Abstract
Rationale
Chronic treatment with dopamine (DA) receptor agonists and antagonists can differentially affect measures of DA D2/D3 receptor number and function, but the effects of chronic treatment with a partial D2/D3 receptor agonist are not clear.
Objective
We used a within-subjects design in male cynomolgus monkeys to determine the effects of repeated (17-day) treatment with the D2/D3 receptor partial agonist aripiprazole (ARI; 0.03 mg/kg and 0.1 mg/kg i.m.) on food-reinforced behavior (n=5) and on D2/D3 receptor availability as measured with positron emission tomography (PET; n=9).
Methods
Five monkeys responded under a fixed-ratio 50 schedule of food reinforcement and D2/D3 receptor availability was measured before and four days after ARI treatment using PET and the D2/D3 receptor-selective radioligand [18F]fluoroclebopride (FCP). Four additional monkeys were studied using [11C]raclopride and treated sequentially with each dose of ARI for 17 days.
Results
ARI decreased food-maintained responding with minimal evidence of tolerance. Repeated ARI administration increased FCP and raclopride distribution volume ratios (DVRs) in the caudate nucleus and putamen in most monkeys, but decreases were observed in monkeys with the highest baseline DVRs.
Conclusions
The results indicate that repeated treatment with a low efficacy DA receptor partial agonist produces effects on brain D2/D3 receptor availability that are qualitatively different from those of both high-efficacy receptor agonists and antagonists, and suggest that the observed individual differences in response to ARI treatment may reflect its partial agonist activity.
Keywords: PET imaging, behavior, dopamine receptors, monkeys
INTRODUCTION
Drugs that directly or indirectly interact with brain dopamine (DA) D2/D3 receptors have long been used in the treatment of a number of psychiatric and neurodegenerative disorders and are frequently abused (for reviews see Volkow et al. 1999 and Beaulieu and Gainetdinov 2011). Long-term exposure to such drugs in clinical situations can lead to neuroadaptations that may affect their therapeutic efficacy and/or abuse potential (Howell and Murnane 2011). Accordingly, brain imaging and autoradiographic studies in humans and laboratory animals have characterized changes in D2/D3 receptor systems that result from chronic treatment with DA receptor agonists and antagonists. Several studies using a variety of techniques, species, dopaminergic drugs and durations of treatment indicate that chronic exposure to indirect DA agonists or direct-acting D2/D3 receptor agonists can decrease the density, availability and/or function of D2/D3 receptors (Volkow et al. 1993; Ginovart et al. 1999; Linazasoro et al. 1999; Nader et al. 2002), whereas chronic receptor antagonist treatment leads to upregulation and increased D2/D3 availability (Burt et al. 1977; Muller and Seeman 1977; Lidow and Goldman-Rakic 1994; Silvestri et al. 2000; Ginovart et al. 2009). Chronic treatment with an antagonist typically results in decreases in operant responding and subsequent increased sensitivity to the behavioral effects of DA agonists (e.g. Howell and Byrd 1992). In our laboratory, we used PET imaging in nonhuman primates to demonstrate that chronic self-administration of the indirect DA receptor agonist cocaine decreased D2/D3 receptor availability (Nader et al. 2006) whereas chronic treatment with the D2/D3 receptor antagonist raclopride led to increases in receptor availability (Czoty et al. 2005).
A noteworthy feature of the studies described above is that, for the most part, they have examined the effects of drugs whose intrinsic efficacy is relatively high (i.e. full agonists) or zero (i.e. receptor antagonists). The clinical usefulness of DA receptor antagonists is lessened due to accompanying extrapyramidal motor side effects that decrease compliance and are thought to result from development of supersensitivity of postsynaptic D2/D3 receptors (Klawans and Rubovits 1972; Farde et al. 1992; Barnes and Edwards 1993; Gerlach 2002). On the other hand, full agonists tend to have abuse liability. To circumvent these limitations, the use of dopamine agonists with lowto- intermediate efficacy (i.e., “partial” agonists) has been proposed (Platt et al. 2002; Lieberman 2004; Tayarani-Binazir et al. 2010). The basis of this recommendation is the hypothesis that such drugs would display agonist-like activity in brain areas or at times when DA is hypofunctional, but would act as antagonists during a hyperdopaminergic state. It is not clear, however, how chronic treatment with D2/D3 receptor partial agonists would affect the function, density or availability of D2/D3 receptors.
