to slow clearance (Figure 3).
Figure 3.
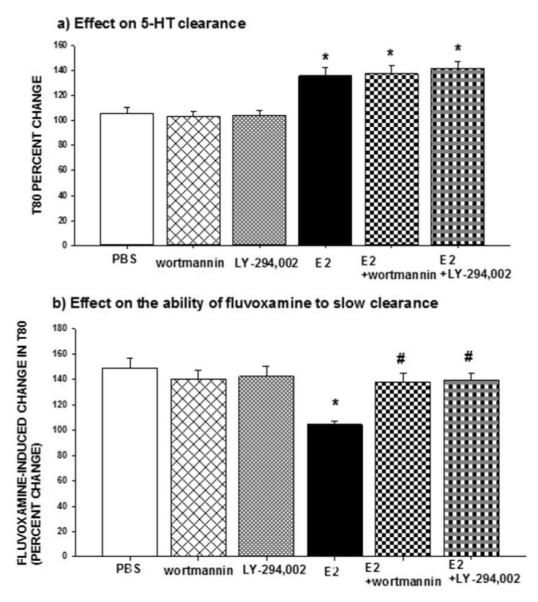
Effects of inhibitors of PI3K/Akt on estradiol’s effects on SERT function. Selective inhibitors of PI3K/Akt, wortmannin (50ng) or LY294,002 (30μg), were applied locally into the CA3 region of the hippocampus 15min prior to local application of 17-β-estradiol (E2; 20 pmol), and their effects on:
(a) The clearance time parameter T80 were measured at 10 min post E2 administration. Bar and brackets represent the T80 value as a percentage of the pretreatment value ± SEM (n = 5–7); Two way analysis of variance was carried out for the percent change in T80 values for E2 and/or inhibitors. There was no significant main effect for inhibitor [F(2,29)=0.148, p=0.863] but there was a significant main effect for E2 [F(1,29)=56.982, *p<0.001] and no significant interaction between inhibitor x E2 [F(2,29)=0.258, p=0.774].
(b) The ability of fluvoxamine to increase the T80 value was measured at 10 min post E2 administration. Bar and brackets represent the percent change in T80 value after fluvoxamine ± SEM (n = 4–12). The data were analyzed by a two-way ANOVA. The percentage change in the T80 value produced by fluvoxamine was the parameter analyzed and the effect of E2 in the absence or presence of the inhibitors was evaluated. There was a significant main effect for inhibitor [F(2,34)=3.114, p=0.05] and for E2 [F(1,34)=9.759, p=0.004] as well as a significant interaction between inhibitor x E2 [F(2,34)=7.782, p=0.002]. Dunnett’s post hoc analysis was carried out. *p < .001, comparing E2 within PBS and #p < .001, comparing value of E2 with inhibitor to value of E2 alone.
2-Effects of antagonism of interacting receptors on estradiol’s effects on SERT function
E2 activates intracellular pathways by binding to ERs which in turn could engage interactions with cell surface receptors for other compounds such as the BDNF receptor, TrkB, the insulin-like growth factor receptor, IGF-1R, and metabotropic glutamate receptors, mGluRs (Meitzen and Mermelstein, 2011; Arevalo et al., 2012). Pharmacological blockade of possible interacting receptors was used to assess their involvement in the effects of estradiol on SERT function.
As in the experiment described above, the antagonists were applied locally into the CA3 region of the hippocampus 15min prior to the application of estradiol (20pmoles) to evaluate their effect on estrogen’s ability to block the SERT as well as its ability to interfere with the inhibitory effect of fluvoxamine on SERT function (Figure 1). None of the antagonists by itself had any effect on SERT function (Figure 4). Blockade of TrkB by K252a (10μg) blocked the increase in the T80 value caused by E2 whereas it did not restore the ability of fluvoxamine to slow clearance (Figures 4a, 4b). Consistent with this, the DPN-induced slowing of 5-HT clearance was also blocked after TrkB inhibition with K252a (Table 1). Thus, these results indicate that the effects of estradiol mediated by ERβ could involve TrkB activation.
Figure 4.
