Abstract
A primary impediment to cardiac tissue engineering lies in the inability to adequately vascularize the constructs to optimize survival upon implantation. During normal angiogenesis, endothelial cells (ECs) require a support cell to form mature patent lumens and it has been demonstrated that pericytes, vascular smooth muscle cells and mesenchymal stem cells (MSCs) are all able to support the formation of mature vessels. In the heart, cardiac fibroblasts (CFs) provide important electrical and mechanical functions, but to date have not been sufficiently studied for their role in angiogenesis. To study CFs role in angiogenesis, we co-cultured different concentrations of various cell types in fibrin hemispheres with appropriate combinations of their specific media, to determine the optimal conditions for EC growth and sprout formation through DNA analysis, flow cytometry and immunohistology. ECs proliferated best when co-cultured with CFs and analysis of immunohistological images demonstrated that ECs formed the longest and most numerous sprouts with CFs as compared to MSCs. However, ECs were able to produce more multicellular sprouts when in culture with the MSCs. Moreover, these effects were dependent on the ratio of support cell to EC in co-culture. Overall, CFs provide a good support system for EC proliferation and sprout formation; however, MSCs allow for more multicellular sprouts, which is more indicative of the in vivo process.
Keywords: Endothelial Cells, Myocardium, Angiogenesis and Vasculogenesis, 3D Cell Culture, Heart Tissue Engineering
INTRODUCTION
The inability to form functional vasculature in vitro is a primary impediment in tissue engineering [1, 2]. The immediate vascularization of an implanted tissue construct is crucial for its survival and function, as it helps to maintain cell viability in the tissue by optimizing oxygen and nutrient delivery to the cells beyond the diffusion limit [3]. While host vasculature ingrowth has been demonstrated in a number of implanted engineered tissues, this process is slow, often on the order of days to weeks [2]. A tissue engineered construct that is prevascularized in vitro can form an interconnected tubular network within 4 days and begin to anastomose to the host within 1 day, allowing for better delivery of oxygen and nutrients [4]. It is known that support cells are critical for the formation of stable endothelial cell (EC) sprouts and subsequent vessels both in vivo and in vitro [5]. Pericytes or vascular smooth muscle cells provide this support in vivo [6, 7]; however, mesenchymal stem cells (MSCs) [8], adipose derived stem cells [9] and fibroblasts [4] have also been studied for their ability to support EC vessel formation, stabilization and maturation over time.
When creating an engineered cardiac tissue, progenitor cells are typically the most clinically relevant source for the formation of functional myocardial tissue as they can be isolated directly from the patient via a biopsy of the right atrial appendage [10]. Another option for cell source is induced pluripotent stem (iPS) cells, which can be derived from the dermal fibroblasts of a patient [11] and subsequently differentiated to cardiomyocytes (CM) [12]. While functioning CM are the critical component of an engineered cardiac tissue, it has been demonstrated that cardiac fibroblasts (CFs) improve contractile force in engineered cardiac constructs [13]. Moreover, when engineered cardiac tissues are created from iPS-derived CM they require the presence of CF-like cells in order to have appropriate tissue formation and function [14]. CFs can be generated in both of the options for cell source described above: either isolated from the heart biopsy or derived via differentiation from the iPS cells. Although studies have been carried out using a co-culture or tri-culture of ECs with CM [15], stem cell derived CM [16], embryonic fibroblasts [16], dermal fibroblasts, lung fibroblasts [4] and other pericyte cells, there are a limited number of studies that have investigated the role (if any) of CFs in the development of vasculature in engineered myocardial tissue. This is especially important given that CFs secrete angiogenic factors necessary for the maturation and stabilization of EC sprouts, including vascular endothelial growth factor (VEGF), heparin-binding epidermal growth factor (HB-EGF), fibroblast growth factor (FGF) and platelet derived growth factor (PDGF) [17].
Our hypothesis was that CFs can support EC sprout formation in engineered tissues. As a benchmark we compared CFs to MSCs, which are considered to be a “gold standard” for the stabilization of EC sprouts in engineered constructs [18]. To test this hypothesis, ECs were co-cultured with either CFs or MSCs in 3-D fibrin hydrogels in order to determine whether CFs provided support for EC sprout formation that was comparable to MSCs. Sprout formation was measured via immunofluorescent image analysis following 10 days in culture with the above mentioned support cell types. Our results indicate that ECs form more numerous sprouts when cultured with CFs, but have more multi-cellular sprouts when cultured with MSCs, indicating that MSCs are better able to support the adhesion and protrusion activities of ECs typically demonstrated during angiogenesis in vivo. Based on this study, it is likely that a combination of both CFs and another pure pericyte population would be beneficial in generating vascularized engineered cardiac tissue.
MATERIALS AND METHODS
Cell Culture
Rat Aortic ECs were purchased from Cell Applications (San Diego, CA) at passage 3 and cultured for use in experiments from P5–P7. The ECs were cultured in complete EC medium (EBM-2; Lonza Walkersville, Walkersville, MD) with the addition of EGM-2 SingleQuots (Lonza Walkersville) at 37°C, 5% CO2. Cardiac cells were isolated from neonatal (2 to 3 day old) Sprague Dawley rats using a type II collagenase solution as previously described [19]. Neonatal CFs were isolated from this mixed cardiac cell population using the pre-plate method [13] and cultured in CF media (DMEM, 15% FBS, 1% penicillin/streptomycin; Invitrogen, Grand Island, NY). Passage 5–10 CFs were used in the experiments described below. When cultured in the fibrin hydrogels, the CF media was supplemented with 1% ε-aminocaproic acid (ACA) to control fibrinolysis. Rat MSCs were purchased from Texas A&M University at passage 6 and cultured in MSC media (alpha-MEM, 20% FBS, 1% penicillin/streptomycin and supplemented daily with 2% L-Glutamine; all from Invitrogen) and used in experiments from passage 8–12. For all three cell types in this study, medium was changed three times per week and cells were harvested at 80% confluence via 0.25% trypsin-EDTA (1x) treatment (Invitrogen) for use in experiments.
