Abstract
Dysfunctions in serotonin (5-hydroxytryptamine, 5-HT) systems have been associated with several psychiatric illnesses, including anxiety, depression, obsessive-compulsive disorders and autism spectrum disorders. Convergent evidence from genetic analyses of human subjects has implicated the integrin β3 subunit gene (ITGB3) as a modulator of serotonergic systems via genetic interactions with the 5-HT transporter gene (SLC6A4, SERT). While genetic interactions may result from contributions of each gene at several levels, we hypothesize that ITGB3 modulates the 5-HT system at the level of the synapse, through the actions of integrin αvβ3. Here we utilized a genetic approach in mouse models to examine Itgb3 contributions to SERT function both in the context of normal and reduced SERT expression. As integrin αvβ3 is expressed in postsynaptic membranes, we isolated synaptoneurosomes, which maintain intact pre- and post-synaptic associations. Citalopram binding revealed significant Slc6a4-driven reductions in SERT expression in midbrain synapses, whereas no significant changes were observed in hippocampal or cortical projections. Expecting corresponding changes to SERT function, we also measured 5-HT uptake activity in synaptoneurosomal preparations. Itgb3 single heterozygous mice displayed significant reductions in 5-HT Vmax, with no chages in Km, in midbrain preparations. However, in the presence of both Itgb3 and Slc6a4 heterozygozity, 5-HT uptake was similar to wild-type levels, revealing a significant Slc6a4 by Itgb3 genetic interaction in the midbrain. Similar findings were observed in cortical preparations, whereas in the hippocampus, most Vmax changes were driven solely by Slc6a4. Our findings provide evidence that integrin αvβ3 is involved in the regulation of serotonergic systems in some, but not all 5-HT synapses, revealing novel contributions to synaptic specificity within the central nervous system.
Keywords: serotonin transporter, integrin, genetic interaction, neuropsychiatric disorders, autism
1. Introduction
Dysfunction in serotonin (5-hydroxytryptamine, 5-HT) neurotransmission has been implicated in the etiology of mood and developmental disorders including anxiety, depression, and autism-spectrum disorders (ASD). Several genetic variants in the 5-HT transporter gene (SERT, SLC6A4) have been associated with behavioral phenotypes manifested in these disorders, especially in the context of genetic interactions or under specific environmental conditions (Caspi et al. 2003, Sutcliffe et al. 2005, Murphy and Moya 2011). Variations in whole blood 5-HT levels, found in several neuropsychiatric disorders, including autism, bipolar disorder and seasonal affective disorder (Velayudhan et al. 1999, Willeit et al. 2008), are associated with non-coding variation in ITGB3 (Weiss et al. 2004). Genetic interaction of ITGB3, which encodes the integrin β3 subunit (forming the integrin αIIbβ3 in platelets and integrin αvβ3 in brain), and SLC6A4, either in mRNA expression or autism susceptibility, further reinforces the suggestion that these two genes may interact to modify 5-HT homeostasis (Weiss et al. 2004). Whereas genetic interactions do not typically translate into functional or biochemical interactions, we have reported a physical and functional association between integrin αIIbβ3 and SERT in platelets (Carneiro et al. 2008). Thus we hypothesize that ITGB3 and SLC6A4 interact to modulate SERT expression and function in the brain. Here we utilized a genetic approach to document unique and interactive contributions of these genes to transporter expression and function in the mouse synaptic preparations.
2. Material and Methods
2.1. Animals
Mouse studies were performed in accordance with humane guidelines established by the Vanderbilt Institutional Animal Care and Use Committee under approved protocol (M/09/198). Both Itgb3−/− (Hodivala-Dilke et al. 1999) and Slc6a4−/− (Bengel et al. 1998) mouse lines were previously backcrossed onto C57BL/6 for more than 20 generations. Slc6a4+/−, Itgb3+/− mice were generated by crossing C57BL/6 Itgb3−/− males and C57BL/6 Slc6a4−/− females. Mice derived from this crossing were not used for experiments to avoid rearing effects caused by Slc6a4−/− dam phenotypes. Instead, the Itgb3+/−, Slc6a4+/− male offspring were paired with wildtype C57BL/6J females producing offspring of four genotypes: Itgb3+/+, Slc6a4+/+ (WT); Itgb3+/−, Slc6a4+/+ (Itgb3+/−); Itgb3+/+, Slc6a4+/− (Slc6a4+/−); and Itgb3+/−, Slc6a4+/−. Male and female offspring were housed by sex with mixed-genotype littermates in groups of 2–5 per cage. Mice were maintained on a 12-hour light-dark cycle, and provided with food and water ad libitum. Littermate males and females were utilized for all biochemical and neurochemical assays.
