Abstract
The monocytic leukemic zinc-finger (MOZ) histone acetyltransferase (HAT) acetylates free histones H3, H4, H2A, and H2B in vitro and is associated with up-regulation of gene transcription. The MOZ HAT functions as a quaternary complex with the bromodomain-PHD finger protein 1 (BRPF1), inhibitor of growth 5 (ING5), and hEaf6 subunits. BRPF1 links the MOZ catalytic subunit to the ING5 and hEaf6 subunits, thereby promoting MOZ HAT activity. Human BRPF1 contains multiple effector domains with known roles in gene transcription, and chromatin binding and remodeling. However, the biological function of the BRPF1 bromodomain remains unknown. Our findings reveal novel interactions of the BRPF1 bromodomain with multiple acetyllysine residues on the N-terminus of histones, and show it preferentially selects for H2AK5ac, H4K12ac and H3K14ac. We used chemical shift perturbation data from NMR titration experiments to map the BRPF1 bromodomain ligand binding pocket and identified key residues responsible for coordination of the post-translationally modified histones. Extensive molecular dynamics simulations were used to generate structural models of bromodomain-histone ligand complexes, to analyze H-bonding and other interactions, and to calculate the binding free energies. Our results outline the molecular mechanism driving binding specificity of the BRPF1 bromodomain for discrete acetyllysine residues on the N-terminal histone tails. Together these data provide insights on how histone recognition by the bromodomain directs the biological function of BRPF1, ultimately targeting the MOZ HAT complex to chromatin substrates.
Keywords: Epigenetics, histone acetyltransferase, nuclear magnetic resonance, isothermal titration calorimetry, molecular dynamic simulations, post translational modification, bromodomain
Introduction
Nucleosomes make up the core unit of chromatin necessary for packaging eukaryotic DNA into higher order and more compact structures. Each nucleosome is composed of four histone proteins (H3, H4, H2A and H2B), forming an octameric structure around which approximately 146 base pairs of superhelical DNA is wrapped1. The N-terminal histone tails protruding from the nucleosome core are the targets of many post-translational modifications (PTMs) including acetylation, methylation, phosphorylation, and ubiquitination, among others2. Collectively, these covalent modifications compose a histone code that is read by other proteins or protein modules to regulate a diverse array of cellular processes including gene transcription, DNA recombination and repair, cell cycle progression and genomic stability3-7. Coordination of DNA compaction, chromatin remodeling and gene expression by the histone code is a complex and dynamic process, and deregulation of the epigenetic machinery often leads to disease, most notably cancer.
The monocytic leukemic zinc-finger (MOZ) (also known as KAT6A) is a histone acetyltransferase (HAT) in the MYST family (MOZ/YBF2/SAS2/TIP60 homology domain) that acetylates free histones H3, H4, H2A, and H2B in vitro8-10. Acetylation of histones located near gene promoters is associated with up-regulation of gene transcription, and the acetylation activity of MOZ has been shown to control expression of homeobox (HOX) genes11. The MOZ HAT also plays a direct role in hematopoiesis and is essential for the development and maintenance of hematopoietic stem cells (HSCs)12. MOZ was first identified as a fusion partner with the CREB binding protein (CBP) HAT in a t(8;16)(p11;p13) translocation found in acute myeloid leukemia (AML), and disruption of the normal acetylation activity of MOZ leads to leukemogenic transformations and oncogenesis13. The MOZ HAT functions as a quaternary complex with the bromodomain-PHD finger protein 1 (BRPF1), inhibitor of growth 5 (ING5) and the human Esa1-associated factor 6 ortholog (hEaf6) subunits (Figure 1A)14. BRPF1 links the MOZ catalytic subunit to the ING5 and hEaf6 subunits in the MOZ HAT complex, thereby promoting its HAT activity14. Human BRPF1 contains multiple effector domains with known functions in gene transcription and chromatin binding and remodeling. These include a double plant homeodomain (PHD) and zinc finger (ZnF) assembly (PZP), a bromodomain, and a chromo/Tudor-related Pro-Trp-Trp-Pro (PWWP) domain (Figure 1B). The PWWP domain is necessary for the association of BRPF1 with condensed chromatin and is able to recognize H3K36me39,15. PHD fingers are a conserved C3HC4 zinc finger motif commonly found in nuclear proteins that regulate transcription and chromatin remodeling. Recently, the first PHD finger (PHD1) of BRPF2 was discovered to recognize the unmodified histone H3 tail, while the second PHD finger (PHD2) interacts non-specifically with DNA16,17. Both the PHD1 domain of BRPF2 and the PWWP domain of BRPF1 are important for targeting the BRPF subunit of MOZ to the HOXA9 gene locus in vivo via recognition of their respective histone PTMs15,16. Bromodomains, initially discovered as acetyllysine binding modules,18 have also been shown to play a crucial role in regulating the function of many transcription factors, chromatin remodeling and cell signaling19-21. More recently, they have emerged as exciting new therapeutic targets because of the development of highly selective and potent bromodomain inhibitors in the BET bromodomain family, and the presence bromodomains in a large number of proteins linked to disease22-25. However, the modifications recognized by the BRPF1 bromodomain, and the specific interactions driving its binding to N-terminal histone acetylation marks remains uncharacterized.
Figure 1.
Function of the BRPF1 bromodomain in the MOZ HAT complex. A. The MOZ HAT complex is a hetero-tetramer composed of the monocytic leukemic zinc-finger (MOZ) catalytic subunit, the bromodomain-PHD finger protein 1 (BRPF1), inhibitor of growth 5 (ING5) and hEaf6 subunits, which carries out acetylation of histones H3, H4, H2A and H2B. B. The BRPF1 subunit contains multiple chromatin reader domains including a double plant homeodomain (PHD) and zinc finger (ZnF) assembly (PZP), a bromodomain and a chromo/Tudor-related PWWP domain. C. The BRPF1 bromodomain binds to H3K14ac, H4K5ac, H4K8ac, H4K12ac and H2AK5ac in a histone peptide array.
In this study we used a combination of biochemical, biophysical and computational methods to characterize the histone binding targets of the BRPF1 bromodomain. Peptide array and nuclear magnetic resonance (NMR) techniques were utilized to identify histone ligands recognized by the BRPF1 bromodomain. The BRPF1 bromodomain binds to multiple acetylated histone peptides, and we show for the first time that these specifically include the H2AK5ac, H4K12ac, H4K8ac, H4K5ac and H3K14ac marks on the N-terminal tails. We measured the binding affinities of the acetylated histone ligands by NMR and isothermal titration calorimetry (ITC) techniques to show it preferentially selects for H2AK5ac, H4K12ac, and H3K14ac. We also used NMR chemical shift perturbation data to map the BRPF1 bromodomain binding pocket and identify key residues involved in the histone binding interaction. The experimental data were reconciled with existing structures using molecular dynamics (MD) simulations, which also provided H-bonding patterns and binding free energies of the BRPF1 bromodomain-histone complexes. Our results establish the intermolecular interactions that determine the binding specificity of the BRPF1 bromodomain for discrete acetyllysine residues on the N-terminal histone tails, and we propose a role for the BRPF1 bromodomain-histone interaction in targeting the MOZ HAT complex to chromatin during normal and disease processes. This information advances our understanding of how the BRPF1 bromodomain recognizes and selects for specific acetyllysine marks, which is important for the development of future therapeutics for AML and other diseases.
Results
The BRPF1 bromodomain recognizes histones H3, H4 and H2A
The BRPF1 subunit of the MOZ HAT contains a bromodomain (Figure 1B), which has previously been identified as an acetyllysine binding domain18,26. The phylogenetic tree of the human bromodomains reported by Filippakopoulos et al. indicates that the BRPF1 bromodomain falls into subfamily IV, which includes the bromodomains of the BRD1, BRD7, BRD9, BRPF1/3, KIAA1240 and ATAD2 proteins (Figure 2C)22,27. Within this subfamily, the histone binding activity of the BRD7 bromodomain has been functionally characterized, and it recognizes acetyllysine residues on the N-terminus of histones H3 and H428. To investigate the function of the bromodomain of BRPF1, we probed its histone binding ability using a peptide array designed to screen the protein domain against a large number of acetylated, methylated and phosphorylated histone tail ligands in a high-throughput assay. We found that the GST-BRPF1 bromodomain recognizes acetylated lysine residues on the N-terminal tails of histones H3, H4 and H2A. As seen from the illuminated red spots in the peptide array (Figure 1C), the BRPF1 bromodomain interacts strongly with histone peptides H4K12ac, and H2AK5ac but more weakly with H4K5ac, H4K8ac, and H3K14ac. Similar to other known bromodomains, the BRPF1 bromodomain has no interactions with methylated or phosphorylated histone modifications. Also, in peptides with double and triple modifications (seen in the bottom left panel of Figure 1C), adjacent methylation and/or acetylation PTMs do not appear to affect acetyllysine recognition by the BRPF1 bromodomain.