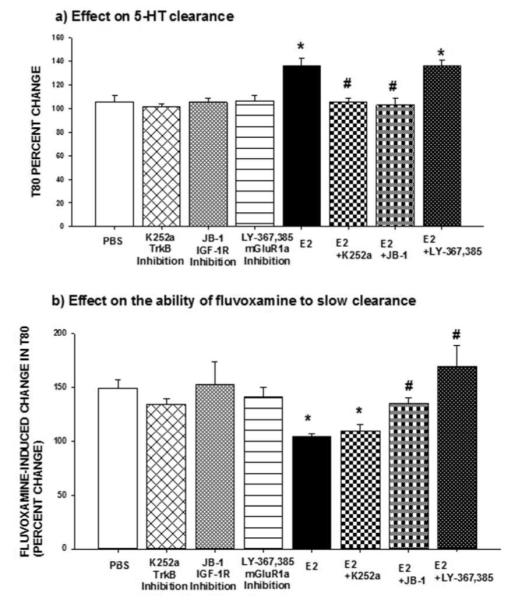
Effects of antagonists of interacting receptors on estradiol’s effects on SERT function. Selective antagonists of TrkB, k252a (10μg); of IGF-1R, JB1(20μg); or that of mGluR1a, LY367385 (20μg) were applied locally into the CA3 region of the hippocampus 15min prior to local application of 17-β-estradiol (E2; 20 pmol), and their effects on:
(a) The clearance time parameter T80 were measured at 10 min post E2 administration. Bar and brackets represent the T80 value as a percentage of the pretreatment value ± SEM (n = 5–11); Two way analysis of variance was carried out for the percent change in T80 values for the E2 and/or inhibitors. There was a significant main effect for inhibitor [F(3,52)=6.604, p<0.001] and for E2 [F(1,52)=15.400, p<0.001] as well as a significant interaction between inhibitor x E2 [F(3,52)=4.903, p=0.004]. Dunnett’s post hoc analysis was carried out. *p < .001, comparing value of E with inhibitor to value of E # 2 2 alone and p < .001, comparing value of E2 with inhibitor to value of E2 alone.
(b) the ability of fluvoxamine to increase T80 were measured at 10 min post E2 administration. Bar and brackets represent the percent change in T80 value after fluvoxamine ± SEM (n = 4–10).
The data were analyzed by a two-way ANOVA. The percentage change in the T80 value produced by fluvoxamine was the parameter analyzed and the effect of E2 in the absence or presence of the inhibitors was evaluated. There was a significant main effect for inhibitor [F(3,45)=4.365, p=0.009] and for E2 [F(1,45)=4.215, p=0.046] as well as a significant interaction between inhibitor x E2 [F(3,45)=4.275, p=0.010]. Dunnett’s post hoc analysis was carried out. *p < .001, comparing E2 within PBS and #p < .05, comparing value of E2 with inhibitor to value of E2 alone.
Blockade of IGF-1R with JB1 (20μg) blocked both effects of E2, as E2 no longer increased the T80 value and fluvoxamine’s ability to increase T80 was restored (Figures 4a, 4b). Similarly it also blocked the effect of DPN on 5-HT clearance and the effect of PPT on fluvoxamine’s ability to slow 5-HT clearance (Tables 1 and 2). Thus, the IGF-1R is implicated in effects of E2 mediated by both ERα and ERβ.
Pretreatment with a selective antagonist of mGluR1a, LY367385 (20μg), blocked the ability of E2 and that of the ERα agonist, PPT, to inhibit the fluvoxamine-induced slowing of 5-HT clearance but had no effect on the E2-increased T80 value (Figures 4a, 4b, table 2) therefore indicating a possible interaction between ERα and the mGluR1 in the effects of E2 on SERT function.
DISCUSSION
These results demonstrate that the effects of E2 on SERT function are mediated by different as well as common signaling pathways. The E2-induced slowing of serotonin clearance via activation of ERβ required MAPK/ERK1/2 signaling pathways and involved interactions both with TrkB and IGF-1R. The E2-induced prevention of the ability of fluvoxamine to slow serotonin clearance, via activation of ERα, required MAPK/ERK1/2 and PI3K/Akt signaling pathways as well as interactions with both IGF-1R and mGluR1.