2.2. Synaptoneurosome Preparation
Synaptoneurosomes were obtained as previously described (Phillips et al. 2001). Briefly, mice were rapidly decapitated and brain regions were dissected onto 0.32 M sucrose in HEPES containing 0.1mM CaCl2 and 1mM MgCl2 at 4°C. Samples were homogenized in a piston-type Teflon® pestle with stainless steel shaft and replaceable grinding vessel and cell debris/nuclei separated by centrifugation at 1,000 x g. Supernatants were collected and spun at 10,000 x g for isolation of crude synaptoneurosomes. Immediately after preparation, synaptoneurosomal protein was measured using a modified Lowry protocol with bicinchoninic acid (BCA Protein Assay Kit, Pierce Chemical Company, Rockford, IL). Approximately 1mg was used immediately for 5-HT saturation kinetic studies of 5-HT uptake, and the remaining was frozen for citalopram binding and western blot studies.
2.3. Saturation Kinetic Studies of [3H] 5-HT Uptake
Synaptoneurosome pellets were resuspended in Krebs-Ringer’s HEPES (KRH) buffer (130mM NaCl, 1.3mM KCl, 2.2mM CaCl2, 1.2mM MgSO4, 1.2 mM KH2PO4, 1.8g/L glucose, 10mM HEPES, pH 7.4 containing 100 μM ascorbic acid and 100 μM pargyline). Synaptoneurosomes (100μg for midbrain and 200μg for hippocampus and cortex) were incubated for 10 min at 37° C in test tubes containing 100μl of KRH buffer, and 50 μl [3H] 5-HT (Concentrations ranging from 12.5–400nM. Hydroxytryptamine Creatinine Sulfate, 5-[1, 2-3H(N)]-(Serotonin). Perkin Elmer, Walthman, MA). An identical set of tubes contained 50 μl of 1μM paroxetine (Sigma Aldrich, Saint Louis, MO) to define SERT-specific uptake. Next, samples were harvested via Brandel tissue harvester and filtered onto GF/B Whatman filters (Brandel, Gaithersburg, MD). Filters were dissolved overnight in scintillation fluid (Econo-Safe™, Research Products International Corp. Mount Prospect, IL) then radioactivity was quantified in a Packard counter by QuantaSmart 4.0 software.
2.4. [3H]-Citalopram Binding
Synaptoneurosomes (100μg for midbrain and 250μg for hippocampus and cortex) were incubated with 5 nM [3H]-citalopram (Racemic citalopram,[N-Methyl-3H]. Perkin-Elmer, Walthman, MA) on ice for 20 min then harvested using a Brandel tissue harvester onto GF/B Whatman filters. An identical set of tubes contained 1μM paroxetine (Sigma Aldrich, Saint Louis, MO) to define SERT-specific binding. Filters were dissolved overnight in scintillation fluid then radioactivity was quantified in a Packard counter by QuantaSmart 4.0 software.
2.5. Western Blotting
Midbrain synaptoneurosomes pellets were resuspended in 1% sodium dodecyl sulfate in phosphate buffered saline pH 7.4 and protein was measured using a modified Lowry protocol with bicinchoninic acid (BCA Protein Assay Kit, Pierce Chemical Company, Rockford, IL). No hippocampal or cortical samples were available for western blots. 50μg of protein were loaded onto 17-well Pierce Protein Gels (Thermo Scientific). Gel electrophoresis was performed at 100v for 3 hours then proteins were transferred overnight at 4°C onto PVDF membranes (Immobilon, Millipore, Billerica, MA). After transfer, membranes were blocked with 5% milk in 1x tris-buffered saline pH 7.4 and incubated with antibodies at 1:250 or 1:1000 dilutions overnight at 4 °C. Secondary antibodies were added at 1:2500 dilution and proteins detected with chemiluminescence. Amersham Hyperfilm ECL films were exposed at 1,5,10, and 30 minutes to address linearity of the data (GE Healthcare, Pittsburgh, PA). Films were scanned in tagged image file format (.tiff) and bands quantified by densitometry using Image J. Antibodies included: rabbit anti-integrin αv and rabbit anti-integrin β3 (Cell Signaling Technology, Denvers, MA), mouse anti-syntaxin (Millipore, Billerica, MA), and guinea pig anti-5-HT transporter (Frontier Science Co., LTD, Hokkaido, Japan).