Figure 2.
Interaction of the BRPF1 bromodomain with acetylated histone ligands. A. 2D 1H-15N HSQC spectra of 15N-labelled BRPF1 bromodomain with the complete HSQC assignments labeled. B. Superimposed 1H-15N HSQC spectra of the 0.469 mM BRPF1 bromodomain, collected during titrating in the indicated histone peptides. The spectra are color-coded according to the protein/peptide ratio. C. A structure based multiple sequence alignment of the bromodomains in subfamily IV created with Expresso64. The locations of structural elements are indicated at the top of the alignment above the BRPF1 bromodomain sequence, which is used as the reference for amino acid numbering. Residues colored red had the largest chemical shift changes upon addition of H2AK5ac. The green boxes highlight residues in the BRPF1 bromodomain that are involved in hydrogen bond interactions with H2AK5ac, as observed in the MD simulation.
To confirm recognition of histone tail peptides seen in the array we further examined binding of the BRPF1 bromodomain by NMR spectroscopy. 1H-15N HSQC spectra of the uniformly 15N-labeled BRPF1 bromodomain were recorded in the absence and presence of the following histone tail peptides; unmodified H3 (residues 1-12), unmodified H4 (residues 4-17), H3K9ac, H3K14ac, H4K5ac, H4K8ac, H4K12ac, H2AK5ac, or acetylated lysine (Kac) (Table 1). Chemical shift perturbations were induced in the BRPF1 bromodomain upon addition of H2AK5ac, H3K14ac, H4K5ac, H4K8ac and H4K12ac (Figure 2B and Supplemental Figure S1). A lack of significant resonance shifts upon addition of Kac, unmodified H3, unmodified H4, or H3K9ac (not shown) peptides indicated that the bromodomain of BRPF1 only recognizes specific epigenetic marks within the context of the histone tail. Together these data reveal that the BRPF1 bromodomain recognizes specific acetylated lysine residues on the N-terminal tail of histones H3, H4 and H2A.
Table 1.
Sequences of the histone peptides studied, and the dissociation constants of their complexes with the BRPF1 bromodomain as measured by NMR titration experiments and ITC.
| Histone peptide | Sequence | NMR Kd (μM) | ITC Kd (μM) |
|---|---|---|---|
| H2A unmodified (1-12) | SGRG K QGGKARA | NA | NA |
| H2AK5ac (1-12) | SGRG Kac QGGKARA | 57 ± 4 | 48.5±1.5 |
| H3 unmodified (1-12) | ARTKQTARKSTG | no binding | NA |
| H3K9ac (1-12) | ARTKQTAR Kac STG | no binding | NA |
| H3 unmodified (8-19) | RKSTGG Kac APRKQ | NA | NA |
| H3K14ac (8-19) | RKSTGG Kac APRKQ | 597 ± 53 | 626±27 |
| H4 unmodified (4-17) | GKGGKGLGKGGAKR | no binding | NA |
| H4K5ac (4-17) | G Kac GGKGLGKGGAKR | 1236±327 | NA |
| H4K8ac (4-17) | GKGG Kac GLGKGGAKR | 697 ± 57 | NA |
| H4K12ac (4-17) | GKGGKGLG Kac GGAKR | 74 ± 18 | 86.5±9.1 |
| Kac | Kac | no binding | NA |
The BRPF1 bromodomain recognizes discrete acetyllysine modifications
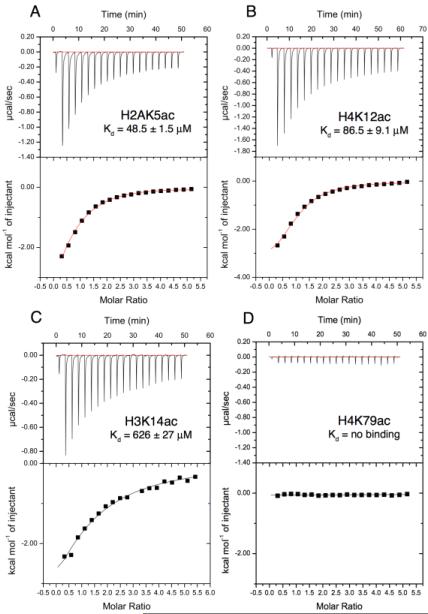
To measure the binding affinity of the BRPF1 bromodomain for the individual histone ligands, the 1H-15N HSQC spectra were recorded on NMR samples containing 15N-labelled BRPF1 bromodomain mixed with increasing concentrations of a histone tail peptide including H2AK5ac, H3K14ac, H4K5ac, H4K8ac, or H4K12ac. As described above, each of these five acetylated peptides induced significant chemical shift changes in the amide resonances of the BRPF1 bromodomain, indicating that they all bind to the bromodomain. Dissociation constants (Table 1) were obtained by fitting the titration curves of residues I27, E36, A84, and I88 upon ligand binding at each of six peptide concentrations to determine the normalized chemical shift change for each residue (Supplemental Figure S1, A-D). The Kd for each of these four residues was averaged to calculate the binding affinity of the histone peptide ligands. The binding dissociation constants ranged from the micro-molar to the low milli-molar, with H2AK5ac having the lowest Kd (57 μM) followed by H4K12ac (74 μM), H3K14ac (597 μM), H4K8ac (697 μM), and H4K5ac (1.24 mM). The dissociation constants of the BRPF1 bromodomain with the H2AK5ac, H4K12ac, and H3K14ac histone peptides were also analyzed by ITC, and the results are shown in Figure 3A-C. The Kd values determined from the ITC titration data, revealed that the H2AK5ac peptide bound to the BRPF1 bromodomain with the highest affinity (48.5 μM), followed by H4K12ac (86.5 μM), and H3K14ac (626 μM), consistent with the NMR binding data seen in Table 1. While this manuscript was in preparation, a report by Filippakopouos et al., found that the BRPF1 bromodomain could recognize histone acetylation marks in the C-terminal globular region of histones H2B, H3 and H4. In their SPOT array very strong interactions were observed with the H3K56ac, H4K44ac, H4K77ac, H4K79ac and H4K91ac histone peptides27. However, when we tested the interaction of the BRPF1 bromodomain with the H4K79ac peptide using ITC no binding interaction was seen (Figure 3D). This result highlights the importance of confirming histone recognition via multiple techniques as we have done in this study.
Figure 3.
ITC measurement of the interaction between the BRPF1 bromodomain and acetylated histone ligands. A-D. Exothermic ITC enthalpy plots for the binding of the BRPF1 bromodomain to H2AK5ac, H4K12ac, H3K14ac and H4K79ac. The calculated binding constants are indicated.
Our results suggest that the binding specificity of the BRPF1 bromodomain is dependent on both the presence of the acetyllysine PTM, and the context of the histone amino acid sequence where this epigenetic mark is located. As seen with other bromodomains, such as those found in BRD7, p300/CBP-associated factor (PCAF) and BRDT (a testis-specific member of the bromodomain and extra-terminal domain protein family), the binding specificity of the BRPF1 bromodomain is not strict, and it is able to recognize acetylated lysine at multiple positions, and on different histones in vitro18,28,29. Interestingly, unlike many bromodomains whose dissociation constants for acetylated histone peptides are in the milli-molar range, the affinity of the BRPF1 bromodomain for H2AK5ac and H4K12ac is in the low micro-molar range similar to the bromodomains of the polybromo-1 protein (Pb1), the transcription factor TAFII250, and the tripartite motif 24 (TRIM24) protein24,30,31.