Characterization of Cell Lines
Flow cytometric analysis was conducted on all cell populations by labeling the cells with FITC-conjugated mouse monoclonal anti-rat CD31 (ab33858; Abcam, Cambridge, MA) diluted to 1:10 (ECs) and APC-conjugated CD90 anti-rat (BioLegend) diluted at 1:5 (MSCs). Antibody labeling was carried out for 30 minutes, incubated at 4°C, followed by two washes in autoMacs® Running Buffer (Miltenyi Biotech, Auburn, CA). Cells were then characterized using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) at the Tufts Medical Center Flow Core facility using CellQuest Software (Becton Dickinson, Franklin, NJ) and data was analyzed using the FlowJo software (FlowJo, Ashland, OR).
Creation of Fibrin Co-Cultured Hydrogels
Fibrin hemisphere constructs (550μl) were made in 12-well tissue culture plates, in order to study the interactions between the ECs and the support cells in 3-D, as previously described [20]. For the medium studies, hemispheres were created with a final gel concentration of 3.3mg/ml with 2.5 x 105 cells/mL initial concentrations. Prior to encapsulation in the constructs, ECs were labeled with PKH26 (Sigma Aldrich) following the protocol from Sigma Aldrich, to later verify co-localization of PKH26 and the staining with EC fluorescent antibodies. Experiments were run in quadruplicates with four samples of each type for each experiment.
In order to determine the best media combination for optimal EC and support cell culture, ECs were cultured at a 1:1 ratio with either CFs or MSCs as support cells in the following media conditions: 100% EC Media, 1:1 EC Media: Support Cell media and 100% Support Cell media. ECs were seeded on their own in fibrin hydrogels with all five media types, as controls. In order to determine the best ratio of support cells to ECs for optimal EC sprout formation, three ratios of ECs to support cells were considered for both CFs and MSCs as support cells: 3:1 Support Cells: ECs, 1:1 Support Cells: ECs and 1:3 Support Cells: ECs. All constructs were cultured in 1:1 EC: appropriate support cell media. ECs were seeded in fibrin hydrogels as a control with 100% EC media and 1:1 EC: Support Cell media. The number of ECs was held constant at 2x105 cells/mL fibrin solution for all of the conditions.
Immunohistochemical and Immunofluorescent Staining
For fluorescent imaging, constructs were fixed in 4% paraformaldehyde at 4°C, permeabilized with 0.1% Triton X-100 solution for 10 minutes and blocked with 5% Donkey Serum for one hour. The constructs were subsequently labeled with α-smooth muscle actin (SMA) (Cat#: ab18147; Abcam Inc., Cambridge, MA), for CFs and MSCs or von Wilibrand’s Factor (vWF) (Cat#: ab6994; Abcam Inc.) and CD31 (Santa Cruz Biotechnology) as EC markers. Alexa Fluor 555 donkey anti-rabbit and Dylight 488 donkey anti-mouse were used as secondary antibodies for vWF and α–SMA, respectively (Jackson ImmunoResearch, West Grove, PA). In all fluorescent images cell nuclei were labeled with a DAPI stain (Hoechst, Sigma Aldrich). The samples were analyzed with an Olympus IX81 inverted fluorescent microscope. The resulting images (n=2–4 per sample, 3–5 samples per condition) were analyzed for EC sprout formation by individuals blinded to the group to which the images they were analyzing belonged using ImageJ (NIH, Bethesda, MD) (see supplemental information for further details).
Characterization of Cell Number
The final cell count of all constructs was quantified using a DNA assay as previously described [21]. In addition, the proliferation of ECs and cellular composition of the constructs after 10 days in culture was assessed by recovering the cells via a modified type-II collagenase digestion on a small subset of constructs (n = 4–6 for each group). The recovered cells underwent the flow cytometry procedure described above to determine the percentage of ECs in each condition after the culture period.
Statistical Analysis
All results are expressed as the mean ± standard deviation. Appropriately dimensioned analysis of variance (ANOVA) was used to compare data, followed by a post hoc Tukeys t-test. Results were considered statistically significant when p<0.05.