2.6. Data Analysis
All data was analyzed in Prism 4.0c (Graphpad Software, Inc., LaJolla, CA). Two-way ANOVA was used with Slc6a4 and Itgb3 as variables to identify contributions of each gene. Dunnett’s multiple comparison tests were used to compare each genotype to wild-type (WT). Kruskal-Wallis test was used to analyze western blot samples as each group of samples was run in a different day and normalized to each individual control (WT =100%). In this particular case we used Dunn’s post-tests to identify statistical significant genotype differences. Saturation data was fit to a one-site non-linear regression model. Scatchard plots were fit by linear regression for calculation of Vmax and Km. A P value of less than 0.05 was considered statistically significant. All data are shown as mean ± standard error of the mean (SEM, represented by error bars).
3. Results and Discussion
3.1. Synaptic SERT expression is reduced in the midbrains of double heterozygous mice
To examine the influence of Itgb3 heterozygosity on SERT expression and uptake activity, we studied Itgb3+/− and Slc6a4+/−, Itgb3+/− mice. Whereas SERT expression patterns in midbrain neurons and in projection areas have been extensively studied (Bengel et al. 1997, Tao-Cheng and Zhou 1999), we have little information on the expression of integrin αvβ3 in the intact brain. Few studies have identified post-synaptic expression of integrin αvβ3 in hippocampal synapses (Cingolani et al. 2008); moreover, it is possible that extracellular-matrix proteins, which bind integrins, maintain synaptic structure and thus pre- and post-synaptic interactions may be essential for proper synaptic function (Wang et al. 2008). Therefore, to examine the influence of Slac6a4 and Itgb3 heterozygozity in synaptic SERT expression and uptake activity, we isolated synaptoneurosomes in the presence of CaCl2 and MgCl2, maintaining N-cadherin, NCAM, and integrin-mediated interactions (Phillips et al. 2001).
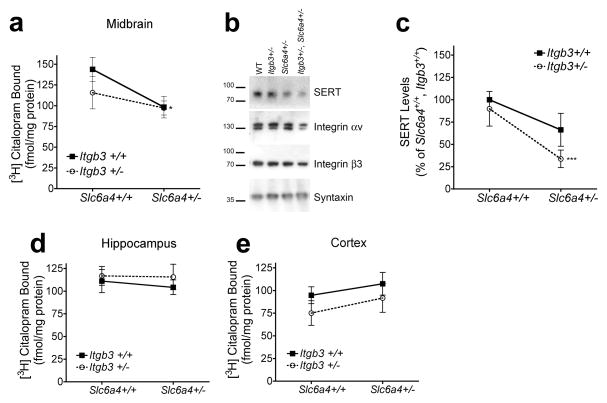
We prepared synaptoneurosomes from midbrain, hippocampus, and cortices dissected from WT, Itgb3+/−, Slc6a4+/−; and Itgb3+/−, Slc6a4+/− littermates and assessed [3H]-citalopram binding. The data revealed a significant a significant reduction in [3H]-citalopram binding in the context of Slc6a4 heterozygosity in midbrain synaptoneurosomes (Figure 1a). We used western blot analysis to determine whether these changes may correspond to reductions in SERT expression in terminals. Our data indicates that Slc6a4 modifies SERT expression in midbrain terminals (Figure 1b, c). Similar findings were found in previous studies of the Slc6a4+/− mice (Bengel et al. 1998). As synapse number/size may be influenced by 5-HT signaling (Udo et al. 2005) or integrin function (Cingolani et al. 2008), we assessed syntaxin expression as a control for pre-synaptic terminal expression. No significant changes were found in integrin αv, integrin β3 or syntaxin expression (Figure 1b). We found no significant alterations in [3H]-citalopram binding in synaptic preparations from two terminal fields: hippocampus and cortex (Figure 1d and 1e, respectively). These findings indicate that, although SERT tissue expression may be influenced by genotype, neither Slc6a4 nor Itgb3 modified synaptic SERT expression in the two terminal fields examined. The discrepancy between midbrain and cortical and hippocampal SERT synaptic expression may be due to differences in the localization of SERT in these brain regions. While SERT is strictly localized to axonal/pre-synaptic terminals in cortex and hippocampus, both at the perisynaptic plasma membrane and in intracellular vesicles, midbrain SERTs localize to both axonal/pre-synaptic and dendritic/post-synaptic terminals (Tao-Cheng and Zhou 1999). It is possible that axonal SERT localization is tightly regulated by trafficking mechanisms, independent of the total protein expressed in the cell body, whereas dendritic expression, predominantly intracellular, may be directly correlated with mRNA/protein expression at the cell body. To determine whether these changes in expression are correlated with changes in SERT function, we performed 5-HT reuptake studies.