Mapping the acetyllysine binding pocket of the BRPF1 bromodomain
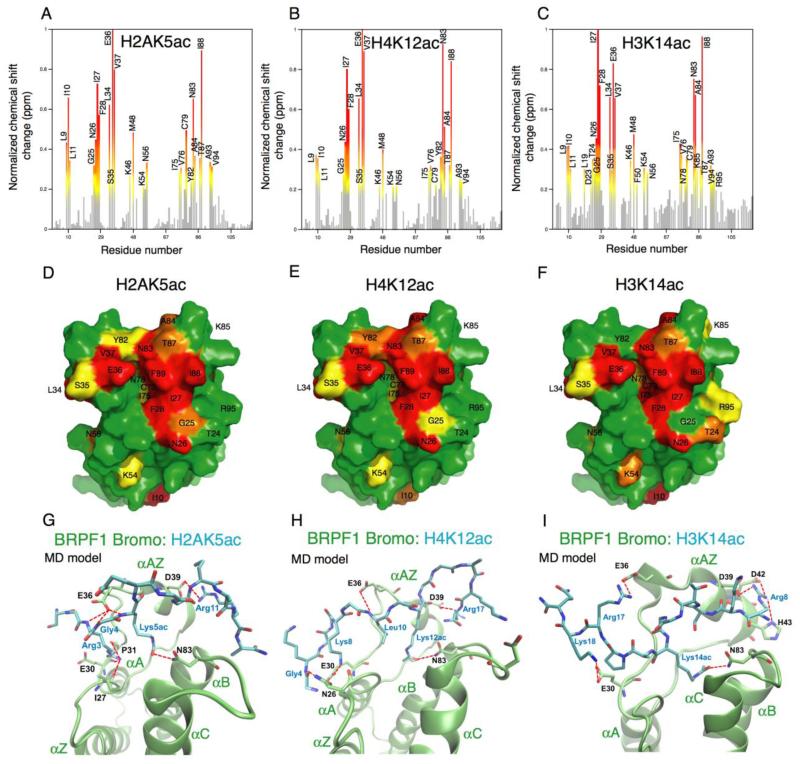
The acetyllysine binding pocket of the BRPF1 bromodomain was analyzed by plotting the NMR chemical-shift perturbations caused by addition of acetylated histone ligands H2AK5ac, H4K12ac, and H3K14ac. Normalized changes in chemical shift were plotted as a bar graph to show the amino acid residues most affected by addition of the respective histone tail peptide in a 1:6.1 ratio of the BRPF1 bromodomain:peptide. The most significant chemical shifts (perturbations greater than 0.4 ppm) are shown in red, while changes of > 0.3 ppm or > 0.2 ppm are shown in orange and yellow, respectively (Figure 4A-C). A total of 12 residues in the BRPF1 bromodomain showed large chemical shift changes upon addition of the H2AK5ac histone ligand including L9, I10, N26, I27, F28, L34, E36, V37, M48, C79, N83 and I88, indicating that these residues are directly or indirectly involved in histone binding. Mapping the positions of these residues onto the surface of the native BRPF1 bromodomain structure (PDB ID 2D9E, unpublished) reveals that the majority of residues with large chemical shift changes are clustered around the acetyllysine binding cavity (Figure 4D). Similar trends of chemical shift perturbations are seen upon addition of the H4K12ac and H3K14ac histone ligands to the BRPF1 bromodomain (Figure 4E-F). Notably, the residues showing the largest chemical shift changes (in red) are conserved among the three different histone ligands, but some differences are apparent in the chemical shift changes of residues that are located more peripherally to the binding pocket. Interestingly, some residues quite distal to the histone binding pocket demonstrated significant chemical shift changes, such as L9, I10, L11, K54 and N56. Closer inspection of the BRPF1 bromodomain structure indicated that these residues are located near the base of the αZ- and αA-helices, where these two helical regions interact. Thus, the chemical shift perturbation of these residues is likely due to conformational changes occurring in the helices in response to ligand binding.
Figure 4.
Identification of the H2AK5ac, H4K12ac and H3K14ac binding sites of the BRPF1 bromodomain, and models of the bromo-H2AK5ac, bromo-H4K12ac, and bromo-H3K14ac complexes. A-C. The histograms show normalized 1H-15N chemical shift changes in backbone amides of the BRPF1 bromodomain upon addition of the H2AK5ac (A), H4K12ac (B), and H3K14ac (C) peptides. Chemical shift changes from 0.2-0.3 ppm are colored yellow, while changes from 0.3-0.4 ppm, and changes greater than 0.4 ppm are colored in orange and red, respectively. D-F. Residues that had significant resonance perturbations upon addition of the acetylated histone ligand H2AK5ac (D), H4K12ac (E) and H3K14ac (F) are mapped onto the surface of the ligand-free BRPF1 bromodomain structure 2D9E. The residues in the binding pocket are labeled in red, orange and yellow depending on the magnitude of the chemical shift change upon ligand addition seen in A-C. G-I. MD models of the BRPF1 bromodomain in complex with H2AK5ac (G), H4K12ac (H) and H3K14ac (I) peptide ligands as obtained using the flexible docking program Flexpepdock followed by MD simulations. The BRPF1 bromodomain structure is shown in green while the peptide ligands are colored in blue. Hydrogen bonds and salt bridges are indicated by red dotted lines.
Molecular dynamics simulations of the bromodomain-histone peptide binding
In order to further understand the critical interactions governing coordination of acetylated histones by the BRPF1 bromodomain protein, molecular dynamic (MD) simulations were carried out for two forms of the studied peptides (Supplementary Table S1 and S2), except Kac. The results for ‘used’ peptides, which had an unmodified (NH3+) N-terminus and an amidated C-terminus, were generated for comparison with the experimental data. We also studied ‘capped’ peptides with an acetylated N-terminus and a methyl-amidated C-terminus in order to approximate the expected behavior of complete histone tails.
The initial structures of the acetylated histone peptides in complex with the BRPF1 bromodomain were obtained by flexible docking of each of the ten peptides listed in Table 1 to the bromodomain protein using the Flexpepdock program32,33 from Rosettacommons34. The MD simulations, which were run using the Amber 12 package35 for 10 ns under isobaric and isothermal conditions, explored the conformational space of the interacting peptides with the protein residues. The overall secondary structures (α helices) of the BRPF1 bromodomain were seen preserved throughout these simulations.
The MD simulation data were processed using an MM/PBSA formalism36, utilizing the normal-mode analysis to obtain vibrational entropy37. The resulting binding free energy values (ΔG) and their components are summarized for ‘used’ and ‘capped’ peptides in Tables S1 and S2, respectively, in the Supplementary Material. Table S1 also contains the experimental binding free energies for the histone peptides (exp. ΔG), which were calculated as ΔG=RTlnKd using the NMR Kd values in Table 1.
The relative energy profiles (Table S1) calculated for the histone peptides show good agreement with the NMR and ITC experimental data (Table 1). The use of vibrational entropy improved the computational predictions. The most favorable binding energies were observed for the H2AK5ac peptide, followed by the H4K12ac and the H3K14ac peptides, respectively. Although the absolute values of ΔG differ significantly, especially for the strong binders (H2AK5ac and H4K12a), the order of binding calculated for the ‘used’ peptides (for ΔG, H2AK5ac < H4K12ac < H3K14ac < H4K8ac < H4K5ac) was correctly predicted and matches the trend observed experimentally. Two of the three non-binding histone peptides were correctly identified (H3 unmodified residues 1-12, and H4 unmodified residues 4-17), and for the third non-binding peptide (H3K9ac residues 1-12), only a very weak binding was predicted. For the other ‘used’ unmodified histone peptides, H2A unmodified (1-12) and H3 unmodified (8-19), no affinity and a weak affinity were predicted, respectively (Table S1). Although there is no experimental data for these peptides, no binding is expected since the other unmodified peptides (H3 unmodified (1-12) and H4 unmodified (4-17) showed no interactions with the BRPF1 bromodomain in the NMR titration and/or ITC experiments.
The trajectories from each of our 10-ns MD simulations (a total of 5000 frames, each representing a 2 ps time interval) were analyzed for the presence of H-bond interactions between the BRPF1 bromodomain protein and the histone peptide residues. The geometries were quantitatively defined in terms of bond length and donor-H-acceptor bond angles. The longevities of the H-bond interactions38 were characterized as the percentage of frames, in which the H-bonds occur. The longevities and average characteristics of the H-bonds, which were present for at least 25% of time in the ‘used’ peptides are summarized in Table 2. The similarity of the H-bond patterns between the ‘used’ peptides, for which the binding was analyzed experimentally, and the ‘capped’ peptides, which better approximate the interactions of the intact histone tails, is conserved for all three peptides, which exhibit the strongest binding to the BRPF1 bromodomain, H2AK5ac, H4K12ac and H3K14ac (Table 2).
Table 2.
