Abstract
Objective
To determine if current retinopathy of prematurity screening guidelines1 adequately identify treatable ROP in a contemporary cohort of extremely low gestation infants.
Study Design
Data from the Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial were used. Inborn infants 24 0/7 to 27 6/7 weeks gestational age with consent prior to delivery were enrolled in 2005-2009. Severe retinopathy of prematurity (Type 1 retinopathy of prematurity or treatment with laser, cryotherapy, or bevacizumab) or death was the primary outcome for the randomized trial. Examinations followed then current American Academy of Pediatrics (AAP) screening recommendations, beginning by 31-33 weeks postmenstrual age.2,3
Results
1316 infants were enrolled in the trial. 997 of the 1121 who survived to first eye exam had final retinopathy of prematurity outcome determined. 137 (14% of 997) met criteria for severe retinopathy of prematurity and 128 (93%) of those had sufficient data (without missing or delayed exams) to determine age of onset of severe retinopathy of prematurity. Postmenstrual age at onset was 32.1 to 53.1 wks. In this referral center cohort, 1.4% (14/997) developed severe retinopathy of prematurity after discharge.
Conclusion
Our contemporary data support the 2013 AAP screening guidelines for ROP for infants 24 0/7 to 27 6/7 weeks gestational age.1 Some infants do not meet treatment criteria until after discharge home. Post-discharge follow-up of infants who are still at risk for severe ROP is crucial for timely detection and treatment.
Keywords: extremely premature infant
Introduction
Timely detection of treatable retinopathy of prematurity (ROP) is necessary to optimize outcomes for infants who meet the current criteria for treatment. The most recently published screening guidelines1,4 are based on natural history data from the CRYO-ROP5 and LIGHT-ROP6 studies. The CRYO-ROP study7 remains the most carefully conducted analysis of the incidence and timing of the onset of ROP, but it was conducted over 20 years ago (1986-1987). The LIGHT-ROP trial enrolled infants from 1995-1997.8 Over the past two decades, survival of lower birth weight infants in the US and other developed countries has increased.9,10 For infants 501-750 g birth weight, survival increased from 41% in 1990-1991 to 55% in 1997-2002.9 The timing of onset of ROP is related to both gestational age (GA) and chronological (postnatal) age.5 It rarely occurs before 30 weeks postmenstrual age (PMA, sum of GA at birth and chronological age) or before 4 weeks chronological age. Current American Academy of Pediatrics (AAP) / American Academy of Ophthalmology (AAO) / American Association of Pediatric Ophthalmology and Strabismus (AAPOS) recommendations are for screening to begin by 31 weeks PMA for infants born at 22-27 weeks.1 The impact of increased survival of extremely low birth weight (ELBW) infants on the incidence and timing of the onset and regression of ROP has not been systematically evaluated.
In the CRYO-ROP and LIGHT-ROP studies, treatment was initiated for threshold ROP (now termed “CRYO-ROP threshold”). In the CRYO-ROP study, the earliest identification of CRYO-ROP threshold disease was 32.6 weeks postmenstrual age.6 Based on the results of the ET-ROP trial, treatment is now recommended for Type 1 ROP, defined as stage 3 in zone I or plus disease with any ROP in zone I, or stage 2 or 3 with plus disease in zone II.11 Since Type 1 ROP occurs earlier in the course than CRYO-ROP threshold ROP, it is important to determine if screening criteria developed for CRYO-ROP threshold ROP are still appropriate for reliable timely identification of Type 1 ROP. There have been several more recent publications of the incidence and timing of ROP onset. The ET-ROP trial12 and a population-based cohort study of infants born 2004-2007 in Sweden13 reported the age of onset of stages 1, 2, and 3 ROP; however, the age distribution of onset of Type 1 ROP was not reported in either publication. A recent publication from Canada reported the age of onset of Type 1 ROP in a cohort of 214 infants ≤ 27 weeks gestation;14 this cohort included only 24 infants with Type 1 ROP. A recent publication from a German cohort15 reported that “No preterm infants required treatment before the 33rd postmenstrual week or 8th postnatal week, respectively”; the age distribution was not reported. We need updated information about the evolution of ROP in a large contemporary cohort to determine when screening must be initiated to capture all infants as Type 1 ROP develops. Type 2 ROP (stage 1 or 2 ROP without plus disease in Zone I, or stage 3 ROP without plus disease in Zone II) is less severe but warrants close follow up for possible progression to Type 1 ROP. Therefore we also looked at the age of onset of Type 2 ROP.
In addition to information about when screening should begin, clinicians need information about when an infant is no longer at risk for severe ROP so that appropriate follow-up can be arranged (particularly for infants who are ready to be discharged from the hospital) or attempts to arrange follow-up can be curtailed. In the CRYO-ROP study, 99% of the infants who reached the CRYO-ROP threshold criteria had done so by 45.9 weeks postmenstrual age.