Figure 1.
SERT expression levels are reduced in midbrain synapses of Itgb3+/+, Slc6a4+/− and Itgb3+/−, Slc6a4+/− mice. (a) Two-way ANOVA reveals significant contributions of Slc6a4 to midbrain synaptoneurosomal [3H]-citalopram binding. WT: 143.8 ± 14.46 fmol/mg, n = 12; Itgb3+/−: 115.6 ± 19.47 fmol/mg, n = 12; Slc6a4+/−: 98.26 ± 13.06 fmol/mg, n = 12; Itgb3+/−, Slc6a4+/−: 97.18 ± 8.43 fmol/mg, n = 12; two-way ANOVA: Slc6a4 P <0.05. (b) Representative western blot showing SERT, integrin αv, integrin β3 and syntaxin (as a presynaptic control) expression in midbrain synaptoneurosomes. (c) Densitometry analysis of western blots for SERT expression levels in midbrain synaptoneurosomes. WT: 100 ± 0%, n = 9; Itgb3+/−, Slc6a4+/+: 89.88 ± 19.4%, n = 12; Itgb3+/+, Slc6a4+/−: 66.26 ± 18.4%, n = 10; Itgb3+/−, Slc6a4+/−: 33.68 ± 9.79%, n = 8; Kruskal-Wallis one-way ANOVA: P < 0.05, Dunn’s post-hoc WT vs. Itgb3+/−, Slc6a4+/−: *P<0.05. Hippocampus (d) and cortex (e) synaptoneurosomal [3H]-citalopram binding is not significantly different across terminal fields examined. Data is shown as means ± SEM.
3.2. Itgb3 and Slc6a4 interact to modulate SERT uptake
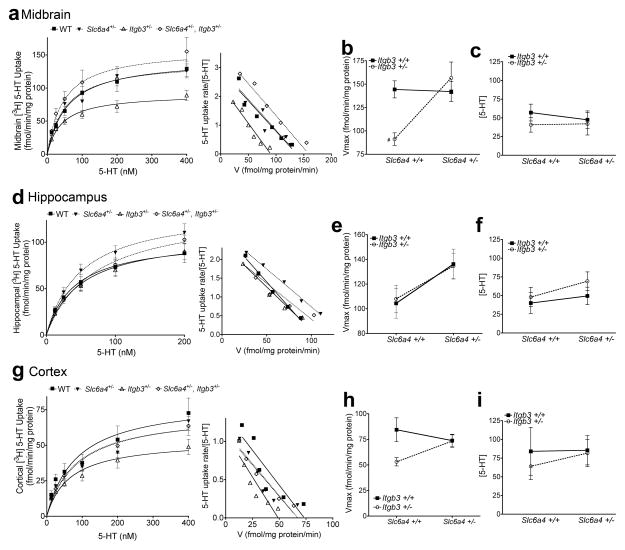
Saturation kinetic analysis of SERT 5-HT uptake from midbrains synaptoneurosomes revealed a significant interaction between Slc6a4 and Itgb3 genes (Figure 2a–c). Itgb3+/− mice exhibited significant reductions in midbrain Vmax (Figure 2b) compared to WT, while Itgb3+/−, Slc6a4+/− mice exhibited normal 5-HT uptake (Figure 2b). No significant changes were observed in Km (Figure 2c). This result supports the hypothesis that axonal/pre-synaptic plasma membrane SERT expression is not reduced in Slc6a4+/− or Itgb3+/−, Slc6a4+/− mice and replicates the initial functional studies performed in Slc6a4+/− mice (Bengel et al. 1998). Our results reveal that heterozygosity in the Itgb3+/− line is sufficient to reduce SERT activity, by a mechanism yet unidentified, as no changes in integrin αvβ3 were observed in the same preparations. It is possible that pre-synaptic levels of integrin αvβ3 are modified, but remain undetected in synaptoneurosomal preparations. Importantly, no 5-HT uptake changes were observed in Itgb3+/− platelets (Carneiro et al. 2008), suggesting a specific neuronal mechanism for integrin αvβ3 modulation of SERT activity in the midbrain.