Summary of H-bond interactions between the strongest binding peptides and the BRPF1 bromodomain protein.
| Histone peptide | Interacting residues | ‘used’ peptidea | ‘capped’ peptideb | |||||
|---|---|---|---|---|---|---|---|---|
| peptide | bromo-domain | % presentd | average bond length (Å) | average bond angle (degree) | % presentd | average bond length (Å) | average bond angle (degree) | |
| H2AK5ac (1-12) |
Lys5ac | N83 | 99.5 | 2.897 | 156.25 | 72 | 2.838 | 157.05 |
| Lys5ac | E36 | 87.0 | 3.015 | 164.93 | 55 | 2.8714 | 163.27 | |
| Arg3 | E36 | 95.1 | 2.915 | 154.17 | 87 | 2.811 | 158.45 | |
| Gly4 | E36 | 93.8 | 2.908 | 151.07 | 79 | 2.834 | 157.57 | |
| Arg11/Lys9c | D39 | 99.9 | 2.792 | 162.03 | 12 | 2.802 | 157.35 | |
| Arg3 | I27 | 36.0 | 2.92 | 135.47 | 3 | 2.817 | 145.17 | |
| H4K12ac | Arg3 | P31 | 29.2 | 2.87 | 144.07 | 15 | 2.825 | 150.16 |
| Lys12ac | N83 | 98.7 | 2.87 | 149.13 | 77 | 2.824 | 156.46 | |
| Arg17 | D39 | 85.7 | 2.83 | 156.46 | 98 | 2.79 | 160.22 | |
| Gly4/Gly7c | N26 | 52.1 | 2.953 | 158.51 | 33 | 3.00 | 153.22 | |
| Lys8 | E30 | 50.7 | 2.81 | 152.52 | 17 | 2.863 | 163.52 | |
| Leu10/Lys16c | E36 | 25.1 | 3.03 | 152.96 | 4 | 2.99 | 146.74 | |
| H3K14ac | Lys14ac | N83 | 98.9 | 2.825 | 149.62 | 96 | 2.9 | 155.03 |
| Arg8 | D39 | 72 | 2.936 | 158.89 | 66 | 2.9187 | 151.09 | |
| Arg17 | E36/E30 | 70 | 2.936 | 151.82 | 74 | 2.879 | 152.52 | |
| Arg8/Lys9c | D42 | 56 | 2.842 | 157.03 | 2 | 2.825 | 151.08 | |
| Arg8 | H43 | 31 | 3.02 | 150.65 | 9 | 3.12 | 139.76 | |
| Lys18 | E30 | 29 | 2.865 | 151.47 | 20 | 2.826 | 156.04 | |
NH3+-peptide-CONH2
CH3CONH-peptide-CONHCH3
indicates the interaction seen in either the used/capped peptides
% of frames, out of 5000 analyzed frames, where the interaction is observed.
The structural models of the H2AK5ac, H4K12ac, and H3K14ac histone peptide ligands in complex with the BRPF1 bromodomain generated from the MD simulations reveal that the histone peptides are bound to the bromodomain via a network of hydrogen bond contacts (Table 2 and Figure 4G-I). The acetyllysine residue in all of the histone peptides is inserted into a deep binding cavity in the BRPF1 bromodomain where the carbonyl of the N-acetyl group forms a strong H-bond to the amide group of N83. The strongest binder, H2AK5ac, shown in Figure 4G, also has significant H-bond interactions between peptide residues Arg3(-2), Gly4(-1) and Arg11(+6) and residues N83, E36, D39 of the bromodomain protein, respectively. All of these interactions were observed in more than 90% of the trajectory (Table 2). In addition, interactions between Arg3(-2) of the peptide and I27 and P31 of the protein were present for about 30% of the trajectory.
For the second strongest binder, H4K12ac (Figure 4H), the MD simulations indicate that Lys12ac interacts with N83, while Arg17(+5) interacts with D39 of the bromodomain protein in a similar way as observed between Arg11(+6) and D39 in the H2AK5ac complex. In addition, Gly4(−8), Lys8(−4), and L10(−2) of the peptide make H-bond interactions with N26, E30 and E36 in the bromodomain, respectively. However, these interactions were observed only during ~50% time frames in the trajectories (Figures 4H and Table 2).
For the weakest binder in this triplet, the H3K14ac peptide, shown in Figure 4I, the MD simulations suggest as the main interaction a H-bond between Lys14ac and N83 of the protein, which is present for 98.9% of simulated time. In addition, Arg8(−6) and Arg17(+3) of this peptide make H-bond interactions with D39 and E36 of the protein, respectively, with a longevity of about 70%. The remaining H-bonds, between Arg8(−6) and D42/H43, and Lys18(+4) and E30, between the peptide and the protein, respectively, are observed for 29-56% of the frames (Table 2). Perhaps one of the most interesting simulation results is that the H3K14ac peptide, and all of the other histone H3 peptides, were oriented in the bromodomain binding pocket with a reverse backbone direction as compared to the histone H2A- and histone H4-related peptides. It remains to be seen whether these data will be confirmed experimentally.
As seen both experimentally and in the MD simulations, the unmodified histone tail peptides do not make significant contacts to the BRPF1 bromodomain. As with other bromodomains, it appears that the histone binding selectivity of the BRPF1 bromodomain is partially driven by recognition of histone residues flanking the acetyllysine modification. However, it is the acetylation of lysine, resulting in a loss of the positive charge on the nitrogen side chain, which leads to a stronger binding interaction between the histone peptide and the bromodomain protein.
While this manuscript was in preparation, an NMR structure of the BRPF1 bromodomain in complex with the H4K5ac ligand was deposited in the Protein Data Bank (2RS9). In this structure the H4K5ac peptide is coordinated via H-bonds in a similar manner predicted by our MD simulation. Lys5ac and Gly6 in the peptide contact the BRPF1 bromodomain at N83 and Y82 through hydrogen bonds, respectively. The directionality of the H4K5ac histone peptide backbone is also consistent with what is observed in our MD simulations of the BRPF1 bromodomain in complex with histone H4-related peptides (Figure 5). Thus, this unpublished NMR solution structure and the qualitative agreement between the experiments and simulation data support our structural models of the BRPF1 bromodomain-peptide complexes.
Figure 5.
Comparison of BRPF1 bromodomain binding to H4K12ac predicted by the MD simulation model with the NMR structure in complex with H4K5ac. The MD model of the BRPF1 bromodomain is shown in green while the H4K12ac ligand is colored blue. The NMR structure of the BRPF1 bromodomain complex (2RS9) in complex with H4K5ac is shown in pink. Directionality of the histone ligands is indicated by residue numbers and labels at the N- and C-terminus (N and C, respectively).
Comparison of histone ligand coordination with other bromodomains
BRD7 is the bromodomain most closely related to BRPF1 in the IV subfamily. The solution structure of the ligand-free BRD7 bromodomain was solved by NMR, and its interaction with histone ligands was characterized by NMR titration experiments28. The BRD7 and BRPF1 bromodomains share some common histone ligands including H3K14ac, H4K8ac and H4K12ac. Interestingly, our results demonstrate that the BRPF1 bromodomain does not recognize H3K9ac or H4K16ac, both of which are ligands for the BRD7 bromodomain. Analysis of the histone peptide sequence reveals that H3K9ac and H4K16ac each contain an arginine residue directly adjacent to the acetylated lysine residue. With the exception of H2AK5ac, all of the histone ligands recognized by the BRPF1 bromodomain contain either a glycine or alanine on either side of the acetyllysine (Table 1). Thus, the bulky arginine side chain at the −1 or +1 position in the histone tail may preclude proper positioning of the acetylated lysine in the BRPF1 bromodomain binding pocket. Mutational analysis of the BRD7 bromodomain indicated that Arg17(+3) in the H3K14ac histone peptide plays a significant role in ligand coordination because the H3K14ac-R17A mutant peptide could still cause chemical shift changes in the bromodomain upon binding, but the dissociation constant was weaker (2.8 mM) than the wild-type H4K14ac peptide (1.19 mM)28. In our model of the BRPF1 bromodomain in complex with the H3K14ac ligand, Arg17(+3) of the peptide also contacts the protein through a hydrogen bond to E36, indicating a similar mode of recognition for this histone ligand. However, without the structure of the BRD7 bromodomain in complex with its ligand(s) it is difficult to determine the mechanisms underlying the different substrate specificities of these two bromodomains.
Discussion
The MOZ HAT is involved in chromosomal translocations found in a subtype of AML associated with a poor prognosis, and a median survival of only 6 months13,39,40. The BRPF1 subunit associates with MOZ in leukemic translocations and is essential for its roles in the regulation of transcription, hematopoiesis, leukemogenesis, and other developmental processes12,14,41,42. BRPF1 also links the MOZ catalytic subunit to the ING5 and hEaf6 subunits in the MOZ HAT complex, thereby promoting its HAT activity. Despite the physiological importance of MOZ HAT-associated proteins, little is known about how these proteins function at the molecular or atomic level. This study uses an innovative approach to investigate how a bromodomain directs the biological function of BRPF1 by interacting with the N-terminal acetylated histones H2AK5ac, H4K12ac, H4K8ac, H4K5ac, and H3K14ac, ultimately targeting the MOZ HAT complex to chromatin substrates. Recently, Filippakopoulos et al. used a SPOT array with peptides covering all possible Kac sites to detect the interaction of multiple bromodomains with post-translationally modified histones27. This assay indicated the BRPF1 bromodomain interacts with H3K56ac, H4K44ac, H4K77ac, H4K79ac and H4K91ac, which had very strong or strong SPOT interactions, and H3K64ac, H3K115ac and H3K122ac, which had weak or very weak SPOT interactions. However, they did not detect any interactions with N-terminal histone acetylation modifications as discovered in our peptide array assay, and confirmed in our NMR and/or ITC experiments. The differing results between these two studies is not surprising as the authors mention that 16 peptides in their SPOT array did not show interactions as expected from previously published bromodomain recognition and binding data27. Moreover, they state these differences could be explained by steric restraints of the immobilized peptides in the SPOT array, or lack of sufficient N- and C-terminal flanking regions in the peptides used. Interestingly, the histone acetylation marks identified by Filippakopouls et al., 2012 are all located in the globular ‘core’ region of histones H3 and H4. These core histone acetylation marks were not tested in our peptide array because their location on the histone region within the nucleosome may inherently prevent any interaction by chromatin reader domains. Indeed, when we tested the interaction of H4K79ac with the BRPF1 bromodomain using ITC no binding was observed. Also, the biological processes mediated by core histone acetylation PTMs are relatively unknown in comparison to their N-terminal counterparts, which are commonly associated with transcriptional activation, and have been shown to disrupt chromatin fiber compaction (H4K16ac)43.