This analysis was designed to describe the natural history of ROP in a recent cohort (born 2005-2009) of inborn infants 24-27 6/7 weeks gestational age who were enrolled in the NICHD Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial (SUPPORT)16 to determine if the current ROP screening guidelines are still appropriate for timely identification of Type 1 ROP in a contemporary cohort of infants.
Methods
In the SUPPORT trial conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, severe ROP [defined as Type 1 ROP or treatment with laser ablation, cryotherapy, bevacizumab (monoclonal antibody to vascular endothelial growth factor) injection, scleral buckle, or vitrectomy] or death before discharge was the primary outcome for the O2 saturation target arm of the factorial design trial. ROP outcome data were prospectively collected for all enrolled infants.16 Inborn infants 24 0/7 – 27 6/7 weeks gestation (no birth weight limits) were eligible for this trial if prenatal consent was obtained, there were no known congenital malformations, and full resuscitation was planned. Study eye examinations were performed by each site's examining ophthalmologists using the International Classification of ROP.17 Ophthalmology exams began no later than 31-33 weeks postmenstrual age, as recommended in the AAP/AAO/AAPOS guidelines that were in place when the study began.2,3 Subsequent inpatient and outpatient exams were conducted according to the ophthalmologists’ established screening procedures at each center, based on the findings of the previous examination. The following data were recorded for each eye at each exam: the date of the eye exam, the highest stage of ROP in the lowest zone, the highest stage of ROP in any zone, the presence of plus disease, and whether the infant met the criteria for Type 1 ROP. Study eye exam data were recorded for each exam until one of the study endpoints: death; severe ROP (Type 1 or worse ROP or ROP first treated with peripheral retinal ablation, vitreoretinal surgery or bevacizumab injection as detailed above) in either eye; or no severe ROP (full vascularization to the ora serrata or vascularization in zone III (without severe ROP) on 2 consecutive exams. Examinations required for the study (including exams after hospital discharge) were curtailed at 55 wks PMA.
Postmenstrual age was calculated as gestational age at birth (weeks+days using the best obstetrical estimate) plus the chronological age in weeks+days at the time of each exam. For this observational study, “age of onset” was defined as the postmenstrual or chronological age at which ROP or ROP of a given severity was detected, with the recognition that onset was some time interval prior to detection. Infants with Type 1 ROP whose first exam with Type 1 ROP was preceded by a gap of more than 2 weeks (or more than 1 week if the previous exam had ROP in zone I) between exams were defined as having an uncertain age of onset. No infants had Type 1 ROP on the initial exam. Infants who did not complete exams according to the study schedule (adjudicated ROP outcomes) were not included in this observational study. In cases where the findings differed between eyes, the age of onset was recorded as the earliest age at which the ROP criteria were met in either eye.
Categorical outcomes were compared using Chi square tests; continuous outcomes were compared using t-tests or Wilcoxon tests where appropriate. Non-parametric confidence limits are provided for continuous data grouped into quantiles.18 Cumulative incidence curves for age of onset of severe ROP and age of maturity were compared by gestational age subgroups (26-27 weeks vs 24-25 weeks) using Kolmogorov-Smirnov tests. All analyses were performed using SAS v. 9.2 (SAS Institute, Cary, NC).
Results
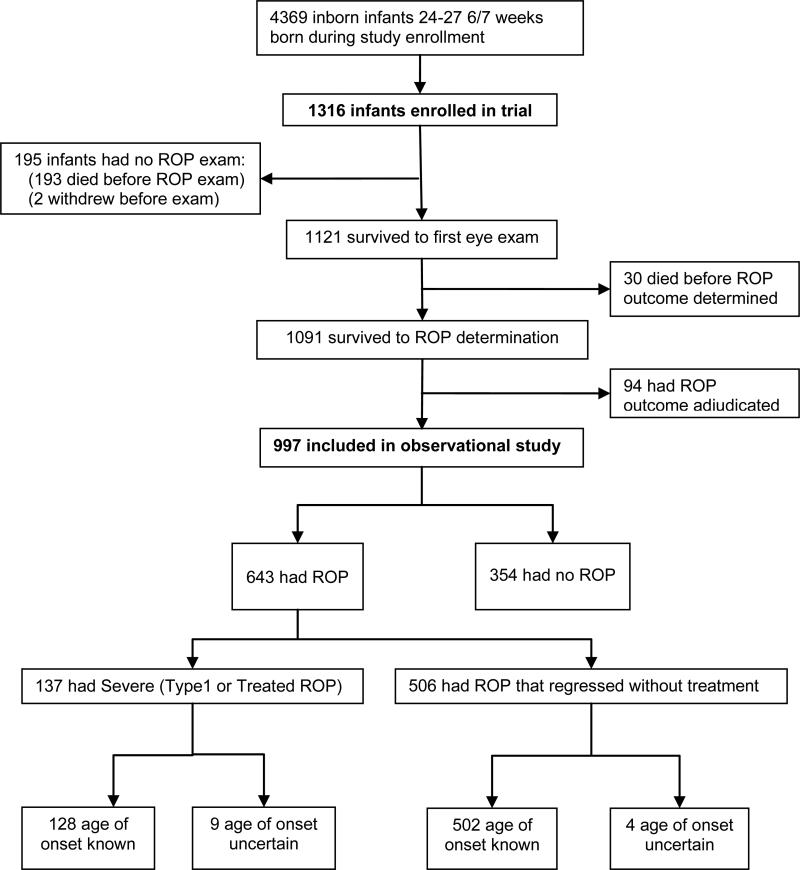
1316 infants were enrolled in the SUPPORT trial from 2005-2009 and 1091 survived to ROP determination (Figure 1). Among infants who survived to ROP determination, 91% (997/1091) had a definitive ROP outcome; 94 of the ROP outcomes were adjudicated. Sixty-four percent (643/997) of these infants developed ROP and 14% (137/997) developed severe ROP. Among infants with severe ROP, 93% (128/137) had sufficient data (no missing or delayed exams prior to “onset” exam) to determine the age of onset of ROP.