Rapid activation of both MAPK and PI3K by E2 has been shown to be important in the effects of estradiol in brain. In neocortical explants, the effects of E2 on neuronal differentiation were associated with increased ERK phosphorylation (Nethrapalli et al., 2001). It was shown that the MAPK cascade plays a central role in E2-induced structural plasticity in cholinergic neurons (Dominguez et al., 2004). E2 also induces rapid activation of ERK in primary cultures of astroglia (Ivanova et al., 2001) and in explants of cerebral cortex (Toran-Allerand et al., 1999, Singh et al., 2000, Setalo et al., 2002). In the rat hippocampus, E2’s activation of MAPK/ERK1/2 protects against quinolone-induced toxicity (Kuroki et al., 2001). Activation of ERK was detected in vivo in the hypothalamus after systemic administration of estradiol (Cardona-Gomez et al., 2002). Consistent with such observations, we found that inhibition of MAPK/ERK1/2 blocked the ability of E2 to slow 5-HT clearance and blocked the ability of E2 to prevent the effect of fluvoxamine on 5-HT clearance (Figure 2a and b).
The PI3K/Akt pathway is involved in a wide array of neuronal functions, including cell survival and long term potentiation (LTP) (Sanna et al., 2002; Sui et al., 2008). Estradiol prevented injury-induced apoptosis via activation of Akt (Wilson et al., 2002). In the rat retina, activation of the PI3K pathway is important in E2-mediated protection against light-induced photoreceptor degeneration (Yu et al., 2004). We also found E2’s activation of PI3K/Akt to be necessary for the effect of the hormone in blocking the ability of fluvoxamine to slow 5-HT clearance (Figure 3b). However, inhibition of PI3K/Akt did not alter the ability of E2 to slow 5-HT clearance (Figure 3a). Effects of estradiol can be mediated by the activation of both MAPK/ERK and PI3K/Akt. For example, phosphorylation of mTOR in the dorsal hippocampus, necessary for E2 -enhanced object recognition and memory consolidation, is dependent on upstream activation of both ERK and PI3K signaling pathways (Fortress et al., 2013). Similarly, we found E2’s inhibition of the effect of fluvoxamine also to be dependent on the activation of both MAPK/ERK1/2 and PI3K/Akt signaling (Figures 2b and 3b). By contrast, E2-induced slowing of 5-HT clearance was dependent only on MAPK/ERK1/2 and not on PI3K/Akt signaling (Figures 2a and 3a). Similarly, the effect of an ERα agonist was dependent on the activation of both MAPK/ERK1/2 and PI3K signaling pathways whereas those of the ERβ agonist involved the MAPK/ERK1/2 but not PI3K/Akt pathways (Tables 1 and 2). Consistent with these data, it has been shown that ERα is involved in the activation of PI3K/Akt whereas ERβ is not (Kahlert et al., 2000; Mendez et al., 2003; 2005). Recently estradiol administration was shown to increase immunoreactivity for phosphorylated Akt at 6h and that of phosphorylated TrkB at 48h after its administration in the hippocampus of mice. These effects were abolished in ERα and ERβ knockout mice and therefore require both ERα and ERβ activation. These results indicate that ERα and ERβ do not always have distinct effects; they can have complementary roles as they may activate distinct signaling pathways that converge onto common downstream targets (Spencer-Segal et al., 2012).
Estrogen and BDNF seem to share common targets, effects, and mechanisms of action in the hippocampus (Scharfman and Maclusky, 2005; 2006). The action of BDNF is mediated through its high affinity tyrosine kinase receptor B (TrkB). Blockade of TrkB prevented the E2-induced slowing of 5-HT clearance but had no effect on the ability of E2 to prevent the effect of fluvoxamine (Figures 4a and 4b). Further evidence was obtained with the ERβ agonist, as DPN no longer slowed the clearance of 5-HT after blockade of TrkB with K252a (Table 1). BDNF has been implicated in the mechanism(s) of action of ADs (see Schmidt and Duman, 2007). Chronic treatment with ADs upregulate the expression of BDNF and TrkB in the hippocampus and cortex; in addition, direct infusion of BDNF into the hippocampus or into cerebral ventricles produces AD-like effects in two animal models of depression (see Schmidt and Duman, 2007). Evidence that activation of the TrkB receptor is required for a behavioral response typically induced by ADs has been shown as well (Saarelainen et al., 2003). Although upregulation of BDNF expression is observed only after chronic AD drug treatments, there is also evidence that acute AD treatment activates TrkB receptors (Saarelainen et al., 2003; Rantamaki et al., 2007; Furmaga et al., 2012). Thus, BDNF could act as an intermediate signaling molecule in the antidepressant-like effect of estradiol mediated by ERβ.