We have identified that the BRPF1 bromodomain recognizes a similar set of N-terminal acetylated histone peptides as the BRD7 bromodomain, which is the only other bromodomain in sub-family IV to be studied in detail28. The BRD7 and BRPF1 bromodomains share some common histone ligands including H3K14ac, H4K8ac and H4K12ac. Interestingly, our results demonstrate that the BRPF1 bromodomain does not recognize H3K9ac or H4K16ac, both of which are ligands for the BRD7 bromodomain. Analysis of the histone peptide sequence reveals that H3K9ac and H4K16ac each contain an arginine residue directly adjacent to the acetylated lysine residue. With the exception of H2AK5ac, all of the histone ligands recognized by the BRPF1 bromodomain contain either a glycine or alanine on either side of the acetyllysine (Table 1). Thus, the bulky arginine side chain at the −1 or +1 position in the histone tail may preclude proper positioning of the acetylated lysine in the BRPF1 bromodomain binding pocket. Mutational analysis of the BRD7 bromodomain indicated that Arg17(+3) in the H3K14ac histone peptide plays a significant role in ligand coordination because the H3K14ac-R17A mutant peptide could still cause chemical shift changes in the bromodomain upon binding, but the dissociation constant was weaker (2.8 mM) than the wild-type H4K14ac peptide (1.19 mM)28. In our model of the BRPF1 bromodomain in complex with the H3K14ac ligand, Arg17(+3) of the peptide also contacts the protein through a hydrogen bond to E36, indicating a similar mode of recognition for this histone ligand. However, without the structure of the BRD7 bromodomain in complex with its ligand(s) it is difficult to determine the mechanisms underlying the different substrate specificities of these two bromodomains.
Recent structural genomic efforts to determine the three dimensional structure of all 61 of the human bromodomains has resulted in the release of 33 new crystal structures, and we now have access to bromodomain structures with representatives in each of the eight bromodomain sub-families (family I-VIII). However, the majority of these structures are of the bromodomain alone, and the histone ligands of human bromodomains remains largely uncharacterized. In addition to the ligands for the BRPF1 and BRD7 bromodomains in family IV, we know that the class I PCAF bromodomain preferentially binds H3K36ac due to major changes in amino acid residues in the ZA loop26. The CREB binding protein (CBP) bromodomain in the class III bromodomain subfamily is known to bind the acetyllysine moiety of residue 382 in p53 as well as H4K20ac19,26, while the more distantly related class II BET (bromodomain and extra-terminal domain) family of bromodomains, which include the bromodomain containing 2 (BRD2) protein, and the class VIII human polybromo bromodomain 2 (PB2), prefer the acetylated ligands H4K12ac and H3K14ac, respectively44,45. The SPOT array employed by Filippakopoulos et al., 2012 identified potential histone ligands for the FALZ, BRDT(1), EP300, BRPF1A, LOC93349, MLL, TAF1L(2) and SMARCA2A bromodomains which are representatives from each of the eight bromodomain subfamilies27. However, this area is still an important and active area of research because as our study shows, bromodomains within the same sub-family do not necessarily have identical histone ligands. It will be essential to identify the histone ligands and characterize the molecular mechanisms driving binding specificity for individual bromodomains to fully understand how they are contributing to the regulation of biological processes and their link(s to disease progression.
Thus, this study demonstrates for the first time that the BRPF1 bromodomain recognizes N-terminal histone acetylation modifications including H2AK5ac, H4K12ac, H4K8ac, H4K5ac, and H3K14ac. These interactions were verified by NMR titration experiments and coupled with ITC to reveal that the BRPF1 bromodomain preferentially selects for the H2AK5ac histone ligand, followed by H4K12ac and H3K14ac. Mapping of the residues involved in histone recognition onto the BRPF1 bromodomain binding pocket was done using NMR chemical shift perturbation data to identify residues involved in ligand coordination, and flexible peptide docking followed by MD simulations were used to create models of the bromodomain in complex with its histone peptide ligands. Our results demonstrate that ligand accessibility to the binding cavity is mediated by hydrophobic and electrostatic interactions with residues F89, Y82, F28, V37, I75, and E36. Once inserted into the binding cleft, the acetyllysine is positioned to make an H-bond with N83. Coordination of the acetyllysine moiety in BRPF1 is generally conserved with other bromodomains, but our data reveal that the BRD7 and BRPF1 bromodomains have slightly different histone ligand binding preferences due to variability in key amino acid residues in the binding site loops28,46.
Importantly, our data indicate that the BRPF1 bromodomain contributes significantly to histone recognition, and is likely involved in targeting the MOZ HAT complex to chromatin in a manner similar to the ING5 PHD finger subunit47. Interestingly, in vitro the MOZ HAT has been shown to acetylate histones H3 and H4 at specific lysine residues including H3K14ac, H4K5ac, H4K8ac, H4K12ac, and H4K16ac48. Our results demonstrate that the BRPF1 bromodomain is able to recognize each of these histone PTMs, with the exception of H4K16ac. H4K16ac is important for the formation of euchromatin, and acts by disrupting the formation of higher order chromatin fibers and inhibiting chromatin remodeling by the ATP-dependent chromatin-assembly factor43. Thus, depending on the epigenetic landscape, the BRPF1 bromodomain could recruit the MOZ HAT complex to acetylate H4K16, and/or the N-terminal tails of histones in the same or adjacent nucleosomes, up-regulating gene expression. In addition to the BRPF1 bromodomain, the MOZ HAT complex contains multiple chromatin reader domains including the ING5 PHD finger, the BRPF1 PWWP domain, the BRPF1 double PHD finger, and the MOZ tandem PHD fingers, which are able to bind H3K4me3, H3K36me3, unmodified H3, and H3K14ac, respectively15-17,47,49. Thus, recognition of multiple PTMs on histone tails by specific modules within the individual subunits is likely to function in a combinatorial manner, regulating the MOZ HAT activity based on the available epigenetic signals.
In conclusion, our results provide the first biochemical and structural characterization of the BRPF1 bromodomain interaction with N-terminal acetylated histones, demonstrate a mechanism for recruitment of the BPRF1 subunit to nucleosomes, and offer new insights into the role of BRPF1 in targeting the MOZ HAT complex to chromatin. This information is critical for advancing our understanding of how bromodomains recognize and select for specific acetyllysine marks. Deregulated acetylation levels have been associated with aberrant transcription, and linked to disease outcomes, particularly in cancer. This study advances our understanding of how the BRPF1 bromodomain recognizes and selects for specific acetyllysine marks. However, it will be necessary to obtain information about the binding specificity of other bromodomains within this subfamily to fully understand the molecular mechanisms driving ligand selectivity. Lastly, because the BRPF1 protein interacts with a region of MOZ that remains intact after leukemic translocations, the mechanistic principles by which the BPRF1 bromodomain recognizes acetylated histones may contribute to the development of future therapeutics for AML and other diseases.
Materials and Methods
Protein purification
Isolated cDNA from human BRPF1 was kindly provided by Xiang-Jiao Yang. Residues 629-742 encoding the 114 amino acid bromodomain were amplified using PCR and cloned into the pDEST15 vector encoding an N-terminal GST (glutathione transferase) tag using the Gateway Cloning technology (Invitrogen). Once the DNA sequence was verified, the wild-type BRPF1 bromodomain was expressed in Escherichia coli Rosetta2 (DE3) pLysS cells grown in TB (terrific broth), or in 15NH4Cl-supplemented, or 15NH4Cl/13C6 D-glucose-supplemented minimal media. Bacteria were harvested by centrifugation after induction with 0.5 mM IPTG overnight at 20°C and lysed by sonication. The unlabeled, 15N-labeled, and 15N, 13C-labeled GST-fusion proteins were purified on glutathione agarose resin (Thermo Scientific). For peptide array analysis the GST tag was left on, and the GST-fusion protein was eluted off the glutathione agarose resin using 50 mM reduced L-glutathione (Fisher Scientific). For proteins used in NMR and ITC experiments, the GST tag was cleaved with PreScission Protease (GE Healthcare). The eluted protein was used in the same buffer with the addition of 1 mM DTT for peptide array analysis, or concentrated into 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 10 mM dithiothreitol (DTT) for NMR spectroscopy.