RESULTS
Total Cell Count in Mixed Media Studies
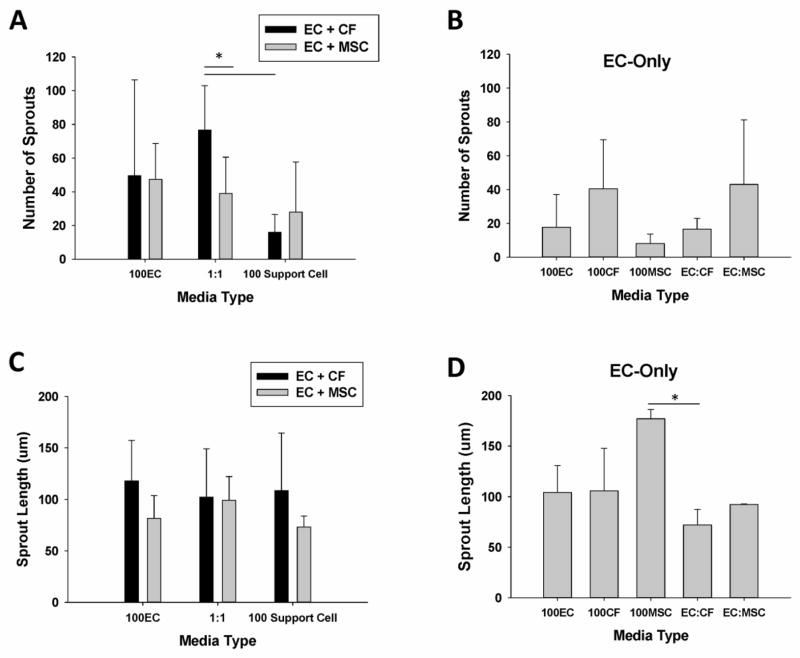
After 7–10 days in co-culture, the total co-culture population appeared to proliferate best when cultured in a 1:1 ratio of EC media: CF media for the CF-EC co-culture (Fig 1A) and in 100% MSC media for the EC-MSC co-culture. While, the highest total EC count averaged by the number of days was found in the 100% MSC medium culture, compared to the 100%CF medium (p<0.003). When the ECs were cultured alone, the highest cell number was found in the EC:MSC group, although there was no statistically significant difference between any of the groups (Fig. 1B).
Figure 1.
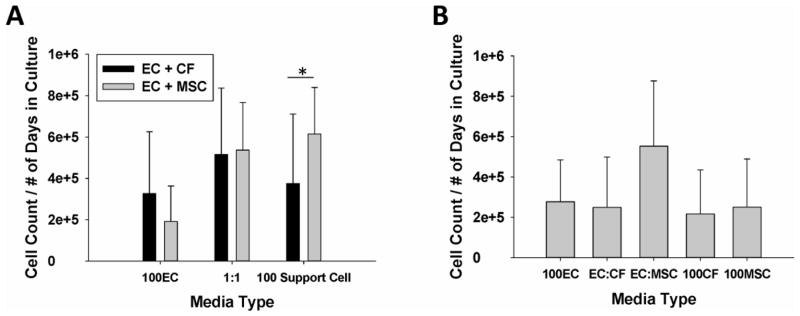
(A) ECs cultured with MSCs and CFs in 100% EC media (100EC), 100% Support Cell media (100 Support Cell) and a 1:1 ratio of EC:Support Cell media (1:1). In 100% Support Cell media, cell number is significantly greater in 100% MSC media vs 100%CF media (p<0.003). (B) ECs cultured alone as a control for cell growth in various media solutions.
Sprout Formation in Mixed Media Study
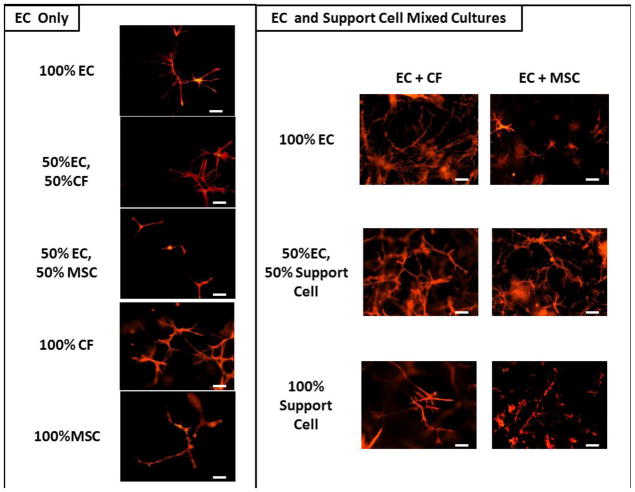
Figure 2 displays representative fluorescent images of the EC sprouts for each condition in the medium optimization study. ECs were pre-labeled with the PKH26 membrane dye and also stained with vWF, while the support cells were labeled with SMA (not shown). The number of EC sprouts formed in each media condition was quantified using ImageJ (Fig. 3A and 3B). After analyzing the fluorescent images (Fig. 2), it was clear that ECs were able to form sprouts in all conditions (Fig. 3); however, the ECs tended to form the longest sprouts when co-cultured with the CFs (Fig. 3C), although this trend was not statistically significant. In co-culture, the average number of EC sprouts was greatest in the 50%CF-50%EC media condition and was signficantly greater than the 50%MSC-50%EC and the 100% CF media conditions (p<0.025 for both).
Figure 2.
Representative images of EC sprout formation in fluorescently stained constructs for EC only cultures (left) and mixed cell cultures (right) for each appropriate media condition. ECs are stained with vWF. Scale bar = 100um.
Figure 3.
(A) The average number of sprouts in EC: CF and EC: MSC fibrin gel co-cultures (* denotes p<0.025). (B) The average number of sprouts when ECs are cultured alone in fibrin gel in the different media types. (C) The average sprout length (in m) in EC: CF and EC: MSC fibrin gel co-cultures. (D) The average sprout length of EC cultured alone in fibrin gel in the different media types. (* denotes p = 0.027).