The objective of the present study was to use a within-subjects design in nonhuman primates to determine whether repeated treatment with the D2/D3 receptor partial agonist aripiprazole (ARI), which is used clinically to treat schizophrenia and unipolar and bipolar depression (Swainston-Harrison and Perry 2004), alters D2/D3 receptor availability as determined with PET imaging. ARI has also been suggested as a potential pharmacotherapy for cocaine and alcohol abuse (Meini et al. 2011; Vergne and Anton 2010). Interestingly, the effects of chronic ARI may be different than those observed following acute administration (e.g. Lile et al. 2005, 2008; Stoops et al. 2007). In vitro and behavioral assays have characterized ARI as an extremely low-efficacy agonist (e.g. Fujikawa et al. 1996; Burris et al. 2002; Hirose et al. 2004; Heusler et al. 2006; Urban et al. 2007; Klewe et al. 2008), with effects on striatal D2/D3 receptors (Takahata et al. 2012; Kim et al. 2013). Thus, we hypothesized that chronic ARI treatment would increase D2/D3 receptor availability in a manner similar to chronic treatment with the D2/D3 antagonist raclopride (Czoty et al. 2005). In the present study, PET scans using the D2/D3 receptor radiotracers [18F]fluoroclebopride [18F]FCP and [11C]raclopride were conducted before and four days after 17 days of once-daily treatment with either 0.03 or 0.1 mg/kg ARI. In addition, in some monkeys the effects of ARI treatment on food-reinforced operant behavior were assessed to provide a behavioral baseline against which to compare effects on brain D2/D3 receptors.
MATERIALS AND METHODS
Subjects
Nine experimentally naive adult male cynomolgus monkeys (Macaca fascicularis) served as subjects. Each monkey lived individually in a 0.33-m3 stainless steel cage (Allentown Caging Co., Allentown, PA), and was fitted with a nylon collar (Primate Products, Redwood City, CA) to facilitate handling. Monkeys were fed daily (Purina monkey chow and fresh fruit) and had unlimited access to water. Body weights were maintained at approximately 95% of free-feeding weights. Animal housing and handling and all experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (2011) and were approved by the Animal Care and Use Committee of Wake Forest University.
Imaging procedures
Magnetic resonance imaging (MRI) scans were acquired for each monkey. Approximately 20 min prior to scanning, subjects were anesthetized with ketamine (10 mg/kg, i.m.) and transported to the MRI facility. Anesthesia was maintained during the scanning procedure with ketamine supplements when necessary. T1-weighted images of the entire brain were acquired with a 1.5-T GE Signa NR scanner (N=5) (GE Medical Systems) or a 3.0-T Siemens SKYRA scanner (N=4). Images were used to anatomically define regions of interest (ROI), including the caudate nucleus, putamen and cerebellum, for later registration with PET images.
Two groups of monkeys were studied. One group (N=5) was studied in operant chambers and PET studies were conducted with [18F]fluoroclebopride (FCP), while a second group (N=4) was scanned with [11C]raclopride. When Group 1 monkeys consistently earned all available reinforcers (see below) and after Group 2 monkeys had been in the lab for at least 6 months, a baseline PET scan was acquired using a GE Advance NXi PET scanner (~4.8 mm3 resolution) and the DA D2/D3 receptor radiotracers [18F]FCP (Mach et al. 1993a,b) or [11C]raclopride (Farde et al. 1985). Approximately 30 min prior to the scan, the monkey was anesthetized with ketamine (10 mg/kg, i.m., which does not affect FCP binding, Nader et al. 1999) and transported to the PET Center. Anesthesia was maintained during the scan by inhaled isoflurane (1.5%). The monkey was placed in the scanner and catheters were inserted into an external vein for tracer injection and fluid replacement throughout the study. Body temperature was maintained at 40° C and vital signs (heart rate, blood pressure, respiration rate and temperature) were monitored throughout the scanning procedure. A 5-min transmission scan was acquired in 2D mode. Next, the monkey received a bolus dose of FCP (3 – 5 mCi) or raclopride (6.5 – 8 mCi) and a 180- or 90-min dynamic acquisition scan was acquired for [18F]FCP and [11C]raclopride, respectively. For [18F]FCP, 26 frames were acquired over 3 hr (5×1 min, 5 × 2 min, 5 × 5 min, 8 × 10 min, 3 × 20 min) in 3D mode (i.e. septa retracted). For [11C]raclopride, 18 frames were acquired over 90 minutes (18 × 5 min) in 3D mode (i.e. septa retracted). Image reconstruction of 3D data was done using the 3D-reprojection method (Kinahan and Rogers 1989) with full quantitative corrections. Once scanning was complete, the transmission scan data were smoothed transaxially using a 4-mm Gaussian filter and segmented (Bettinardi 1999). Emission data were corrected for attenuation and reconstructed into 128 × 128 matrices using a Hanning filter with a 4-mm cutoff transaxially and a ramp filter with an 8.5-mm cutoff axially. Data analysis including image registration was conducted using PMOD Biomedical Image Quantification Software (version 3.1; PMOD Technologies, Zurich, Switzerland). Distribution volume ratios (DVRs) for the caudate nucleus and putamen were calculated by implementing the “Logan method” of analysis in PMOD (Logan et al. 1996) using the cerebellum as our reference region. Monkeys were not studied in behavioral experiments on the day of a PET scan.