Peptide array analysis
For the peptide array, biotinylated histone peptides were synthesized at the Yale W. M. Keck facility (Shi et al., 2007). Peptides were printed in three replicates onto Streptavidin-coated slides (PolyAn, Germany) using the VersArray Compact Microarrayer (BioRad). Slides were air-dried overnight prior to use. Directly before use, unbound Streptavidin sites were blocked with free biotin (Sigma; 1 mg/mL). Slides were incubated at 4°C overnight with GST-BRPF1 bromodomain diluted in peptide binding buffer (50 mM Tris-HCl 7.5, 150 mM NaCl, 0.1% NP-40, 20% fetal bovine serum). Slides were washed 6 times with peptide binding buffer and probed with anti-GST antibody (Millipore) diluted in PBS containing 0.1% Tween-20 (PBST) and 20% FBS at room temperature for 1 h. Slides were washed with PBST six times, then incubated for 30 min with Alexa Fluor 647 chicken anti-rabbit IgG (Invitrogen) diluted in (PBST with 20% FBS). Lastly, slides were washed with PBST six times, briefly rinsed with PBS and air-dried. A GenePix 4000 scanner (Molecular Devices) was used to scan the arrays, and data images were analyzed by GenePix Pro Version 56.0 1 software.
NMR spectroscopy
Chemical shift perturbation experiments were conducted using uniformly 15N-labeled BRPF1 bromodomain prepared at 0.469 mM in buffer containing 20 mM Tris-HCl pH 6.8, 150 mM NaCl, 10 mM DTT and 10% D2O. 35 μL titration mixtures of the protein and peptide were made at concentration ratios of 1:0, 1:0.3, 1:0.6, 1:1.2, 1:2.9, and 1:6.1 for the 12-mer and 14-mer unlabeled histone tail peptides containing specific acetyllysine modifications (H3K14ac, H4K5ac, H4K8ac, H3K18ac, Kac, H2AK5ac, and H4K12ac). The histone peptides were synthesized by the Peptide Core Facility at the University of Colorado Denver, and the 1-mer acetyllysine was purchased from Sigma. These mixtures were then transferred into 1.7 mm NMR tubes (Bruker) using disposable polyethylene tubing (Intramedic, O.D. 0.965 mm, #427410, Becton Dickinson and Company, Sparks, MD).
2D 15N HSQC (heteronuclear single quantum coherence) experiments for all samples were run at 25 °C on a 600 MHz Bruker AVANCE III spectrometer equipped with a z-gradient 1.7 mm TCI probe at the National Magnetic Resonance Facility at Madison (NMRFAM). The NMR data were collected with 1H and 15N radio-frequency pulses applied at 4.745 parts per million (ppm) and 116 ppm, respectively. 1024 × 128 complex data points with spectral widths of 16 ppm and 30 ppm, respectively, were collected along the 1H and 15N dimensions, with 32 scans per FID and an inter-scan delay of 1.0 sec, resulting in a total experimental time of about 160 min for each HSQC spectrum. Water suppression for each sample was manually optimized by adjusting the power level of soft water selective pulse in water-gate. Transformed spectra at each titration point were overlaid and analyzed to characterize the relevant residues affected by the interaction between the BRPF1 bromodomain and the histone peptides. Kd values were calculated by a nonlinear least-squares analysis in KaleidaGraph using the equation:
where [L] is the concentration of the peptide, [P] is the concentration of the protein, Δδ is the observed chemical shift change and Δδmax is the normalized chemical shift change at saturation. Normalized chemical shift changes were calculated using the equation:
where ΔδH and ΔδN are the proton and nitrogen change in chemical shift in ppm, respectively.
To obtain the backbone resonance assignments, the 15N, 13C double labeled BRPF1 bromodomain was prepared at about 1 mM in buffer containing 20 mM Tris-HCl pH 6.8, 150 mM NaCl, 10 mM DTT and 10% D2O. The Bruker version of ADAPT-NMR (Assignment-directed Data collection Algorithm utilizing a Probabilistic Toolkit in NMR) was used to optimize simultaneous fast data collection and automated NMR assignment of the HNCO, HN(CA)CB, HNCA, HN(CO)CA, HN(CA)CO, CBCA(CO)NH and C(CCO)NH spectra by achieving reduced dimensionality (2D) (Lee et al. manuscript accepted in Journal of Magnetic Resonance).
These experiments were run at 25 °C on a 500 MHz Bruker AVANCE III spectrometer equipped with a z-gradient 5 mm TCI probe. The NMR data for all experiments were collected with the universal carrier position of 1H, 15N, 13Ca (shaped pulse), 13Caliphatic (13Ca/b or 13Cb, shaped pulse), 13C’ (shaped pulse) applied at 4.76 ppm (H2O frequency), 118 ppm, 56 ppm, 45 ppm, and 176 ppm respectively. 1024, 32, 64, 64, and 64 complex data points with spectral widths of 16 ppm, 36 ppm, 32 ppm, 70 ppm and 22 ppm, respectively, were collected along the 1H, 15N, 13Ca, 13Caliphatic and 13C’ dimensions. Processing and analysis for all data was conducted automatically with ADAPT-NMR. After one day of the ADAPT-NMR run, an 84.7% assignment level was achieved. To generate visualized assignment labels on the 2D 1H-15N HSQC spectra, PINE-SPARKY50 and SPARKY51 were used.
Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) experiments were conducted at 5° C with a MicroCal ITC200 instrument (GE Healthcare). The BRPF1 bromodomain and histone peptide samples were prepared in a 20 mM Tris-HCl pH 8.0 and 150 mM NaCl solution, except for the H4K79ac peptide and bromodomain sample which was prepared in 20 mM NaH2PO4 pH 7.0 and 150 mM NaCl. All titrations were performed as follows: 203 μM bromodomain solution was added to the sample cell, which then received one preliminary injection of 0.5 μL of 5 mM histone peptide sample followed by 19 injections of 2 μL of peptide at time intervals of 150 sec. Control experiments were performed under identical conditions to determine the heat of dilution of the titrant peptides into the experimental buffer (see Supplemental Figure S2). This was subtracted from the experimental data as part of data analysis. Data were analyzed using the software ORIGIN 7.0 (OriginLab Corporation). All experiments where binding occurred were performed in triplicate, while non-binding experiments were performed in duplicate.
In silico protein and peptide preparation
The published NMR solution structure (PDB code 2D9E) of the bromodomain of human peregrin was taken from the Protein Data Bank52. The 2D9E structure was modified with Biopolymer structure preparation tool in Sybyl-X1.153: the N-terminal and the C-terminal residues were capped with N-acetyl and N-methyl amide groups; protonation types were set for His (ε-N protonated), Glu (negatively charged), and Lys (positively charged) residues; hydrogen atoms were added and charges were loaded using the AMBER ff99SB54 force field. The resulting structure was briefly (100 steps) minimized only for H-atoms by the Powell conjugate gradient method in Sybyl-X1.153. The peptide structures (Table 1) were built in Sybyl-X1.153. Geometry optimization and Mulliken charge55 calculations were done in the Gaussian 09 program56 using the DFT/B3LYP method57 with the 6-31G** basis set58,59. Charge and spin multiplicity were entered manually for different protonation states.
Flexible protein-peptide docking
The starting structures for MD simulations were obtained by flexible protein-peptide docking using Rosetta Flexpepdock,32,33 a high resolution docking program, available through an online web service at (http://flexpepdock.furmanlab.cs.huji.ac.il/). The docking protocol incorporates iterative cycles of optimization and energy minimization that include full flexibility and rigid body orientation for the peptide backbone, as well as side chain flexibility for both the peptide and the receptor protein. The resulting models are ranked based on Rosetta full-atom energy function (Rosetta score12) available within the Rosetta modeling framework34. The models with the highest scores were used for the preparation of MD simulations.
MD simulations
Molecular dynamics simulations were performed using the Amber 12 package35 under isothermal/isobaric (NPT) conditions with the Amber ff99SB force field60 for protein and peptide molecules. The force field parameters for acetylated lysine were obtained by following the standard procedure used in the AMBER force field development utilizing the Mulliken charges calculated above. The backbone torsion parameters are the same as those of the natural lysine residue in the AMBER ff99SB force field.