Figure 1.
Flow diagram of subjects in the original trial and current analysis
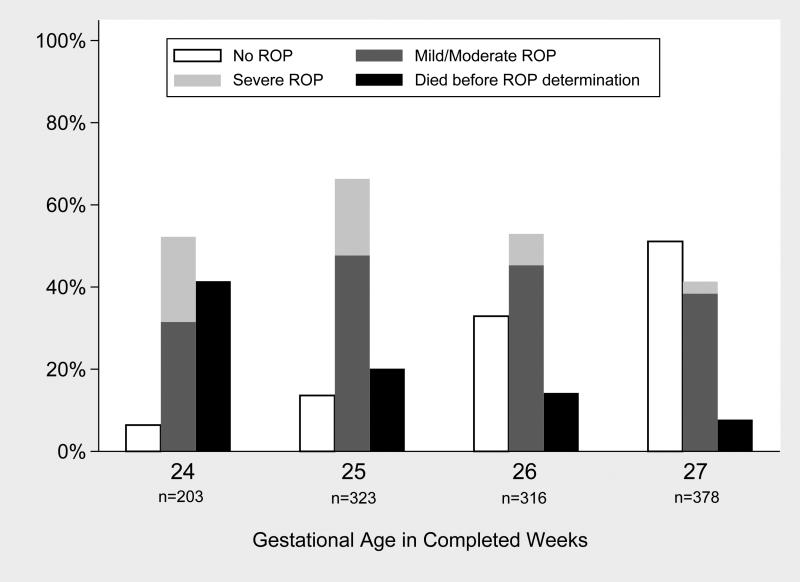
The baseline demographic characteristics of the infants with and without various ROP categories are shown in Table 1. As expected, infants with ROP were lower birth weight and more frequently non-Hispanic White as compared to infants without ROP. The risk of ROP by gestational age, in this cohort, is depicted in Figure 2. As expected, the likelihood of having no ROP increased and the likelihood of having severe ROP decreased with each increasing week of completed gestation at birth (Figure 2).
Table 1.
Baseline characteristics of infants in SUPPORT Trial and observational study
| Infants Enrolled in SUPPORT Trial | Infants Included in Observational Study (Reached Final ROP1 Outcome) |
||||
|---|---|---|---|---|---|
| All ROP Outcomes | By ROP Outcome Category |
||||
| No ROP | Mild/Moderate ROP | Severe (Type 1 or Treated) ROP | |||
| n | 1316 | 997 | 354 | 506 | 137 |
| Gestational age, wks [mean (SD2)] | 26.2 (1.1) | 26.3 (1.1) | 26.8 (0.9) | 26.2 (1.0) | 25.4 (0.9) |
| Birth weight, g [mean (SD)] | 830 (193) | 849 (190) | 943 (173) | 823 (180) | 704 (142) |
| Small for gestational age3 [n (%)] | 173 (13) | 117 (12) | 22 (6) | 65 (13) | 30 (22) |
| Race/ethnicity [n (%)] | |||||
| Non-Hispanic Black | 489 (37) | 374 (38) | 154 (44) | 179 (35) | 41 (30) |
| Non-Hispanic White | 521 (40) | 398 (40) | 125 (35) | 212 (42) | 61 (45) |
| Hispanic | 259 (20) | 190 (19) | 69 (19) | 93 (18) | 28 (20) |
| Other | 47 (4) | 35 (4) | 6 (2) | 22 (4) | 7 (5) |
| Male [n (%)] | 712 (54) | 529 (53) | 195 (55) | 256 (51) | 78 (57) |
| Antenatal steroids [n (%)] | 1265 (96) | 955 (96) | 341 (96) | 480 (95) | 134 (98) |
| Multiple birth [n (%)] | 337 (26) | 253 (25) | 91 (26) | 121 (24) | 41 (30) |
Retinopathy of prematurity
Standard deviation
Based on Olsen25 growth curves
Figure 2.