Monkeys were scanned at baseline and again 4 days after the last ARI treatment. Behavioral time course data indicate that 0.1 mg/kg ARI has no behavioral effects 18 hrs after administration (Czoty and Nader 2013); thus a 4-day washout from ARI was chosen prior to the PET study to allow sufficient time to assure that ARI was not occupying D2/D3 receptors during the scan. For monkeys studied with [18F]FCP, this was following either 0.03 mg/kg (N=2) or 0.1 mg/kg (N=3) ARI. Monkeys studied with [11C]raclopride were scanned after 17 days of 0.03 mg/kg ARI and after 17 additional days of treatment with 0.1 mg/kg ARI.
Behavioral procedures
For five monkeys, behavioral experiments were conducted 5 days per week. Each monkey was seated in a primate chair and placed into a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, East Fairfield, VT) equipped with a retractable response lever (5 cm wide) positioned within easy reach of the monkey. Three small stimulus lights located horizontally 14 cm above the lever provided information to the monkey regarding the schedule of reinforcement. Adjacent to the lever was a food receptacle with an attachment site for tygon tubing which connected to a feeder located on the top of the chamber for delivery of 1-g food pellets. Monkeys were trained to respond on the lever under a 50-response fixed-ratio (FR 50) schedule of food (1-g banana-flavored pellet) presentation. Daily sessions lasted until 60 min had elapsed or 30 reinforcers had been earned.
Once rates of responding had stabilized and a baseline [18F]FCP PET scan had been conducted, monkeys received an i.m. injection of either 0.03 (n=2) or 0.1 (n=3) mg/kg ARI 30 minutes before the start of a behavioral session. These doses were based on initial dose-finding experiments in which these doses moderately decreased response rates when administered acutely (data not shown). Monkeys were treated with the same dose of ARI for 17 days. A follow-up PET scan was conducted four days after ARI administration was terminated.
Drugs
ARI was obtained from Toronto Research Chemicals, Inc (Ontario, Canada) and was dissolved in a vehicle of 4% Tween 80 in sterile water up to a concentration of 1.0 mg/ml.
Data Analysis
Behavioral data are shown as mean session response rates (response/second) for baseline and each day of ARI treatment (Figure 1). For both [18F]FCP and [11C]raclopride PET studies, DVRs in the caudate nucleus and putamen were not different between left and right sides, so mean data for each monkey are presented in Figure 2. In order to compare between studies using different PET cameras, mean values for the caudate nucleus and putamen were calculated and referred to as the striatum (Tables 1 and 2).
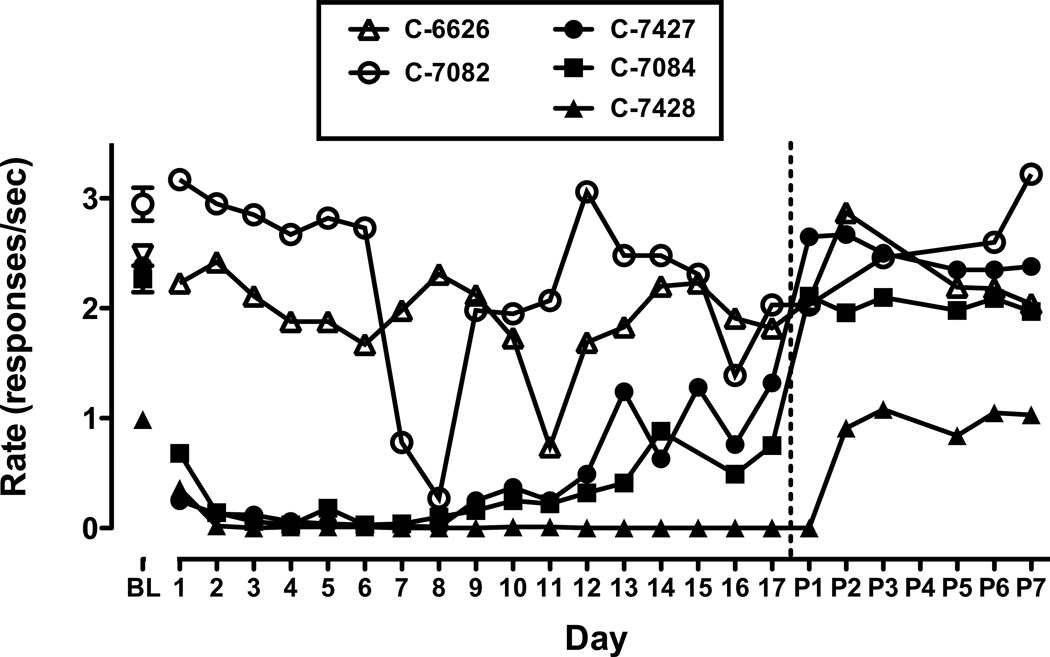
Figure 1.