When considering the average lengths of the sprouts in each condition there was no statistically significant effect of either media type (p=0.125) or support cell type (p=0.883), independent of the other factor. When ECs were cultured alone, they tended to form the longest sprouts in the 100% MSC media condition, which was significantly greater than the 50%CF-50%EC media condition (p=0.027). When considering the mean values for all media conditions for the EC-only culture, the differences in the mean sprout lengths is greater than would be expected by chance as determined by one-way ANOVA (p=0.035)
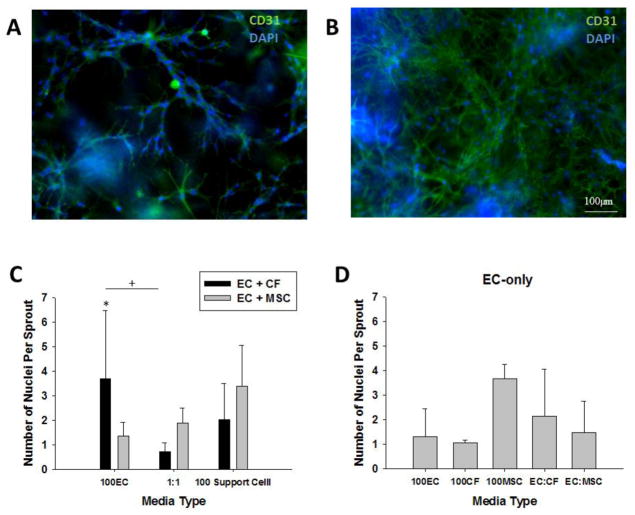
The formation of multi-cellular EC sprouts, which is indicative of the in vivo angiogenic process, is demonstrated for MSCs (Fig. 4A) and CFs (Fig. 4B). The average number of nuclei per sprout (Fig. 4C, D) was found to be dependent on the support cell type present within a specific media type. There is a statistically significant interaction between the support cell type and the media type as determined by two-way ANOVA (p=0.009). For the support cell co-culture conditions, the EC:CF condition had most nuclei per sprout when the constructs were cultured with 100% EC media, which was significantly greater than the EC:MSC condition in 100% EC medium (p=0.007) and the EC:CF condition (p=0.002) in the 50%CF-50%EC media condition. The EC-only culture demonstrated the most abundant number of nuclei per sprout when cultured with 100%MSC media and in the 50%CF-50%EC media condition, although there was not a statistically significant difference between groups. Based on these results, the 1:1 medium ratio was used for all subsequent studies.
Figure 4.
(A) A representative image of the formation of a multi-cellular sprout in the EC: MSC co-culture condition in 50/50% MSC medium. (B) A representative image of the formation of a multi-cellular sprout in the EC: CF co-culture condition in 50/50% CF medium. Scale bar = 100um. Blue = DAPI, Green = CD31 and Red = vWF. (C) Quantification of the number of nuclei per sprout for EC: CF and EC: MSC co-cultures in fibrin gels (* p=0.007, + p=0.002). (D) Quantification of the number of nuclei per sprout for EC-only culture in fibrin gels in the various media formulations.
Total Cell Count in Varied Ratio Studies
Similar to the mixed media studies detailed above, the total cell number after 10 days in culture was calculated using a DNA assay (Fig. 5A). Because the initial number of cells seeded in the constructs varied by condition even though the EC number was held constant (Table 1), the total number of doublings for each condition was also calculated based on the initial seeding number (Fig. 5B). When compared to the 3:1 condition for both support cell types, the 1:1 and 1:3 ratio of support cells to ECs resulted in a statistically greater number of doublings (p<0.001). Within the MSC support cell condition, the 1:3 ratio of Support Cell to EC resulted in a significantly greater number of doublings than the 3:1 and 1:1 conditions (p<0.001 and p=0.027, respectively. While within the CF support cell type, the 1:3 and 1:1 condition both lead to a significantly greater number of doublings compared to the 3:1 condition (p=0.003 and p=0.035, respectively).
Figure 5.
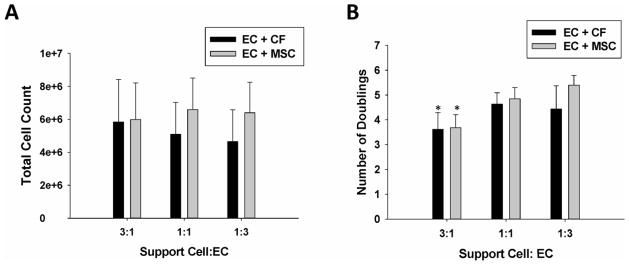
(A) Total cell count of varied ratio of support cell to EC for EC: CF and EC: MSC co-cultures. (B) The number of doublings in varied co-culture conditions for the three Support Cell: EC ratios. Note that for both EC: CF and EC: MSC co-cultures that the number of doublings in the 3:1 Support Cell: EC ratio is significantly less than the other two ratios for each co-culture type (p<0.001).
Table 1.
Ratio of Support Cells to ECs with the total initial cell number and the number of each cell type seeded in the constructs.