Risk of ROP by gestational age at birth (in completed weeks) among all SUPPORT trial infants with known outcome (997 survivors + 223 infants who died)
Several previously reported risk factors for ROP are shown in Table 2.19,20,21 Consistent with prior observational studies, as compared to infants without ROP, infants with ROP had a longer duration of supplemental oxygen and more frequently had late onset sepsis, fungal sepsis, intraventricular hemorrhage, necrotizing enterocolitis, and patent ductus arteriosus (p<0.05 for all comparisons of no ROP vs any ROP).
Table 2.
Risk factors for ROP1
| Risk Factor | No ROP2 | Any ROP (Mild, Moderate, or Severe) | Mild/Moderate ROP | Severe (Treated or Type 1) ROP |
|---|---|---|---|---|
| n | 354 | 643 | 506 | 137 |
| Days on supplemental oxygen3 [median (IQR4)] | 33 (10, 60) | 66 (39, 100)5 | 59 (31, 94) | 95 (68, 119) |
| Late-onset sepsis (+ culture) [(n (%)] | 75 (21) | 247 (38) | 171 (34) | 76 (55) |
| Fungal sepsis [n (%)] | 2 (0.6) | 23 (4)5 | 155 (3.0) | 8 (5.8) |
| Grade 3-4 intraventricular hemorrhage or periventricular leukomalacia [n (%)] | 29 (8) | 98 (15)5 | 695 (14) | 29 (21) |
| Proven necrotizing enterocolitis6 [n (%)] | 20 (6) | 72 (11) | 54 (11) | 18 (13) |
| Patent ductus arteriosus (medical or surgical) [n (%)] | 123 (35) | 365 (57) | 271 (54) | 94 (69) |
Retinopathy of prematurity
p<0.05 for all comparisons of No ROP vs Any ROP (mild, moderate, or severe)
Tabulated until 120 days or discharge if discharged sooner, among infants who survived to discharge, transfer or 120 days
Interquartile range
Missing data for 1 infant
Modified Bell's stage II or III26
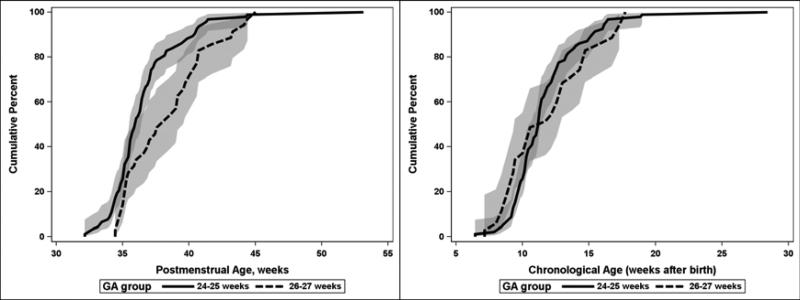
For infants who had any stage of ROP and had a known age of onset, the cumulative distribution for the age of onset is displayed in Table 3. Of note, 6.4 weeks was the minimum (youngest) chronologic age at which severe ROP was seen; 95% of cases had occurred by 17 weeks chronologic age. Also, 25% of severe ROP was identified after 38.6 weeks postmenstrual age and 5% was identified after 43.3 weeks. For the 9 infants with severe ROP and uncertain age of onset, the age of identification ranged from 33.7-40.0 weeks PMA. For the 64 infants who had an exam with Type II ROP prior to the first exam with Type I ROP (and known time of onset for both), the interval between these observations ranged from 0.1 to 17.9 weeks [median (IQR) 1.9 (0.9-3.0) weeks]. There were also 85 infants who had Type II ROP that regressed and 64 infants who developed Type I ROP without have a prior exam that met the criteria for Type II. The distributions for onset of ROP were examined separately (not shown) for infants in each of the treatment arms (lower oxygen saturation and higher oxygen saturation target ranges) and the distributions were similar so only the combined data are shown. The distributions for age of onset of severe ROP for each two-week interval of completed gestation at birth are shown in Figure 3. In contrast to prior studies,5 our data did not show an inverse relationship between gestational age at birth and chronological age at onset of treatable ROP. PMA of onset of severe ROP is significantly later for GA groups 26-27 weeks vs. 24-25 weeks (p<0.01). There is no significant difference in the distribution of chronologic age of onset between these two GA groups.
Table 3.