Mean ± SD response rates (responses per second) for sessions before (baseline; BL), during 17 days of aripiprazole treatment (0.03 or 0.1 mg/kg per day) and after treatment (P1–P7) for each monkey. Vertical dotted line indicates termination of aripiprazole treatment. Open symbols represent data from monkeys treated with 0.03 mg/kg ARI and filled symbols represent data for 0.1 mg/kg ARI. PET scans were conducted on P4.
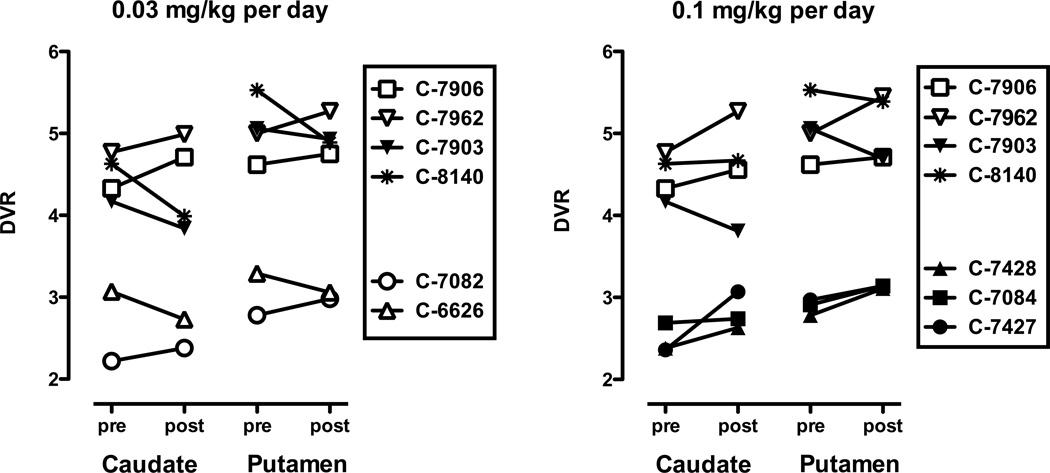
Figure 2.
Individual subject data for the effects of 17 days of 0.03 mg/kg (left panel) or 0.1 mg/kg (right panel) aripiprazole on DA D2/D3 receptor availability as measured with [18F]FCP DVR.
Table 1.
Effects of chronic aripiprazole on D2 receptors in the striatumf.
| FCP DVR | Raclopride DVR | |||||
|---|---|---|---|---|---|---|
| Monkey | Pre¶ | Post§ | Monkey | Pre¶ | Post1 | Post2 |
| C-7082 | 2.50 | 2.68 (+7.2%)1 | C-7906 | 4.48 | 4.73 (+5.7) | 4.63 (+3.5) |
| C-7428 | 2.58 | 2.87 (+11.2%)2 | C-7903 | 4.61 | 4.39 (−4.9) | 4.25 (−7.8) |
| C-7427 | 2.67 | 3.11 (+16.5%)2 | C-7962 | 4.88 | 5.13 (+5.0) | 5.36 (+9.8) |
| C-7084 | 2.80 | 2.94 (+5.0%)2 | C-8140 | 5.08 | 4.44 (−12.6) | 5.03 (−1.1) |
| C-6626 | 3.18 | 2.90 (−9.0%)1 | ||||
| Mean | 2.75 | 4.76 | ||||
| SEM | 0.13 | 0.16 | ||||
Striatum represents the mean DVRs from the caudate nucleus and putamen (see Fig. 2)
Monkeys are listed from lowest to highest baseline (Pre) DVRs
Numbers in parenthesis represent % change = [(Post DVR-Pre DVR)/Pre DVR]×100
Dose of aripiprazole was 0.03 mg/kg
dose of aripiprazole was 0.1 mg/kg
Table 2.