The interaction of estradiol and IGF-1R in neuroprotection has been characterized in experimental models of excitotoxicity, Parkinson’s disease and stroke (Jover-Mengual et al., 2007; Quesada et al., 2008; Lebesgue et al., 2009; Garcia-Segura et al., 2010; Selvamani and Sohrabji, 2010). It was shown that the inhibition of brain IGF-1 signaling in these models resulted in attenuation of the neuroprotective effects of estradiol. Blockade of IGF-1R with JB-1, a selective competitive antagonist of IGF-1 autophosphorylation, attenuated the neuroprotective effects of both E2 and IGF-1, suggesting that the neuroprotective actions of E2 depend on the co-activation of both ERs and IGF-IR (Quesada and Micevych, 2004). Moreover, intracerebroventricular infusion of JB-1 inhibits the estrogen-induced LH surge and sexual behavior in ovariectomized rats (Quesada and Etgen, 2002). In addition to interactions between ERs and IGF-1R, IGF-1 has been implicated in the mechanism of action of antidepressants, perhaps through a mechanism involving serotonin. For example, chronic administration of fluoxetine to rats increased protein levels of IGF-1 in hippocampus (Khawaja et al., 2004). Acute intraventricular delivery of IGF-1 to mice had an antidepressant-like effect in the tail suspension test (Malberg et al., 2007). IGF-1 induced AD-like effects in the FST three days after its intraventricular administration and this effect required the presence of 5-HT (Hoshaw et al., 2008). We found that antagonism of the IGF-1R with JB-1 blocked both E2- or DPN-induced slowing of 5-HT clearance and the ability of either E2 or PPT to prevent the inhibitory effect of fluvoxamine on 5-HT clearance (Figures 4a and 4b, Tables 1 and 2). Collectively, these findings support functional interactions between ER subtypes and IGF-1R in the effects of estradiol on SERT function. These interactions could be complex as it was shown in neurons that estradiol induces incorporation of ERα into a macromolecular complex in which IGF-1R and several components of IGF-1R signaling are associated and lead to the regulation of neuroprotection and improvement of brain homeostasis (Garcia-Segura et al., 2010; Alonso and Gonzalez, 2012).
In addition to ER cross-talk with IGF-1 receptors, there is evidence for interaction between ER and metabotropic glutamate receptor (mGluRs) signaling in hippocampal, hypothalamic and striatal neurons (Boulware et al., 2005; Dewing et al., 2007; Micevych and Mermelstein, 2008; Grove-Strawser et al., 2010). Estradiol binding to the ER promotes transactivation of mGluR, initiating mGluR signaling without the need for glutamate (Boulware et al., 2005; Dewing et al., 2007; Kuo et al., 2010). Such a receptor–receptor interaction of ERs and mGluRs is supported by co-immunoprecipitation experiments that indicate, for example, that ERα can directly interact with mGluR1a (Dewing et al., 2007). The ER/mGluR interactions are dependent upon caveolin (CAV) proteins that are essential for the trafficking and clustering of signaling molecules (Boulware et al., 2007). Interestingly, there is a brain region specific ER–CAV interaction (Meitzen and Mermelstein, 2011). In hippocampal neurons, ERα interaction with either mGluR1 or mGluR2/3 was dependent upon CAV3 or CAV1, respectively. Conversely, ERβ interacts with mGluR2/3 via CAV3 (Boulware et al., 2007). In striatal neurons, ERα via CAV1 activates mGluR5 (Grove-Strawser et al., 2010). We found that an interaction between ERα and mGluR1a seems to be important in the effects of estradiol on SERT function. Antagonism of mGluR1a blocked the ability of E2 and that of the ERα agonist PPT to inhibit the effects of fluvoxamine but did not alter E2’s ability to slow 5-HT clearance, implying that ERβ does not require an interaction with this receptor (Figures 4a and 4b, Table 2). It is not known which of the caveolin proteins are associated with the effect of estradiol mediated by ERα on SERT function. It is also worth noting that a role for mGluR in anxiety and depression models has been shown and that several mGluR selective agents are described to modulate stress, anxiety and depressive behavior (See Artigas, 2013).