Postmenstrual and chronological age of onset1 [with 95% confidence intervals (CI2)] of any stage ROP3 (among infants with ROP age of onset determined)
| Cumulative Percent with Onset of ROP (by age of onset and ROP category) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROP type | n | Min4 | 1% | 5% | 25% | 50% | 75% | 95% | 99% | Max4 |
| Postmenstrual Age (weeks) | ||||||||||
| Any ROP (95%CI) | 634 | 29.3 | 30.4 (29.6-30.7) | 31.4 (31.1-31.4) | 32.7 (32.4-32.9) | 33.9 (33.7-34.0) | 35.1 (34.9-35.4) | 38.0 (37.3-38.7) | 41.0 (39.9-43.6) | 46.7 |
| Type 2 ROP5 (95%CI) | 158 | 29.3 | 29.7 (29.3-30.7) | 31.1 (30.6-31.7) | 34.3 (33.6-34.9) | 36.1 (35.7-36.9) | 38.1 (37.6-38.7) | 40.4 (39.9-43.7) | 46.4 (43.3-46.9) | 46.9 |
| Severe (Type 1/treated) ROP (95% CI) | 128 | 32.1 | 32.7 (32.1-32.7) | 33.9 (32.7-34.3) | 35.1 (34.7-35.4) | 36.4 (35.7-36.9) | 38.6 (37.4-40.0) | 43.3 (41.0-45.0) | 45.0 (44.4-53.1) | 53.1 |
| Cumulative Percent with Onset of ROP (by age of onset and ROP category) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROP type | n | Min | 1% | 5% | 25% | 50% | 75% | 95% | 99% | Max |
| Chronological Age (weeks) | ||||||||||
| Any ROP (95%CI) | 634 | 4.0 | 4.6 (4.1-4.7) | 5.4 (5.0-5.6) | 6.9 (6.6-6.9) | 8.0 (7.9-8.1) | 9.4 (9.1-9.6) | 11.9 (11.3-13.0) | 15.3 (14.4-18.0) | 19.7 |
| Type 2 ROP3 (95%CI) | 158 | 4.4 | 4.6 (4.4-5.6) | 6.3 (4.7-6.6) | 8.7 (7.9-9.6) | 10.8 (10.3-11.4) | 12.6 (12.0-13.1) | 15.0 (14.1-19.6) | 21.0 (17.0-22.7) | 22.7 |
| Severe (Type 1/treated) ROP (95% CI) | 128 | 6.4 | 7.1 (6.4-7.9) | 8.4 (7.1-8.9) | 9.8 (9.3-10.3) | 11.3 (10.6-11.7) | 13.1 (12.4-14.4) | 17.0 (16.1-19.0) | 19.0 (18.9-28.4) | 28.4 |
Age of onset is defined as the age at which the specified type of ROP was first observed while following the study monitoring protocol. For “Any ROP”, this is the first exam with any stage of ROP in any zone.
Confidence interval
Retinopathy of prematurity
Min = minimum age at which designated severity of ROP was identified; max = maximum age.
Type 2 ROP is defined as stage 3 in zone II, no plus disease or stage 1 or 2 in zone I, no plus disease. (85 of these infants had ROP that regressed and 73 infants later developed severe ROP.)
Figure 3.
Postmenstrual and chronological age of onset for severe (Type 1 or treated) ROP (among infants with age of onset determined) by gestational age at birth with 95% confidence intervals (shaded areas)
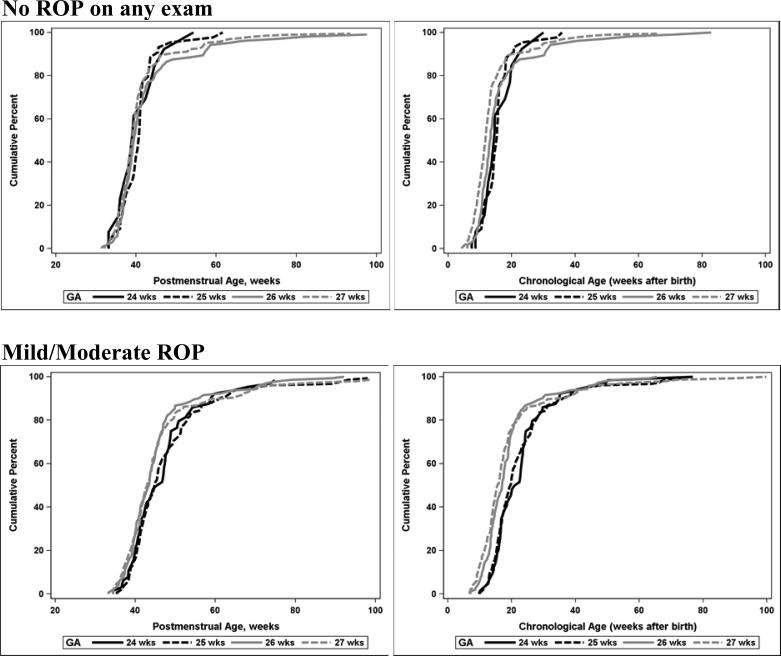
The age at which the retinal vessels matured to the point of minimal risk of progression to severe ROP (to the ora serrata or two consecutive exams with vessels in zone III without stage 3 or plus disease) is shown in Figure 4 for infants who never had ROP and for infants who had mild or moderate ROP (ROP that did not meet criteria for severe ROP). The cumulative distributions are shown by postmenstrual age and by chronological age, plotted separately for each completed week of gestation at birth. Among infants who had one exam with vessels recorded as in Zone III (but not to the ora serrata), 2/251 infants subsequently developed severe ROP. Retinal vessels reached final favorable status several weeks later in infants with mild or moderate ROP as compared to infants who never had ROP. The distributions of PMA and chronologic age at maturity were significantly different for infants with mild/moderate ROP vs. infants with no ROP, both overall and within GA groups (p< .0001).