Effects of raclopride and cocaine on [18F]FCP DVRs in Striatum of Monkeys
| Monkey | Pre | Post (% change) |
|---|---|---|
| Chronic raclopride (1 month)¶ | ||
| C-7082 | 2.18 | 2.51 (+14.91) |
| C-7079 | 2.84 | 3.17 (+11.82) |
| C-7081 | 2.93 | 3.55 (+21.16) |
| Chronic cocaine (1 month)§ | ||
| R-1276 | 2.15 | 1.71 (−20.47) |
| R-1289 | 2.35 | 1.79 (−23.83) |
| R-1247 | 2.52 | 1.99 (−21.03) |
| R-1277 | 2.54 | 2.54 (0.0) |
| R-1284 | 2.63 | 2.30 (−12.55) |
From Czoty et al. (2005)
From Nader et al. (2006)
RESULTS
Food-maintained responding
Under baseline conditions, monkeys consistently made the maximum number of responses in each behavioral session and earned the maximum available reinforcers. In the two monkeys who received 0.03 mg/kg ARI, baseline ± SD rates of responding (i.e., total number of responses/total session time, averaged across the three days prior to the start of ARI treatment) were 2.95 ± 0.26 and 2.49 ± 0.07 responses/sec (Fig. 1, open symbols) while baseline response rates for the three monkeys who received 0.1 mg/kg ARI were 0.99 ± 0.04, 2.27 ± 0.21 and 2.39 ± 0.11 responses/sec (Fig. 1, closed symbols). Repeated daily treatment with 0.03 mg/kg ARI resulted in a gradual reduction in response rates over the first 8 days; rates remained below baseline across the remaining 9 sessions (Fig. 1, open symbols). Daily treatment with 0.1 mg/kg ARI almost completely eliminated responding and reduced the number of food pellets received to near zero in all three monkeys (Fig. 1, closed symbols). A modest degree of tolerance to these effects was observed, but tolerance was not complete by the time treatment was terminated. Although pronounced decreases in food-reinforced responding were observed, no noticeable unconditioned behaviors (e.g. ataxia or bradykinesia) were induced by repeated ARI exposure. By the second day after ARI treatment had been terminated, response rates had returned to baseline levels in all monkeys.
D2/D3 receptor availability
Baseline [18F]FCP DVRs in the caudate nucleus ranged from 2.22 to 3.07 and putamen DVRs ranged from 2.78 to 3.29 (Fig. 2). Baseline [11C]raclopride DVRs in the caudate nucleus ranged from 4.17 to 4.77 and putamen DVRs ranged from 4.62 to 5.53 (Fig. 2). While the absolute values for the DVRs are approximately 1.5 times higher for [11C]raclopride compared to [18F]FCP, the relative numbers appear to be equally distributed between the two groups of monkeys. For both radiotracers, repeated aripiprazole treatment affected DVRs in both brain regions in a similar manner (Fig. 2). That is, if repeated ARI resulted in increases in caudate nucleus DVR in a monkey, it most likely also increased putamen DVR (Fig. 2). Striatal DVRs (mean from caudate nucleus and putamen) were calculated for each monkey (Table 1). Although not statistically significant, repeated ARI tended to increase striatal DVRs in monkeys that had relatively low baseline measures prior to initiating treatment and decreased striatal DVRs in monkeys with high baseline DVRs (Table 1). Of the nine monkeys studied, monkeys C-7903 and C-7962 were the exceptions.
DISCUSSION
The primary aim of the present study was to determine the effects of daily treatment with aripiprazole (ARI), a low-efficacy D2/D3 receptor agonist, on D2/D3 receptor availability. Concurrent effects of aripiprazole on schedule-controlled behavior were assessed in one group of monkeys and the influence of repeated ARI on D2/D3 receptor availability was determined using two different PET radiotracers. Baseline D2/D3 receptor measures were higher with [11C]raclopride compared with [18F]FCP and for both tracers the values were higher in the putamen compared to the caudate nucleus. Repeated ARI decreased food-maintained responding, with little tolerance developing to this effect over the 17-day treatment period. At both doses of ARI, there was evidence of increases and decreases in D2/D3 receptor availability with both radiotracers. The relationship between changes in D2/D3 receptor availability following repeated ARI appeared to depend on baseline D2/D3 receptor measures. That is, in general repeated ARI decreased D2/D3 receptor availability in monkeys with the highest baseline values and increased D2/D3 receptor availability in monkeys with lower baseline values, although a larger sample size would be needed to make definitive conclusions in this regard.