| Ratio of Support Cell: Endothelial Cell | Initial Support Cell Number | Initial Endothelial Cell Number | Total Cell Number |
|---|---|---|---|
| 3:1 | 330,000 | 110,000 | 440,000 |
| 1:1 | 110,000 | 110,000 | 220,000 |
| 1:3 | 36,630 | 110,000 | 146,630 |
Flow Cytometry of Constructs in Varied Co-Culture Ratios
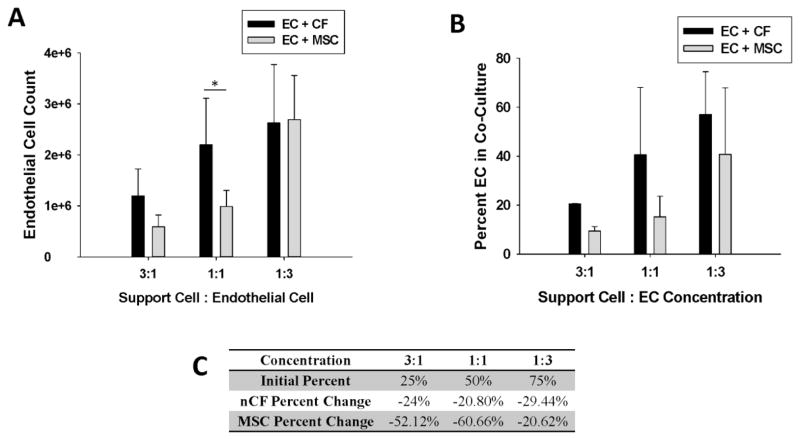
The proliferation of ECs in each of the conditions was determined by combining flow cytometry measurements (see supplemental data, Fig. S1) on the digested constructs to determine cell percentages with the DNA data (Fig. 6A). After 10 days in culture, there was a significantly greater number of ECs in the CF:EC constructs than the MSC:EC constructs in the 1:1 ratio (p=0.001). This is especially significant since the conditions were initially run with an equal number of ECs to start. As a support cell, CFs generally allowed for a significantly greater proliferation of ECs by Day 10 than MSCs did (p=0.014) irrespective of support cell to EC ratio, as determined by one-way ANOVA. While CFs demonstrated an increase in the percentage of total cells that were ECs after 10 days in culture for all conditions (Fig. 6B), these results were not statistically significant. Figure 6C illustrates the initial percent of ECs in each co-culture condition, the final percent of ECs in the CF and MSC conditions and the percent change of ECs in co-culture. The results are depicted as the change in the percent of ECs in culture (as shown by the negative values). The total percent of ECs in culture decreased for all conditions; however, the decrease was lowest in the CF conditions.
Figure 6.
(A) EC proliferation in EC: CF and EC: MSC co-cultures of varying ratios (* denotes p=0.001). (B) ECs as a percentage of the total cell population present in culture after 10 days for EC: CF and EC: MSC co-cultures in the various support cell to EC ratios. (C) Tabulated data of the initial percent of ECs in the various conditions and the percent change of ECs after 10 days in culture in the varied support cell ratios.
Sprout Formation in Varied Co-Culture Ratio Study
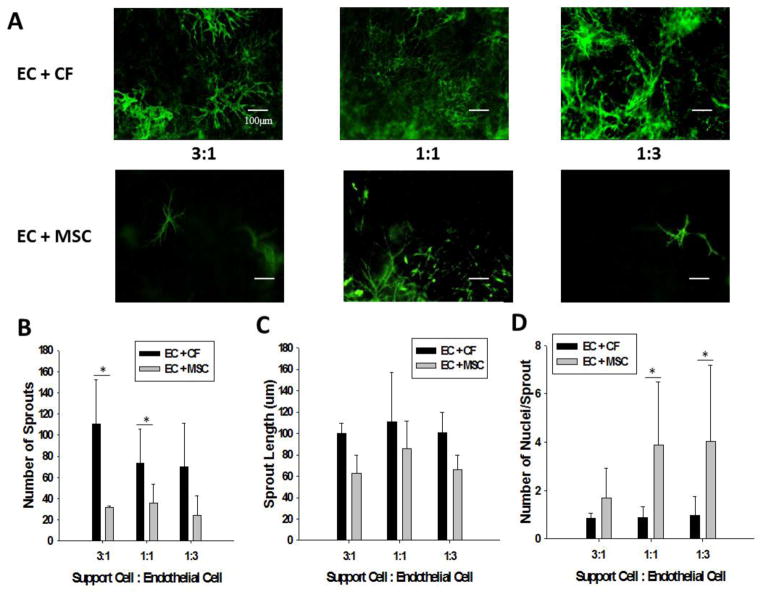
Figure 7A shows representative stained fluorescent images of ECs in co-culture with both CFs and MSCs. In quantifying these sprouts as in the previous experiment, the total number of sprouts was found to be greater for all concentrations of CFs to ECs, as compared to the conditions where the MSCs served as support cells. Results from the ANOVA indicate that there is a significant interaction between the support cell type and the ratio of support cells to ECs (p<0.001). In the 1:1 condition of Support Cells: ECs, the CFs allowed for a significantly greater number of sprouts to grow versus the MSCs (p=0.007), while in the 3:1 condition the difference in the number of sprouts was even greater between the CFs and the MSCs (p=0.005). There was no significant difference at the 1:3 ratio between cell types.
Figure 7.
(A) Representative fluorescent images of EC sprout formation in varied concentration co-culture with CFs (top row) and MSCs (bottom row). (B) Total number of sprouts in the varied support cell co-culture conditions. CFs as a support cell allow for a significantly greater number of sprouts compared to MSCs both in the 3:1 and 1:1 conditions (* denotes p<0.005). (C) The average sprout length for the EC: CF and EC: MSC co-cultures at varying ratios of support cell to EC. (D) The number of nuclei per sprout in varied support cell ratio co-culture studies. Note that the EC: MSC co-cultures have significantly more nuclei per sprout at both the 1:1 and 1:3 support cell to EC ratios (* denotes p<0.02).
Although none of the support cell conditions demonstrated a significant difference in sprout length, the CF support cell conditions tended to have longer sprouts (Fig. 7C). The results from the one-way ANOVA of sprout length by support cell type, irrespective of support cell to EC ratio indicated that CFs have significantly longer sprouts than the MSCs (p=0.023). With regards to the average number of nuclei per sprout the ECs are able to form the most multicellular sprouts when cultured with MSCs (Fig. 7D). The number of multi-cellular sprouts is significantly greater in the 1:1 MSC:EC and 1:3 MSC:EC conditions as compared to the CF-EC co-culture of the same ratios (p<0.001 and p=0.019, respectively). There was no significant difference in the number of multi-cellular sprouts in the 3:1 condition, however, MSCs tended to support more multi-cellular sprouts than when ECs were cultured with CFs.