Figure 4.
Postmenstrual and chronological age of “favorable outcome” (vessels to the ora serrata or vessels in Zone III on two consecutive exams) by gestational age at birth
The proportions of infants who had severe (Type 1 or treated) ROP identified after discharge home are shown in Table 4. Infants with severe ROP identified after discharge had onset of ROP at a later postmenstrual age and were discharged at an earlier postmenstrual age than infants who had severe ROP identified before discharge. In this referral center cohort of 997 infants, 1 infant (0.1%; or 0.7% of 137 infants with severe ROP) was diagnosed with severe ROP after back transfer to a lower acuity neonatal intensive care unit (while still in the hospital); 14 (1.4% of the cohort; or 10% of infants with severe ROP) reached severe ROP after discharge home. Among the 14 infants with severe ROP identified after discharge home, 4 had been transferred to lower acuity neonatal intensive care units prior to discharge home. To explore whether infants at high risk of developing severe ROP after discharge could be identified before discharge, we compared the last pre-discharge exams (Table 5) and clinical risk factors (Table 6) for infants who did and did not develop severe ROP after discharge (among infants whose exams had not reached final favorable status at the time of discharge). While infants with vessels in Zone I or with ROP in Zone II on the last pre-discharge exam were at the highest risk for developing severe ROP after discharge (1/4=25% and 10/206=5%, respectively), 1 case of severe ROP after discharge (1/82=1%) occurred in an infant with ROP in Zone III on the last exam before discharge. Infants who developed severe ROP after discharge were slightly lower birth weight and lower gestational age and treated with supplemental oxygen longer, but we did not identify any clinical risk factors in our data that clearly identify infants at risk to develop severe ROP after discharge.
Table 4.
Postmenstrual age of severe ROP1 onset and discharge for infants with severe ROP determined before and after discharge home
| Infants with Severe ROP N=137 | First exam with severe ROP occurred before discharge to home n=123 | First exam with severe ROP criteria occurred after discharge to home2 n=14 |
|---|---|---|
| Postmenstrual age at first occurrence of severe ROP: weeks [median, range] | 36.0 (32.1-45.0) | 40.9 (37.9-53.1) |
| Postmenstrual age at discharge: weeks [median, range] | 42.5 (37.7-78.3) | 38.3 (36.4-51.3) |
Retinopathy of prematurity
Among these 14 infants, 4 had been transferred back to lower acuity neonatal intensive care units prior to discharge home.
Table 5.
ROP1 exam (most recent) prior to discharge for infants with final ROP status determined after discharge home
| Worst findings in either or both eyes on last exam prior to discharge: | Severe ROP Group N=14 | No Severe ROP Group N=535 |
|---|---|---|
| Vessels in zone I [n (%)] | 1 | 3 |
| Lowest zone of vessels=II and any stage ROP in any zone [n (%)] | 10 | 196 |
| Lowest zone of vessels=II and no ROP [n (%)] | 2 | 126 |
| Lowest zone of vessels=III and any stage ROP in any zone [n (%)] | 1 | 81 |
| Lowest zone of vessels=III and no ROP [n (%)] | 0 | 121 |
| Plus disease [n (%)] | 0 | 0 |
| No exam prior to discharge [n (%)] | 0 | 3 |
| Unknown (missing or incomplete information on exam prior to discharge) [n (%)] | 0 | 5 |
Retinopathy of prematurity
Table 6.