To prepare the structures, the Leap program from Antechamber tools, AmberTools 1361 was used to create the parameter/topology (prmtop) and input coordinate (inpcrd) files. The net charge of the protein-peptide complexes varied from +2 to +4 depending upon the overall charge of the peptide, and was neutralized by adding Cl− ions at positions of high positive electron potential around the complexes. The system was immersed in a truncated octahedral box of pre-equilibrated TIP3P water molecules62 in a way that no atoms in the protein-ligand complexes were closer than 16 Å to any of the sides of the water box. The counter ions and solvent molecules were briefly minimized for 2500 steps to remove any bad contacts in the complexes, whereby the protein and peptides were position-restrained using force constant of 100 kcal/(mol×Å2), followed by another 2500 steps minimization of the whole solvated complex.
To allow for the readjustment of solvent molecules to the potential field of the protein-peptide complex, the solvent equilibration step was performed in three stages. During the first heating phase, MD simulation was carried out for 10 ps, with constant volume periodic boundaries with an initial temperature of 0 K, allowing it to heat up to 100 K. The second heating phase was performed for 40 ps under constant pressure periodic boundaries with an average pressure of 1 atm. Isotropic position scaling was used to maintain the pressure, and a relaxation time of 2 ps was used. The system was allowed to heat up from the initial temperature 100 K to 200 K, and from 200 K to the final temperature 300 K, for 20 ps each. During the heating phases, the protein-ligand complexes were position-restrained with a force constant of 10 kcal/(mol×Å2). Langevin dynamics was used in all stages to control the temperature using a collision frequency of 1.0 ps−1. The final solvent equilibration step was performed for 50 ps under constant pressure with periodic boundary conditions as mentioned above. The production phase with the entire system was carried out under isothermal/isobaric conditions for 10 ns. The SHAKE algorithm63 was used to constrain bonds involving hydrogen, allowing a time step of 2 fs, for a total of 5,000,000 steps. The trajectory file was written for every 1000 steps resulting in 5000 frames. The cutoff for non-bonded interactions was set to 12 Å in all steps.
The MD trajectory analysis for H-bond interactions was carried out on the 10 complexes from the production phase using the cpptraj program in the AmberTools 13 suite35,61. The default cutoff values for distance (3.2 Å) and angle (135 degrees) were chosen. The pair-wise interactions were monitored between the protein (residues 20-51 and 69-99) and all peptide residues.
MM/PBSA binding free energy calculation
The Molecular Mechanics/Poisson Boltzmann Surface Area (MM/PBSA) method36 combines the molecular mechanical energies with the continuum solvent approaches. The molecular mechanical energies represent the internal energy (bond, angle and dihedral), and van der Waals and electrostatic interactions. The nonpolar contribution to the solvation free energy is determined with solvent-accessible surface area dependent terms: the attractive (dispersion) and repulsive (cavity) interactions. The estimates of vibrational entropies were made using the normal mode analysis or NAB module from Amber. The calculations were done using the recently published MMPBSA.py program37 from Amber Suite.
Supplementary Material
Acknowledgements
We thank XJ Yang for providing us with the BRPF1 cDNA. We also thank C. Francklyn and J. Stein for editorial assistance and discussions. Research reported in this publication was supported by an award from the American Heart Association to KCG (10BGIA3420014), the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R15GM104865 to KCG and R01GM80508 to SB, and by a grant from the Welch Foundation to XS (Welch G1719). DNA sequencing was performed in the Vermont Cancer Center DNA Analysis Facility. This study made use of the National Magnetic Resonance Facility at Madison, which is supported by National Institutes of Health grants P41RR02301 (Biomedical Research Technology Program, National Center for Research Resources) and P41GM66326 (National Institute of General Medical Sciences). Equipment in the facility was purchased with funds from the University of Wisconsin, the National Institutes of Health (P41GM66326, P41RR02301, RR02781, RR08438), the National Science Foundation (DMB-8415048, OIA-9977486, BIR-9214394), and the U.S. Department of Agriculture.
Abbreviations
- ADAPT-NMR
Assignment-directed Data collection Algorithm utilizing a Probabilistic Toolkit in NMR
- AML
acute myeloid leukemia
- BRDT
bromodomain containing protein testis specific
- BRPF1
bromodomain-PHD finger protein 1
- CBP
CREB binding protein
- HAT
histone acetyltransferase
- hEAF6
human Esa1-associated factor 6 homolog
- HOX
homeobox
- HSCs
hematopoietic stem cells
- ING5
inhibitor of growth 5
- ITC
isothermal titration calorimetry
- MOZ
monocytic leukemic zinc-finger
- NMR
nuclear magnetic resonance
- PCAF
p300/CBP-associated factor
- PHD
plant homeodomain
- PTM
post-translational modification
- PWWP
proline-tryptophan-tryptophan-proline
- RUNX1/2/3
runt-related transcription factors 1, 2 or 3
- TIF2
transcriptional intermediary binding factor 2
- TRIM24
tripartite motif 24
- ZnF
zinc finger
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers:
The NMR assignments of the BRPF1 bromodomain are deposited in the BMRB under accession number 19125. The solution structure of the human bromodomain of peregrin (2D9E) was used to model the coordination of acetylated histone ligands.
REFERENCES
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 4.Utley RT, Lacoste N, Jobin-Robitaille O, Allard S, Côté J. Regulation of NuA4 histone acetyltransferase activity in transcription and DNA repair by phosphorylation of histone H4. Mol Cell Biol. 2005;25:8179–90. doi: 10.1128/MCB.25.18.8179-8190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi SK, Young DW, Montecino M, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Architectural epigenetics: mitotic retention of mammalian transcriptional regulatory information. Mol Cell Biol. 2010;30:4758–66. doi: 10.1128/MCB.00646-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 7.Guetg C, Lienemann P, Sirri V, Grummt I, Hernandez-Verdun D, Hottiger MO, Fussenegger M, Santoro R. The NoRC complex mediates the heterochromatin formation and stability of silent rRNA genes and centromeric repeats. EMBO J. 2010;29:2135–46. doi: 10.1038/emboj.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne N, Pelletier N, Yang XJ. The monocytic leukemia zinc finger protein MOZ is a histone acetyltransferase. Oncogene. 2001;20:404–9. doi: 10.1038/sj.onc.1204114. [DOI] [PubMed] [Google Scholar]
- 9.Laue K, Daujat S, Crump JG, Plaster N, Roehl HH, Kimmel CB, Schneider R, Hammerschmidt M. The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development. 2008;135:1935–46. doi: 10.1242/dev.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holbert MA, Sikorski T, Carten J, Snowflack D, Hodawadekar S, Marmorstein R. The human monocytic leukemia zinc finger histone acetyltransferase domain contains DNA-binding activity implicated in chromatin targeting. J Biol Chem. 2007;282:36603–13. doi: 10.1074/jbc.M705812200. [DOI] [PubMed] [Google Scholar]
- 11.Camos M, Esteve J, Jares P, Colomer D, Rozman M, Villamor N, Costa D, Carrio A, Nomdedeu J, Montserrat E, Campo E. Gene expression profiling of acute myeloid leukemia with translocation t(8;16)(p11;p13) and MYST3-CREBBP rearrangement reveals a distinctive signature with a specific pattern of HOX gene expression. Cancer Res. 2006;66:6947–54. doi: 10.1158/0008-5472.CAN-05-4601. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Campo FM, Borrow J, Kouskoff V, Lacaud G. The histone acetyl transferase activity of monocytic leukemia zinc finger is critical for the proliferation of hematopoietic precursors. Blood. 2009;113:4866–74. doi: 10.1182/blood-2008-04-152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrow J, Stanton VP, Jr., Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dube I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 14.Ullah M, Pelletier N, Xiao L, Zhao SP, Wang K, Degerny C, Tahmasebi S, Cayrou C, Doyon Y, Goh SL, Champagne N, Cote J, Yang XJ. Molecular architecture of quartet MOZ/MORF histone acetyltransferase complexes. Mol Cell Biol. 2008;28:6828–43. doi: 10.1128/MCB.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Gottgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat Struct Mol Biol. 2010;17:617–9. doi: 10.1038/nsmb.1797. [DOI] [PubMed] [Google Scholar]