Of particular interest was to compare the effects of the partial agonist ARI on D2/D3 receptor availability to the known effects of repeated treatment with indirect-acting DA agonists and D2/D3 receptor antagonists. Previous studies using several techniques and species, including PET imaging in monkeys, have shown that chronic treatment with indirect or high-efficacy direct agonists decreases D2/D3 receptor availability, whereas chronic receptor antagonist treatment increases D2/D3 receptor availability (e.g. Czoty et al. 2005; Nader et al. 2006). Because ARI has been shown to be an agonist with very low efficacy (e.g. Semba et al. 1995; Klewe et al. 2008), we hypothesized that the effects of repeated ARI treatment would be similar to those previously observed with the D2/D3 receptor antagonist raclopride (Czoty et al. 2005). In order to better compare results of the present study with earlier research, mean striatal [18F]FCP DVRs were calculated in monkeys treated chronically with the DA D2/D3 receptor antagonist raclopride (Czoty et al. 2005) and the indirect-acting DA agonist cocaine (Nader et al. 2006); these values and percent changes are shown in Table 2. Irrespective of baseline DVR, chronic raclopride increased DVRs (Table 2). Chronic administration (in this case, self-administration of cocaine) resulted in uniform effects and were not dependent on baseline DVR – only decreases were observed (Table 2). On the whole, the effects of repeated ARI on D2 receptor availability were distinct from high-efficacy agonists and antagonists which, in previous studies (see Table 2) decreased or increased D2 receptor availability, respectively, in nearly all monkeys studied. In the present experiments, the effects of ARI on D2 receptor availability were less uniform and generally appeared to depend on baseline DVRs in striatal areas, perhaps reflecting the partial agonist activity of the drug.
Daily ARI administration decreased food-maintained responding in a manner that was related to dose and duration of treatment. Although some measure of tolerance developed in the monkey who was more profoundly affected (C-7082), average response rates had not recovered fully to baseline at the end of the 17-day treatment period. The modest amount of tolerance observed here is similar to the lack of tolerance observed after 5 days of ARI treatment in rats self-administering food and cocaine under a choice procedure (Thomsen et al. 2008), although tolerance to the effects of ARI on the number of cocaine injections earned was observed. Taken together, the behavioral data suggest that 0.03 mg/kg ARI represents a moderately effective ARI dose whereas 0.1 mg/kg should be considered a high dose. In contrast to the effects of ARI observed in the present study, chronic treatment with the D2/D3 antagonist raclopride produced decreases in responding that were intermediate in magnitude to those of the two ARI doses used in the present study, and tolerance developed more quickly and was more complete (Czoty et al. 2005).
A goal of the present study was to extend the characterization of dopaminergic compounds on D2/D3 receptor availability to another radiotracer, [11C]raclopride. The binding profile of raclopride and FCP are very similar, with affinities at D2 and D3 receptor subtypes of 1.8 and 3.5 nM for raclopride (Sokoloff et al. 1990) and 0.95 and 5.46 nM for FCP (Mach et al. 1993a,b). A particular advantage of extending our characterization to [11C]raclopride is the extensive literature in human and animal studies using this radiotracer. There are many factors that influence the final measure of receptor availability (see Logan et al. 1996), and it is not appropriate to directly compare different radiotracers in terms of the absolute values for the DVRs. One limitation of the present study was that we did not scan the same monkey with both [11C]raclopride and [18F]FCP.
The individual differences in the effects of ARI on D2/D3 DVR observed in the present study may result from the intermediate efficacy of ARI as opposed to the more consistent effects of full agonists and antagonists observed in previous studies (see Takahata et al. 2012). A partial agonist would be expected to have different effects based on factors such as endogenous dopaminergic tone and D2/D3 receptor density. In PET imaging studies, increases in DVR after some manipulation is performed could be due to changes in either of these variables. Thus, it is possible that repeated ARI treatment resulted in changes in D2/D3 receptor densities, endogenous DA tone, or both. In addition, partial agonist activity of ARI at serotonin 5-HT1A receptors is thought to play a role in the antipsychotic efficacy of ARI (e.g. Shapiro et al. 2003; Koener et al. 2011; Tanahashi et al. 2012). Further studies are needed to determine the extent to which the alteration in D2/D3 receptor measures that occurs during repeated ARI treatment is mediated by 5-HT1A receptors or other receptors.