Risk factors for ROP1 for infants with final ROP status determined after discharge home
| Risk Factor | Severe ROP Group N=14 | No Severe ROP Group N=535 |
|---|---|---|
| Birth weight, g [mean (SD)] | 701 (103) | 872 (185) |
| GA2 at birth, wks [mean (SD)] | 25.7 (0.9) | 26.4 (1.0) |
| Days on oxygen [mean (SD)] | 59 (27) | 47 (33)3 |
| Early onset sepsis [n (%)] | 0 | 10 (2) |
| Late onset sepsis [n (%)] | 7 (50) | 148 (28) |
| Fungal sepsis [n (%)] | 1 (7) | 12 (2)3 |
| Grade 3-4 intraventricular hemorrhage or periventricular leukomalacia [n (%)] | 0 | 59 (11.1)3 |
| Proven necrotizing enterocolitis [n (%)] | 1 (7) | 36 (7) |
| Patent ductus arteriosus [n (%)] | 11 (79) | 258 (48) |
| Discharge on oxygen [n (%)] | 2 (14) | 88 (16) |
Retinopathy of prematurity
Gestational age
N=534 (1 missing)
Discussion
Current screening guidelines are based on studies conducted over 20 years ago. Earlier treatment of ROP has been recommended since 2003,11 so updated information regarding the timing of onset of ROP is needed. While our study findings differ from previous studies5 in that the chronologic age of ROP onset was not later in lower GA infants, our findings still support the 2013 screening guidelines for infants 24-27 6/7 weeks gestation at birth.
In the CRYO-ROP natural history study,5 lower birth weight infants developed treatable ROP at a later chronological age than larger infants, such that the incidence curves for birth weight strata were superimposed when plotted by postmenstrual age. This observation led to a recommendation by the AAP/AAO/AAPOS that ROP screening could be delayed until 31 weeks postmenstrual age, regardless of gestational age at birth,1 albeit with a caution that the data supporting the recommendation included very few 22-23 week infants. This relationship (later postnatal onset in lower gestational age infants) was not apparent in our data. There are several potential explanations for this difference. Firstly, the gestational age range of infants in our study was relatively narrow because our cohort was selected by gestational age. The CRYO-ROP cohort was selected by birth weight (≤1250 g) and therefore included a wider gestational age range and a relatively high proportion (20%) of infants who were small for gestational age.22 Although both the CRYO-ROP and SUPPORT trials used obstetrical criteria, if available, for assigning gestational age, the more recent SUPPORT trial relied more heavily on early ultrasound criteria. If the CRYO-ROP trial more often used pediatric exam criteria, this could have resulted in a systematic overestimate of gestational age23 and in a systematic bias toward more stable lower risk infants having gestational age overestimated. In our data, age of onset was related to chronological age as well as PMA such that onset of severe ROP occurred at a slightly earlier postmenstrual age in more immature infants.
The more recent studies of the timing of onset of ROP have had inconsistent findings regarding the relationship of onset with chronologic vs postmenstrual age. In the study by Austeng et al,13 which included 22-26 week GA infants, the more immature infants developed ROP (any ROP) at an earlier PMA than more mature infants. The study by Isaza et al14 included 23-27 week infants; infants ≤25 weeks GA developed any ROP at the same mean PMA (later mean chronologic age) than infants >25 weeks. In this study, the onset of Type 1 ROP occurred at an earlier PMA and at an earlier chronologic age in the less mature infants. In our data, the median age of onset of Type 1 ROP (50% cumulative incidence in Figure 3) occurred at an earlier PMA in the less mature (24-25 week) infants, whereas the medians for chronologic age are similar.
For the purpose of screening (not missing cases of treatable ROP), the earliest and latest ages of onset of Type 1 ROP are more important than the mean or median age. We did not observe severe ROP before 6 weeks chronological age or before 32 weeks PMA. These findings are consistent with the other recent studies. In the Canadian study,14 the earliest onset of Type 1 ROP was 6 weeks chronological age or 32.7 weeks PMA. In the study by Muether et al15 that included 767 infants 22-35 weeks gestation, no infants required treatment before 8 weeks chronologic age or 33 weeks PMA. Together these studies provide no evidence that current screening guidelines should be changed to accommodate earlier (Type 1 ROP) treatment, although we still have limited data for 22-23 week GA infants.
For clinicians who care for infants in tertiary referral centers, an important question is whether infants are still at risk for treatable ROP when they are otherwise ready to be discharged to home. We have not identified any other studies that report the risk of treatable ROP occurring after discharge home. While it was not a common occurrence in our study (1.4% of the cohort and 14% of the infants with severe ROP), the potential consequences could be severe if infants who are still at risk for treatable ROP are lost to follow up after discharge. We were not able to identify any risk factors or combination of risk factors that would distinguish these infants from others who did not develop Type 1 ROP after discharge.
This observational study has several important limitations. We were unable to generate true population incidence data from this cohort because only consented inborn infants were included. This consented enrolled cohort differed from the non-enrolled populations in participating sites in that the proportion receiving antenatal steroids was higher and the proportion of Caucasians was higher.24 The SUPPORT trial inclusion criteria limit the generalizability of these data to infants < 24 weeks gestation who are at even higher risk of ROP or to infants >27 6/7 weeks. The ophthalmology exams for this study were performed by each unit's examining ophthalmologists according to AAP/AAO/AAPOS recommendations using the international classification of ROP but with no formal certification for the study. This might lead to more inconsistency or random error than would occur under strict study exam protocols, but it more closely reflects what typically occurs in clinical practice.