E2 activation MAPK/ERK1/2 and PI3K/Akt signaling pathways involve not only ERs but also interactions with other receptors. Given the similarity of the intracellular signaling pathways activated by ER with those activated by mGluR, IGF-1R and TrkB, it is possible that the interacting receptors may serve to facilitate activation of kinases by E2.
At this time, it is unclear how the ER-induced activation of the signaling pathways in question lead to the alterations observed in SERT function. SERT proteins exhibit posttranslational regulation (Steiner et al., 2008). Serotonin transporter regulation can occur via phosphorylation dependent and independent posttranslational modifications (Ramamoorthy et al., 2011). In human embryonic kidney cells, protein kinase C (PKC) activation and PP2A inhibition trigger SERT phosphorylation correlated with decreased serotonin uptake function that occurs with a loss of SERT surface density (Qian et al., 1997; Ramamoorthy et al., 1998; Ramamoorthy and Blakely, 1999). Supporting an acute SERT regulation, adenosine A3 receptor stimulation increased SERT uptake function in rat basophilic leukemia (RBL-2H3) cells and mouse mid-brain and hippocampal synaptosomes. This effect is thought to be mediated by both a PKG-dependent surface density increase of the SERT, and p38-MAPK dependent activation of SERT intrinsic activity (Samuvel et al., 2005; Zhu et al., 2005). Multiple signaling pathways seem to contribute to the regulation of SERT mediated 5-HT clearance (Blakely et al., 2005). It is therefore possible that the downstream signaling pathways of estradiol could induce SERT phosphorylation and therefore decrease its uptake function. Future studies are needed to delineate the changes occurring in SERT (i.e. phosphorylation, internalization or conformational changes) and that would account for the effects of estradiol.
In conclusion, as shown previously (Benmansour et al., 2009; 2012), E2 has two opposing effects with respect to the functioning of the SERT: one is to inhibit the SERT so as to produce an antidepressant-like effect but the other is to inhibit the ability of SSRIs to block the SERT. These two effects are mediated by different types of ERs interacting with similar as well as different receptors and using different as well common signaling pathways. Targeting the ER subtypes and/or their interacting receptors/ signaling pathways may reveal a strategy to permit only beneficial effects of estrogen without its deleterious effect on SSRI-efficacy. Further delineating the signaling mechanisms involved in the effects of estradiol on SERT function could lead to a better understanding of the effects of endogenous and exogenous estrogens on mood.
ACKNOWLEDGEMENTS
This research was supported by funds from NARSAD to SB, the Department of Veterans Affairs and the National Institute of Mental Health (MH090386) to AF.
Dr. Frazer has been on advisory boards for Cyberonics, Inc., H. Lundbeck A/S and Takeda Pharmaceuticals America, Inc. and he has consulted and/or received research support for preclinical studies from Forest Research Institute, Eli Lilly and Company, Wyeth Pharmaceuticals, and H. Lundbeck A/S. No support for this study was received from any pharmaceutical company.