Importantly, the present results highlight the feasibility of using PET to document druginduced changes in receptor availability using a within-subject design. Assessment of such substrates before and during treatment with a pharmacological agent may help to predict and direct pharmacotherapy. For example, Martinez et al. (2011) found a relationship between D2/D3 receptor availability and treatment success in a group of cocaine abusers. In a recent preclinical study, it was noted that the effects of repeated ARI on cocaine self-administration were influenced by the social rank of the monkey (Czoty and Nader 2013). The present findings suggest that discrete neurobiological substrates may underlie differences in treatment effectiveness, and caution that the effects of partial agonists may not be identical across patients or subjects in preclinical studies.
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Drug Abuse (DA 10584, DA 12460 and DA 14637). The authors thank Michael Coller, Michelle Icenhower Bell and Michael Bounds for their assistance in conducting these experiments.
REFERENCES
- Barnes TR, Edwards JG. The side-effects of antipsychotic drugs. I. CNS and Neuromuscular effects. Antipsychotic drugs and Their Side-Effects. In: Barnes TRE, editor. Neuroscience Perspectives. Academic Press, Harcourt Brace & Company, Publishers; 1993. pp. 213–247. [Google Scholar]
- Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bettinardi V. An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med. 1999;26:447–458. doi: 10.1007/s002590050410. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196:326–328. doi: 10.1126/science.847477. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse. 2005;58:215–219. doi: 10.1002/syn.20200. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. J Pharmacol Exp Ther. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedstrom C-G, Litton J-E, Sedvall G. Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci. 1985;82:3863–3867. doi: 10.1073/pnas.82.11.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Fujikawa M, Nagashima M, Inoue T, Yamada K, Furukawa T. Partial agonistic effects of OPC-14597, a potential antipsychotic agent, on yawning behavior in rats. Pharmacol Biochem Behav. 1996;53:903–909. doi: 10.1016/0091-3057(95)02096-9. [DOI] [PubMed] [Google Scholar]
- Gerlach J. Improving outcome in schizophrenia: the potential importance of EPS and neuroleptic dysphoria. Ann Clin Psychiatry. 2002;14:47–57. doi: 10.1023/a:1015276028425. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Farde L, Halldin C, Swahn CG. Changes in striatal D2-receptor density following chronic treatment with amphetamine as assessed with PET in nonhuman primates. Synapse. 1999;31:154–162. doi: 10.1002/(SICI)1098-2396(199902)31:2<154::AID-SYN9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Hussey D, Houle S, Kapur S. D2-receptor upregulation is dependent upon temporal course of D2-occupancyL a longitudinal [11C]-raclopride PET study in cats. Neuropsychopharmacology. 2009;34:662–671. doi: 10.1038/npp.2008.116. [DOI] [PubMed] [Google Scholar]
- Heusler P, Newman-Tancredi A, Castro-Fernandez A, Cussac D. Differential agonist and antagonist profile of antipsychotics at D2L receptors coupled to GIRK potassium channels. Neuropharmacology. 2007;52:1106–1113. doi: 10.1016/j.neuropharm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Hirose T, Uwahodo Y, Yamada S, Miwa T, Kitagawa H, Burris KD, Altar CA, Nabeshima T. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J Psychopharmacol. 2004;18:375–383. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Enhanced sensitivity to the behavioral effects of cocaine after chronic administration of D2-selective dopamine antagonists in the squirrel monkey. J Pharmacol Exp Ther. 1992;262:907–915. [PubMed] [Google Scholar]
- Howell LL, Murnane KS. Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther. 2011;337:324–334. doi: 10.1124/jpet.108.136689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Howes OD, Turkheimer FE, Kim B-H, Jeong JM, Kim JW, Lee JS, Jang I-J, Shin S-G, Kapur S, Kwon JS. The relationship between antipsychotic D2 occupancy and changes in frontal metabolism and working memory: A dual [11C]raclopride and [18F]FDG imaging study with aripiprazole. Psychopharmacology. 2013;227:221–229. doi: 10.1007/s00213-012-2953-0. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. Analytic 3-D image reconstruction using all detected events. IEEE. Trans Nucl Sci. 1989;36:964–968. [Google Scholar]
- Klawans HL, Jr, Rubovits R. An experimental model of tardive dyskinesia. J Neural Transm. 1972;33:235–246. doi: 10.1007/BF01245320. [DOI] [PubMed] [Google Scholar]