DISCUSSION
The primary impediment in the translation of most tissue engineering research is the inability to form a functional vascular network in order for the tissue to anastomose to the host, which is crucial for the survival of the tissue post-implantation. Several groups have demonstrated that the pre-vascularization of tissue constructs by EC network formation prior to implantation aids in more rapid anastomosis of the construct to the host. While many studies have established the necessity of support cells in the formation of EC sprouts and vascularization, little work has been done to investigate CFs as a support cell, even though they have been shown to secrete angiogenic factors [3, 4, 22] and are a critical part of engineered myocardial tissue formation and contractile function [13]. Although CFs may be more difficult to isolate from an allogeneic source than MSCs or Adipose derived-Stem Cells, this cell line is more relevant because they are one of the largest cell populations present in the heart. In this study we compared the in vitro culture of EC-only constructs, and constructs fabricated with ECs in co-culture with CFs and MSCs as support cells to study the potential of CFs to serve as a support for EC sprout formation in 3D. The primary hypothesis of these studies was that neonatal CFs would provide as good of a support for EC sprout formation as MSCs, which are the “gold standard” of support cells for EC maturation in vitro [17].
The first set of experiments in the mixed media study focused on determining the optimal medium concentration to support the growth of both the ECs and support cells in mixed culture. The results of the first part of these experiments (Fig. 1) indicated that ECs were able to grow in all culture medium formulations; however, the ECs demonstrated a higher number of cells in the 50%EC-50%MSC media condition. One likely explanation for these findings could be that EC media contains 2% fetal bovine serum, while MSC medium contains 20% FBS. When the mediums are mixed, the ECs are receiving 11% serum, a much greater amount than in their normal growth medium, and higher serum levels have been demonstrated to induce ECs to proliferate at a more rapid rate [23].
Analysis of sprout formation in the mixed media study demonstrated that ECs formed significantly more sprouts when cultured with CFs in the 1:1 ratio of CF to EC media (Figure 3A). When cultured on their own, the ECs formed the longest sprouts with the 100% MSC media, which could again be related to the amount of serum in the MSC media. When considering the multi-cellularity of the ECs sprouts in the media studies, the ECs formed the most multi-cellular sprouts when cultured with the CFs in 100% EC media. The multi-cellularity of the CF-EC co-culture in the 100% EC media was significantly greater than the MSC-EC co-culture in the same media (p=0.002) and the CF-EC co-culture in the 50/50 CF-EC media (p=0.007), indicating that CFs are able to support multi-cellular spout formation when cultured in 100% EC medium. It has been determined that in order to create a stable vascular system, multiple endothelial cells must form a sprout structure [8, 28]. As ECs form stable vascular lumens a second endothelial cell, the stalk cell, connects behind the leading EC, known as a tip cell. As more mature vessels form, additional ECs form along the sprout, thus creating more multi-cellular sprouts. The creation of a perfusable vascular network within a tissue structure requires the formation of these multi-cellular sprout structures; therefore it can be inferred that CFs will be able to support a mature EC vascular network in a pre-vascularized engineered tissue. All in all, since there appeared to be no altered effects on EC proliferation in the 1:1 mixed medium, the proliferation in mixed cell populations was best for each support cell type in these formulations and sprout formation was generally best in, for the remaining studies we used the 1:1 Support Cell-EC medium.
The second set of experiments in this manuscript focused on determining any changes in the response of ECs to alterations in the ratios of support cells to ECs. In analyzing EC specific proliferation in the various groups in this study following 10 days of culture in our system via flow cytometry, it became evident that ECs proliferated best and maintained a higher percentage of the total cells in the CF co-culture conditions (Fig. 6A, B). When ECs were cultured with the MSCs as a support cell there was a significant decrease in the percent of ECs remaining in the constructs after ten days (Fig. 6C). One possible explanation for these results could be that the MSCs are proliferating at a greater rate than the ECs and inhibiting EC growth via contact inhibition. Indeed, it has been previously demonstrated that VEGF induces MSC proliferation [24], and since the constructs for this study were all cultured in 1:1 EC to support cell medium, there would be VEGF present from the EC medium. This seems to agree with the complete data set, as although the total cell count was higher when ECs were cultured with MSCs (Fig. 5A), the EC proliferation was much lower in the cultures that had higher initial support cell numbers (Fig. 6B). Higher ratios of MSC:EC in culture have also led to EC apoptosis via contact-mediated release of reactive oxygen species [25], which could also negatively impact EC number in co-culture with MSC.
From the perspective of the CFs in these ratio studies, it has been previously demonstrated that CF conditioned medium can promote EC proliferation via growth factor release [17], which may also explain the higher retention of ECs in the CF conditions. Another previous study that qualitatively investigated the effects of CFs in fibrin gels on EC sprout formation found that CFs at low concentrations (1:1) promote EC sprouts [26]. However, this same study also found that co-culture of CFs with ECs at a higher ratio (1:5 EC to CF ratio) significantly reduced sprouting. While this finding seems to be in conflict with the results reported here, it is important to note that the 1:5 EC to CF ratio was higher than any of the ones examined in this study. In addition, the CFs used in this previous work were epicardial in origin and taken from older porcine hearts, while those used here were myocardial CFs from neonates. The age of the CF donor tissue can be especially critical given that it is known that the growth factor expression of CFs changes significantly with age [27]. Although there is a trend that the CFs aid in longer EC sprout formation, culture with MSCs resulted in significantly more multicellular sprouts in the ratio study (Fig. 7D), which is more indicative of the in vivo process [28]. MSCs have been previously demonstrated to aid in vessel stability during in vitro co-culture with ECs [29]. Moreover, MSCs are known to produce TGF which can lead to decreased EC migration/ proliferation and enhanced vessel maturity via Alk1 and Alk5 receptors [8].