Current AAP/AAO/AAPOS screening guidelines, published in 2013,1 recommend that ROP screening should begin by 31 weeks postmenstrual age and continue until vessels have reached zone III for infants without previous zone I or II ROP, until full vascularization to the ora serrata for infants treated with bevacizumab, until 50 weeks postmenstrual age for infants without prethreshold ROP, or until ROP has regressed. In our cohort, the postmenstrual age at onset of severe ROP ranged from 32.1 to 53.1 wks, although only 1 infant developed severe ROP after 45 weeks. Our data therefore do not support a change in the 2013 screening guidelines. In this referral center cohort of 997 infants, 0.1% (0.7% of those with severe ROP) were diagnosed with severe ROP after back transfer to a lower acuity neonatal intensive care unit; 1.4% (10% of infants with severe ROP) reached severe ROP after discharge home. Post-discharge follow-up of infants who are still at risk for severe ROP is crucial for timely detection and treatment.
Future population-based studies are needed to better inform the optimal windows for ROP screening in extremely premature infants, particularly those less than 24 weeks and more than 27 weeks gestation at birth. These studies are difficult because they require strict adherence to screening protocols and careful documentation of all eye exams in a large number of infants to identify the full spectrum of age at onset. While randomized trials most often employ such rigorous data collection methods, they are often limited by selection bias that is introduced by the consent process for trials.24
Supplementary Material
Acknowledgments
Funding source: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network's SUPPORT trial.
Footnotes
Conflict of Interest: The authors declare that they have no financial interests related to the work described in this manuscript.
References
- 1.American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–576. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies A. Retinopathy of prematurity: Recommendations for screening. Paediatr Child Health. 2010;15:667–674. doi: 10.1093/pch/15.10.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, et al. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JD, Dobson V, Quinn GE, Fielder AR, Palmer EA, Saunders RA, et al. the CRYOROP and LIGHT-ROP Cooperative Study Groups. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–1476. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]
- 7.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity; preliminary results. Pediatrics. 1988;81:697–706. [PubMed] [Google Scholar]
- 8.Reynolds JD, Hardy RJ, Kennedy KA, Spencer R, van Heuven WA, Fielder AR. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) Cooperative Group. N Engl J Med. 1998;338:1572–1576. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 9.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. the NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Ob Gynecol. 2007;196:147, e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC. the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 12.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. the Early Treatment for Retinopathy of Prematurity Cooperative Group. The incidence and course of retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity Study. Pediatrics. 2005;116:15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 13.Austeng D, Kallen KB, Hellstrom A, Tornqvist K, Holmstrom GE. Natural history of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol. 2010;128:1289–1294. doi: 10.1001/archophthalmol.2010.234. [DOI] [PubMed] [Google Scholar]
- 14.Isaza G, Arora S. Incidence and severity of retinopathy of prematurity in extremely premature infants. Can J Ophthalmol. 2012;47:296–300. doi: 10.1016/j.jcjo.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Muether PS, Kribs A, Hahn M, Schumacher J, Eifinger F, Kirchhof B, et al. No advanced retinopathy of prematurity stages 4 or 5 in a large high-risk German cohort. Br J Ophthalmol. 2012;96:400–404. doi: 10.1136/bjo.2011.203125. [DOI] [PubMed] [Google Scholar]
- 16.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Target ranges of oxygen saturation in extremely preterm infants. New Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics An international classification of retinopathy of prematurity. Pediatrics. 1984;74:127–133. [PubMed] [Google Scholar]
- 18.Hahn GJ, Meeker WQ. Statistical Intervals: A Guide for Practitioners. John Wiley & Sons; 1991. [Google Scholar]
- 19.Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989-1997. Pediatrics. 1999;104:e26–33. doi: 10.1542/peds.104.3.e26. [DOI] [PubMed] [Google Scholar]
- 20.Liu PM, Fang PC, Huang CB, Kou HK, Chung MY, Yang YH, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22:115–120. doi: 10.1055/s-2005-837276. [DOI] [PubMed] [Google Scholar]
- 21.Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. 2009;148:451–458. doi: 10.1016/j.ajo.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Phelps DL, Brown DR, Tung B, Cassady G, McClead RE, Purohit DM, et al. 28-day survival rates of 6676 neonates with birth weights of 1250 grams or less. Pediatrics. 1991;87:7–17. [PubMed] [Google Scholar]
- 23.Donovan EF, Tyson JE, Ehrenkranz RA, Verter J, Wright LL, Korones SB, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. J Pediatr. 1999;135:147–152. doi: 10.1016/s0022-3476(99)70015-6. [DOI] [PubMed] [Google Scholar]
- 24.Rich W, Finer NN, Gantz MG, Newman NS, Hensman AM, Hale EC, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129:480–484. doi: 10.1542/peds.2011-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 26.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.