Footnotes
STATEMENT OF INTEREST Dr. Benmansour, Mr. Privratsky and Mr. Adeniji have no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in viva. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Alonso A, Gonzalez C. Neuroprotective role of estrogens: relationship with insulin/IGF-1 signaling. Front Biosci (Elite Ed) 2012;1:607–619. doi: 10.2741/403. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Ruiz-Palmero I, Scerbo MJ, Acaz-Fonseca E, Cambiasso MJ, Garcia-Segura LM. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: Recent advances. J Steroid Biochem Mol Biol. 2012;131:52–56. doi: 10.1016/j.jsbmb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Artigas F. Developments in the field of antidepressants, where do we go now? Eur Neuropsychopharmacol. 2013 doi: 10.1016/j.euroneuro.2013.04.013. S0924-977X(13)00144-2 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Benmansour S, Deltheil T, Piotrowski JP, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–8. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34:555–64. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry. 2012;71:633–641. doi: 10.1016/j.biopsych.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–31. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front Neuroendocrinol. 2012;33:169–78. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev. 2008;60:1504–11. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–14. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor–alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Jalali C, de Lacalle S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J Neurosci. 2004;24:982–990. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Liu R, Baudry M. 17-Beta-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-D-aspartate receptor phosphorylation in cortical synaptoneurosomes. J Neurochem. 2007;101:232–40. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–70. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duric V, Duman RS. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci. 2013;70:39–53. doi: 10.1007/s00018-012-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–28. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Eaton SA, Jane DE, Jones PL, Porter RH, Pook PC, Sunter DC, Udvarhelyi PM, Roberts PJ, Salt TE, Watkins JC. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur J Pharmacol. 1993;244:195–197. doi: 10.1016/0922-4106(93)90028-8. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–55. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One. 2012;7:e34844. doi: 10.1371/journal.pone.0034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arévalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Hill TI, Crowley JJ, Malberg JE, Khawaja X, Rosenzweig-Lipson S, Schechter LE, Lucki I. Antidepressant-like behavioral effects of IGF-I produced by enhanced serotonin transmission. Eur J Pharmacol. 2008;594:109–116. doi: 10.1016/j.ejphar.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Karolczak M, Beyer C. Estrogen stimulates the mitogen-activated protein kinase pathway in midbrain astroglia. Brain Res. 2001;889:264–9. doi: 10.1016/s0006-8993(00)03149-8. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–5. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Khawaja X, Xu J, Liang JJ, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J Neurosci Res. 2004;75:451–60. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Neuroprotection by estrogen via extracellular signal-regulated kinase against quinolinic acid-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13:472–6. doi: 10.1046/j.0953-816x.2000.01409.x. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular Functions of the Plasma Membrane Estrogen Receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Platt B, Rizzo SJ, Ring RH, Lucki I, Schechter LE, Rosenzweig-Lipson S. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32:2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;4:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms. J Endocrinol. 2005;185:11–7. doi: 10.1677/joe.1.06058. [DOI] [PubMed] [Google Scholar]
- Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramaugé M, Courtin F, Pierre M. MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24:207–16. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD. Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–5148. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; New York, NY: 1986. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor. Cancer Res. 1992;52:6447–6451. [PubMed] [Google Scholar]
- Polter AM, Li X. Glycogen Synthase Kinase-3 is an Intermediate Modulator of Serotonin Neurotransmission. Front Mol Neurosci. 2011;4:31. doi: 10.3389/fnmol.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of alpha 1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Melikian HE, Qian Y, Blakely RD. Biosynthesis, N-glycosylation, and surface trafficking of biogenic amine transporter proteins. Methods Enzymol. 1998;296:347–70. doi: 10.1016/s0076-6879(98)96026-8. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–6. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–38. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E. Pharmacologically Diverse Antidepressants Rapidly Activate Brain-Derived Neurotrophic Factor Receptor TrkB and Induce Phospholipase-Cgamma Signaling Pathways in Mouse Brain. Neuropsychopharmacology. 2007;32:2152–62. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Margálet V, Goldfine ID, Vlahos CJ, Sung CK. Role of phosphatidylinositol-3-kinase in insulin receptor signaling: studies with inhibitor, LY294002. Biochem Biophys Res Commun. 1994;204:446–452. doi: 10.1006/bbrc.1994.2480. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–65. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;2:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Frontiers in Neuroendocrinology. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sétáló G, Jr, Singh M, Guan X, Toran-Allerand CD. Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (Hsp90) and MEK2. J Neurobiol. 2002;50:1–12. doi: 10.1002/neu.10000. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Sétáló G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–76. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007;39:531–44. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Sétáló G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20(2):97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Brain Res Mol Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J Biol Chem. 2004;279:13086–94. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–58. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]