- 16.Qin S, Jin L, Zhang J, Liu L, Ji P, Wu M, Wu J, Shi Y. Recognition of Unmodified Histone H3 by the First PHD Finger of Bromodomain-PHD Finger Protein 2 Provides Insights into the Regulation of Histone Acetyltransferases Monocytic Leukemic Zinc-finger Protein (MOZ) and MOZ-related factor (MORF) J Biol Chem. 2011;286:36944–55. doi: 10.1074/jbc.M111.244400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Qin S, Zhang J, Ji P, Shi Y, Wu J. Solution structure of an atypical PHD finger in BRPF2 and its interaction with DNA. J Struct Biol. 2012;180:165–73. doi: 10.1016/j.jsb.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 19.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13:251–63. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Grummt I. The PHD finger/bromodomain of NoRC interacts with acetylated histone H4K16 and is sufficient for rDNA silencing. Curr Biol. 2005;15:1434–8. doi: 10.1016/j.cub.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 21.Lamonica JM, Deng W, Kadauke S, Campbell AE, Gamsjaeger R, Wang H, Cheng Y, Billin AN, Hardison RC, Mackay JP, Blobel GA. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc Natl Acad Sci U S A. 2011;108:E159–68. doi: 10.1073/pnas.1102140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciro M, Prosperini E, Quarto M, Grazini U, Walfridsson J, McBlane F, Nucifero P, Pacchiana G, Capra M, Christensen J, Helin K. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 2009;69:8491–8. doi: 10.1158/0008-5472.CAN-09-2131. [DOI] [PubMed] [Google Scholar]
- 24.Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, Tsai CY, Shi X, Schwarzer D, Plunkett W, Aronow B, Gozani O, Fischle W, Hung MC, Patel DJ, Barton MC. TRIM24 links a non-canonical histone signature to breast cancer. Nature. 2010;468:927–32. doi: 10.1038/nature09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng L, Zhang Q, Gerona-Navarro G, Moshkina N, Zhou MM. Structural basis of site-specific histone recognition by the bromodomains of human coactivators PCAF and CBP/p300. Structure. 2008;16:643–52. doi: 10.1016/j.str.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, Gingras AC, Arrowsmith CH, Knapp S. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–31. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun H, Liu J, Zhang J, Shen W, Huang H, Xu C, Dai H, Wu J, Shi Y. Solution structure of BRD7 bromodomain and its interaction with acetylated peptides from histone H3 and H4. Biochem Biophys Res Commun. 2007;358:435–41. doi: 10.1016/j.bbrc.2007.04.139. [DOI] [PubMed] [Google Scholar]
- 29.Moriniere J, Rousseaux S, Steuerwald U, Soler-Lopez M, Curtet S, Vitte AL, Govin J, Gaucher J, Sadoul K, Hart DJ, Krijgsveld J, Khochbin S, Muller CW, Petosa C. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature. 2009;461:664–8. doi: 10.1038/nature08397. [DOI] [PubMed] [Google Scholar]
- 30.Kupitz C, Chandrasekaran R, Thompson M. Kinetic analysis of acetylation-dependent Pb1 bromodomain-histone interactions. Biophys Chem. 2008;136:7–12. doi: 10.1016/j.bpc.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson RH, Ladurner AG, King DS, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–5. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 32.London N, Raveh B, Cohen E, Fathi G, Schueler-Furman O. Rosetta FlexPepDock web server--high resolution modeling of peptide-protein interactions. Nucleic Acids Res. 2011;39:W249–53. doi: 10.1093/nar/gkr431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raveh B, London N, Schueler-Furman O. Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins. 2010;78:2029–40. doi: 10.1002/prot.22716. [DOI] [PubMed] [Google Scholar]
- 34.Das R, Baker D. Macromolecular modeling with rosetta. Annu Rev Biochem. 2008;77:363–82. doi: 10.1146/annurev.biochem.77.062906.171838. [DOI] [PubMed] [Google Scholar]
- 35.Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, robers B, Hayik S, Roitberg A, Kolossváry I, Seabra G, swails J, Wong KF, Paesani F, Vanicek J, Wolf RM, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, cai q., Ye X, Wang J, hsieh m. j., Cui G, roe d. r., Mathews DH, Seetin MG, Sagui C, salomon-ferrer R, Sagui C, luchko t., gusarov s., Kovalenko A, Kollman PA. Amber12 and AmberTools. 2012:13. [Google Scholar]
- 36.Srinivasan J, Cheatham TE, III, Cieplak P, Kollman PA, Case DA. Continuum Solvent Studies of the Stability of DNA, RNA, and Phosphoramidate-DNA Helices. J. Am. Chem. Soc. 1998;120:9401–9409. [Google Scholar]
- 37.Miller BR, McGee TD, Swails JM, Homeyer N, Gohlke H, Roitberg AE. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. Journal of Chemical Theory and Computation. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 38.Bissantz C, Kuhn B, Stahl M. A medicinal chemist’s guide to molecular interactions. J Med Chem. 2010;53:5061–84. doi: 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown T, Swansbury J, Taj MM. Prognosis of patients with t(8;16)(p11;p13) acute myeloid leukemia. Leuk Lymphoma. 2012;53:338–41. doi: 10.3109/10428194.2011.614703. [DOI] [PubMed] [Google Scholar]
- 40.Panagopoulos I, Fioretos T, Isaksson M, Samuelsson U, Billstrom R, Strombeck B, Mitelman F, Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13) Hum Mol Genet. 2001;10:395–404. doi: 10.1093/hmg/10.4.395. [DOI] [PubMed] [Google Scholar]
- 41.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–59. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibiya K, Katsumoto T, Kondo T, Kitabayashi I, Kudo A. Brpf1, a subunit of the MOZ histone acetyl transferase complex, maintains expression of anterior and posterior Hox. Dev Biol. 2009;329:176–90. doi: 10.1016/j.ydbio.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–7. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 44.Umehara T, Nakamura Y, Jang MK, Nakano K, Tanaka A, Ozato K, Padmanabhan B, Yokoyama S. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J Biol Chem. 2010;285:7610–8. doi: 10.1074/jbc.M109.062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlop-Powers Z, Zeng L, Zhang Q, Zhou MM. Structural insights into selective histone H3 recognition by the human Polybromo bromodomain 2. Cell Res. 2010;20:529–38. doi: 10.1038/cr.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000;19:6141–9. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champagne KS, Saksouk N, Pena PV, Johnson K, Ullah M, Yang XJ, Cote J, Kutateladze TG. The crystal structure of the ING5 PHD finger in complex with an H3K4me3 histone peptide. Proteins. 2008;72:1371–6. doi: 10.1002/prot.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitabayashi I, Aikawa Y, Nguyen LA, Yokoyama A, Ohki M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. EMBO J. 2001;20:7184–96. doi: 10.1093/emboj/20.24.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu Y, Liu L, Zhao C, Han C, Li F, Zhang J, Wang Y, Li G, Mei Y, Wu M, Wu J, Shi Y. Combinatorial readout of unmodified H3R2 and acetylated H3K14 by the tandem PHD finger of MOZ reveals a regulatory mechanism for HOXA9 transcription. Genes Dev. 2012;26:1376–91. doi: 10.1101/gad.188359.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee W, Westler WM, Bahrami A, Eghbalnia HR, Markley JL. PINE-SPARKY: graphical interface for evaluating automated probabilistic peak assignments in protein NMR spectroscopy. Bioinformatics. 2009;25:2085–7. doi: 10.1093/bioinformatics/btp345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goddard TD, Kneller DG. Sparky3. University of California; San Francisco: [Google Scholar]
- 52.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripos I. SYBYL-X 1.1. Tripos International, 1699 South Hanley Rd., St. Louis, Missouri, 63144, USA: 2010. [Google Scholar]
- 54.Ren P, Ponder JW. Consistent treatment of inter- and intramolecular polarization in molecular mechanics calculations. J Comput Chem. 2002;23:1497–506. doi: 10.1002/jcc.10127. [DOI] [PubMed] [Google Scholar]
- 55.Mulliken RS. Electronic population analysis on LCAO-MO [linear combination of atomic orbital-molecular orbital] molecular wave functions. Journal of Chemical Physics. 1955;23:1833–1840. [Google Scholar]
- 56.Frisch MJ. Gaussian 09 Revision A.1. Gaussian Inc.; Wallingford, CT 06492, USA: 2011. [Google Scholar]
- 57.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. Journal of Chemical Physics. 1993;98:5648–5652. [Google Scholar]
- 58.Hay PJ, a. W. WR. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms scandium to mercury. Journal of Chemical Physics. 1985;82:270–283. [Google Scholar]
- 59.Wadt W. R. a. H., P. J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements sodium to bismuth. Journal of Chemical Physics. 1985;82:284–298. [Google Scholar]
- 60.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–25. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Case DA, Cheatham TE, 3rd, Darden T, Gohlke H, Luo R, Merz KM, Jr., Onufriev A, Simmerling C, Wang B, Woods RJ. The Amber biomolecular simulation programs. J Comput Chem. 2005;26:1668–88. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. Journal of Chemical Physics. 1983;79:926–935. [Google Scholar]
- 63.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. Journal of Computational Physics. 1977;23:327–341. [Google Scholar]
- 64.Armougom F, Moretti S, Poirot O, Audic S, Dumas P, Schaeli B, Keduas V, Notredame C. Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acidss Res. 2006;34:W604–8. doi: 10.1093/nar/gkl092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.