- Klewe IV, Nielsen SM, Tarpo L, Urizar E, Dipace C, Javitch JA, Gether U, Egebjerg J, Christensen KV. Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into antipsychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology. 2008;54:1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koener B, Goursaud S, Van De Stadt M, Calas A-G, Jeanjean AP, Maloteaux J-M, Hermans E. Pharmacological blockade of dopamine D2 receptors by aripiprazole is not associated with striatal sensitization. Naunyn-Schmied Arch Pharmacol. 2011;383:65–77. doi: 10.1007/s00210-010-0577-7. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci USA. 1994;91:4353–4356. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18:251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Hays LR, Rush CR. The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance II: increased aripiprazole dose and maintenance period. Am J Drug Alcohol Abuse. 2008;34:721–729. doi: 10.1080/00952990802308262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus effects of d-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Linazasoro G, Obeso JA, Gomez JC, Martinez M, Antonini A, Leenders KL. Modification of dopamine D2 receptor activity by pergolide in Parkinson’s disease: an in vivo study by PET. Clin Neuropharmacol. 1999;22:277–280. [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time- activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Mach RH, Elder ST, Morton TE, Nowak PA, Evora PH, Scripko JG, Luedtke RR, Unsworth CD, Filtz T, Rao AV, et al. The use of [18F]4-fluorobenzyl iodide (FBI) in PET radiotracer synthesis: model alkylation studies and its application in the design of dopamine D1 and D2 receptor-based imaging agents. Nucl Med Biol. 1993a;20:777–794. doi: 10.1016/0969-8051(93)90165-q. [DOI] [PubMed] [Google Scholar]
- Mach RH, Luedtke RR, Unsworth CD, Boundy VA, Nowak PA, Scripko JG, Elder ST, Jackson JR, Hoffman PL, Evora PH, et al. 18F-labeled benzamides for studying the dopamine D2 receptor with positron emission tomography. J Med Chem. 1993b;36:3707–3720. doi: 10.1021/jm00075a028. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: Link between neurochemistry and response to treatment. Am J Psych. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, Simoni G, Ameglio M, Pellegrini M, Forgione RN, Rucci P. Safety, tolerability, and self-rated effects of aripiprazole and ropinirole treatment for cocaine dependence: a pilot study. Am J Addict. 2011;20:179–180. doi: 10.1111/j.1521-0391.2010.00106.x. [DOI] [PubMed] [Google Scholar]
- Muller P, Seeman P. Brain neurotransmitter receptors after long-term haloperidol: dopamine, acetylcholine, serotonin, alpha-noradrenergic and naloxone receptors. Life Sci. 1977;21:1751–1758. doi: 10.1016/0024-3205(77)90155-2. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Grant KA, Gage HD, Ehrenkaufer RL, Kaplan JR, Mach RH. PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuropsychopharmacology. 1999;21:589–596. doi: 10.1016/S0893-133X(98)00101-8. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Semba J, Watanabe A, Kito S, Toru M. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34:785–791. doi: 10.1016/0028-3908(95)00059-f. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Silvestri S, Seeman MV, Negrete J-C, Houle S, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology. 2000;152:174–180. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature (Lond) 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. Am J Drug Alcohol Abuse. 2007;33:769–776. doi: 10.1080/00952990701651556. [DOI] [PubMed] [Google Scholar]
- Swainston-Harrison T, Perry CM. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64:1715–1736. doi: 10.2165/00003495-200464150-00010. [DOI] [PubMed] [Google Scholar]
- Takahata K, Ito H, Takano H, Arakawa R, Fujiwara H, Kimura Y, Kodaka F, Sasaki T, Nogami T, Suzuki M, Nagashima T, Shimada H, Kato M, Mimura M, Suhara T. Striatal and extrastriatal dopamine D2 receptor occupancy by the partial agonist antipsychotic drug aripiprazole in the human brain: a positron emission tomography study with [11C]raclopride and [11C]FLB457. Psychopharmacology. 2012;222:165–172. doi: 10.1007/s00213-011-2633-5. [DOI] [PubMed] [Google Scholar]
- Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Dopamine D2 and serotonin1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology. 2012;62:765–744. doi: 10.1016/j.neuropharm.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Tayarani-Binazir K, Jackson MJ, Rose S, McCreary AC, Jenner P. The partial dopamine agonist pardoprunox (SLV308) administered in combination with l-dopa improves efficacy and decreases dyskinesia in MPTP treated common marmosets. Exp Neurol. 2010;226:320–327. doi: 10.1016/j.expneurol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DPD, Wortwein G, Sager TN, Holm R, Pepe LM, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine selfadministration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–54. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropharmacology. 2007;32:1106–1113. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- Vergne DE, Anton RF. Aripiprazole: a drug with a novel mechanism of action and possible efficacy for alcohol dependence. CNS Neurol Disord Drug Targets. 2010;9:50–54. doi: 10.2174/187152710790966731. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J. Comp Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]