We next sought to explore two potential mechanisms for the differences in sprout formation and number between CF and MSC cultures via some preliminary experiments. We first investigated the effects of medium conditioned by each of the support cell types on EC proliferation (supplementary materials Figure S2). In general there was not much of an effect of conditioned medium on EC proliferation. We also looked at co-localization of the support cells with ECs as this has been shown to be an important aid in sprouting vasculogenesis, but saw no obvious co-localization in either support cell type (supplementary material Figure S4). One other potential mechanism that could influence these results is the ECM secreted by support cells. Several studies have elucidated the importance of ECM, and particularly type I Collagen, in promoting vasculogenesis of ECs (reviewed in [34]). Ultimately, we would expect that there is not much ECM production in our system, especially of Collagen I. This is because we use a fibrinolytic inhibitor in the CF medium (epsilon-aminocaproic acid) and the breakdown of the fibrin is usually required in this system to initiate other matrix production by cells. In addition, there is no ascorbic acid in any of the culture medium formulations, which is required for fibrous collagen formation. However, there could certainly be other ECM proteins being produced that play a role (e.g. Laminin which is known to be secreted by both CFs and MSCs), and future studies with this system will seek to investigate this as a potential mechanism.
The use of fibrin as a scaffold should also be taken into consideration when discussing EC sprout formation as it has many intrinsic angiogenic properties [30]. Fibrin scaffolds can be used as temporary scaffolds that mimic the natural wound healing response and induce other cell types to produce their own ECM. As the CFs degrade the fibrin over time, they produce ECM. In our cultures, a fibrinolytic inhibitor, ε-aminocaproic acid (ACA), was added to the culture medium to control the rate of degradation of the fibrin. Fibrin degradation products (FDPs) have been demonstrated to be angiogenic signaling molecules through both clotting dependent and clotting independent mechanisms [31]. Furthermore, it has been shown that the degradation of fibrin enhances the proliferation of cells (e.g. vascular smooth muscle cells [32]). It is possible that the CFs degrade the fibrin at a faster rate than the MSCs, which would result in the release of more FDPs, thus increasing EC proliferation and sprout formation. An increase in the amount of FDPs could explain the increase in the nuclei per sprout in the EC: CF co-cultures that were cultured in 100% EC medium (Fig. 4C), as this medium contains no ACA. Moreover, it has been shown that FDPs can enhance the angiogenic effects of VEGF and bFGF [33], indicating a possible synergistic effect that could enhance multicellular sprout formation.
The results of this study indicate that ECs are able to proliferate and form sprouts when co-cultured with both CFs and MSCs. In mixed media conditions, ECs formed the longest sprout length in all CF media conditions, and formed more multicellular cells in culture with EC media; however, ECs cultured with the MSC had more multicellular sprouts in all conditions. Furthermore, with respect to the varied co-culture experiments, the ECs in culture with the CFs were able to form the longer sprouts compared to ECs cultured on their own or with MSCs. When considering EC proliferation, the ECs had the greatest proliferation after ten days when cultured with the CFs as compared to the ECs cultured with the MSCs. In conclusion, CFs are able to provide as good a support for EC sprout formation, compared to MSCs, in terms of sprout number and formation. However, MSCs are able to better aid in the formation of multi-cellular EC sprouts, better mimicking the process of angiogenesis in vivo. These results also further underline the importance of utilizing mixed cell populations that contain CFs and other potential pericyte cells in cardiac tissue engineering approaches. As previously mentioned non-myocyte cells such as cardiac fibroblasts make up 70% of the composition of the myocardium and play a key role in the contractile properties of the heart [13]. For this reason, the cell source must be taken into account when constructing a tissue engineered myocardial scaffold. Future studies can expand on this work by considering other components such as the extracellular matrix produced by CFs, which is primarily made up of fibronectin, collagen and laminin and whether the paracrine signaling between CFs, ECM and ECs plays a role in EC growth and sprout formation.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH (R00HL093358, to LB). Some of the materials were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine through a grant from the NIH (P40RR017447). The authors also thank Dr. Dean Glettig for his help with flow cytometry.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in Cancer and Other Diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering Vascularized Tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 3.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, et al. Angiogenesis in Tissue Engineering: Breathing Life into Constructed Tissue Substitutes. Tiss Eng. 2006;12:2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, et al. Prevascularization of a Fibrin-Based Tissue Construct Accelerates the Formation of Functional Anastomosis with Host Vasculature. Tiss Eng Part A. 2009;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergers G, Song S. The Role of Pericytes in Blood-vessel Formation and Maintenance. Neuro-Oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhardt H, Betsholtz C. Endothelial-pericyte Interactions in Angiogenesis. Cell Tiss Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 7.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of Vascular Morphogenesis and Homeostasis through the Angiopoietin–Tie System. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 8.Holderfield MT, Hughes CCW. Crosstalk Between Vascular Endothelial Growth Factor, Notch, and Transforming Growth Factor-β in Vascular Morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 9.Lovett M, Lee K, Edwards A, Kaplan D. Vascularization Strategies for Tissue Engineering. Tiss Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis DR, Kizana E, Terrovitis J, Barth AS, Zhang Y, Smith RP, et al. Isolation and Expansion of Functionally-competent Cardiac Progenitor Cells Directly from Heart Biopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent Stem Cell-Derived Cardiac Tissues with Advanced Structure and Function. Biomaterials. 2011;32:9180–9187. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gai G, Leung EL, Costantino PD, Aguila JR, Nguyen DM, Fink LM, et al. Generation and Characterization of Functional Cardiomyocytes Using Induced Pluripotent Stem Cells Derived from Human Fibroblasts. Cell Biol Int. 2009;33:1184–1193. doi: 10.1016/j.cellbi.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Naito H, Melnychenko I, Didié M, Schneiderbanger K, Schubert P, Rosenkranz S, et al. Optimizing Engineered Heart Tissue for Therapeutic Applications as Surrogate Heart Muscle. Circulation. 2006;114:I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 14.Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla H. Expression and Adhesive Properties of Basement Membrane Proteins in Cerebral Capillary Endothelial Cell Cultures. Cell Tissue Res. 2002;310:19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 15.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. Endothelial Cell Co-culture Within Tissue-Engineered Cardiomyocyte Sheets Enhances Neovascularization and Improves Cardiac Function of Ischemic Hearts. Circulation. 2008;118:S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 16.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, et al. Transplantation of a Tissue-Engineered Human Vascularized Cardiac Muscle. Tiss Eng Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Eghbali-Webb M. Release of Pro- and Anti-angiogenic Factors by Human Cardiac Fibroblasts: Effects on DNA Synthesis and Protection under Hypoxia in Human Endothelial Cells. BBA - Molecular Cell Research. 2001;1538:273–82. doi: 10.1016/s0167-4889(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 18.Neofytou EA, Chang E, Patlola B, Joubert LM, Rajadas J, Gambhir SS, et al. Adipose tissue-derived stem cells display a proangiogenic phenotype on 3D scaffolds. J Biomed Mater Res Part A. 2001;98:383–393. doi: 10.1002/jbm.a.33113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye KY, Sullivan KE, Black LD. Encapsulation of Cardiomyocytes in a Fibrin Hydrogel for Cardiac Tissue Engineering. J Vis Exp. 2011;55:3251. doi: 10.3791/3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black LD, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel–Based Engineered Myocardium via Gap Junction Modification. Tiss Eng Part A. 2009;15:3099–3108. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams C, Johnson SL, Robinson PS, Tranquillo RT. Cell Sourcing and Culture Conditions for Fibrin-Based Valve Constructs. Tiss Eng Part A. 2006;12:1489–1502. doi: 10.1089/ten.2006.12.1489. [DOI] [PubMed] [Google Scholar]
- 22.Valarmathi M, Davis J, Yost M, Goodwin R, Potts J. A Three-dimensional Model of Vasculogenesis. Biomaterials. 2009;30:1098–1112. doi: 10.1016/j.biomaterials.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 23.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and Characterization of an Injectable Pericardial Matrix Gel: A Potentially Autologous Scaffold for Cardiac Tissue Engineering. Tiss Eng Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, et al. VEGF Improves Survival of Mesenchymal Stem Cells in Infarcted Hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent Inhibition of Angiogenesis by Mesenchymal Stem Cells. Blood. 2009;113:4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehls V, Herrmann R, Huhnken M, Palmetshofer A. Contact-Dependent Inhibition of Angiogenesis by Cardiac Fibroblasts in Three-Dimensional Fibrin Gels In Vitro: Implications for Microvascular Network Remodeling and Coronary Collateral Formation. Cell Tissue Res. 1998;293:479–488. doi: 10.1007/s004410051140. [DOI] [PubMed] [Google Scholar]
- 27.Noseda M, Schneider MD. Fibroblasts Inform the Heart: Control of Cardiomyocyte Cycling and Size by Age-Dependent Paracrine Signals. Dev Cell. 2009;16:161–162. doi: 10.1016/j.devcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Szabó A, Czirók A. The Role of Cell-Cell Adhesion in the Formation of Multicellular Sprouts. Mathematical Modelling of Natural Phenomena. 2010;5:106. doi: 10.1051/mmnp/20105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy GP, Ahsan T, O’Brien T, Barry F, Nerem RM. Bone Marrow–Derived Mesenchymal Stem Cells Promote Angiogenic Processes in a Time- and Dose-Dependent Manner. Tiss Eng Part A. 2009;15:2459–2470. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 30.Hinsbergh VWM, Collen A, Koolwijk P. Role of Fibrin Matrix in Angiogenesis. Ann N Y Acad Sci. 2001;936:426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 31.Rickles FR, Patierno S, Fernandez PM. Tissue Factor, Thrombin, and Cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 32.Ahmann KA, Weinbaum JS, Johnson SL, Tranquillo RT. Fibrin Degradation Enhances Vascular Smooth Muscle Cell Proliferation and Matrix Deposition in Fibrin-Based Tissue Constructs Fabricated In Vitro. Tiss Eng Part A. 2010;16:3261–3270. doi: 10.1089/ten.tea.2009.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bootle-Wilbraham CA, Tazzyman S, Thompson WD, Stirk CM, Lewis CE. Fibrin Fragment E Stimulate the Proliferation, Migration and Differentiation of Human Microvascular Endothelial Cells In Vitro. Angiogenesis. 2001;4:269–275. doi: 10.1023/a:1016076121918. [DOI] [PubMed] [Google Scholar]
- 34.Davis GE, Senger DR. Endothelial Extracellular Matrix: Biosynthesis, Remodeling, and Functions During Vascular Morphogenesis and Neovessel Stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.