Figure 2.
3[H] 5-HT uptake in synaptoneurosomes reveals differential changes in midbrain and cortices of Itgb3+/− mice. (a) Saturation kinetic studies and Scatchard plot for midbrain synaptoneurosomes. (b) Two-way ANOVA of Vmax for 5-HT uptake in the midbrain reveals a significant statistical interaction between Slc6a4 and Itgb3. WT: 144.4 ± 4.58 fmol/min/mg protein, n = 4; Itgb3+/−: 91.19 ± 3.33 fmol/min/mg protein, n = 4; Slc6a4+/−: 142.1 ± 5.3 fmol/min/mg protein, n = 4; Itgb3+/−, Slc6a4+/−: 156.7 ± 8.45 fmol/min/mg protein, n = 4; Two-way ANOVA: Interaction P = 0.0183; Dunnett’s post-hoc WT vs. Itgb3+/−: #P < 0.05. (c) No changes in Km were observed in the midbrain. (d) Saturation kinetics studies and Scatchard plots for SERT-mediated 5-HT uptake from hippocampal synaptoneurosomes. (e) Two-way ANOVA analysis of Vmax values obtained in d reveal significant contributions of Slc6a4 to 5-HT Vmax in the hippocampus. WT: 160 ± 12 fmol/min/mg protein, n = 4; Itgb3+/−: 119.1 ± 4.55 fmol/min/mg protein, n = 4; Slc6a4+/−: 150 ± 4.99 fmol/min/mg protein, n = 4; Itgb3+/−, Slc6a4+/−: 148.5 ± 3.5 fmol/min/mg protein, n = 4; two-way ANOVA: Slc6a4: P = 0.0330. (f) No significant changes were observed in Km. (g) Saturation kinetics studies and Scatchard plots for SERT-mediated 5-HT uptake in cortical synaptoneurosomes. (h) Two-way ANOVA analysis of cortical SERT Vmax reveals significant reductions in Itgb3+/− samples. WT: 84.37 ± 5.79 fmol/min/mg protein, n = 4; Itgb3+/−: 53 ± 2 fmol/min/mg protein, n = 4; Slc6a4+/− : 73.76 ± 3.1 fmol/min/mg protein, n = 4; Itgb3+/−, Slc6a4+/− : 73.76 ± 3.02 fmol/min/mg protein, n = 4; two-way ANOVA: Interaction P = 0.0731; Dunnett’s post-hoc Itgb3+/−, Slc6a4+/− vs. Itgb3+/−: #P < 0.05. (i) No significant changes were observed in SERT Km in cortical preparations. Data is shown as means ± SEM.
5-HT uptake was differentially modulated in the two terminal fields studied. In the hippocampus (Figure 2d–f), we found significant Vmax increases driven by Slc6a4 (Figure 2e). No changes in Km were observed (Figure 2f). In cortical preparations, Vmax reductions were found in Itgb3+/− mice compared to WT (Figure 2h). These changes in hippocampal uptake are not replicated in other studies; most laboratories report reductions in hippocampal Vmax in Slc6a4+/− mice (Murphy and Moya 2011). These differences may result from different fractionation methods used to isolate 5-HT synapses i.e. synaptosomes vs. synaptoneurosomes. Thus, we have detected changes in SERT transport activity, increases driven by genetic alterations in Slc6a4 in the hippocampus, and decreases driven by Itgb3. Together, in Itgb3+/−, Slc6a4+/− preparations, we observe no significant changes in SERT uptake activity.
How can we interpret alterations in SERT uptake vs. expression levels in Itgb3+/−, Slc6a4+/− mice? One possibility is the presence of two distinct SERT populations at the plasma membrane. One population has high capacity for 5-HT uptake, which is preferentially expressed at the plasma membrane of Slc6a4+/− mice. The second population, perhaps with a low-capacity uptake, is absent in Slc6a4+/− mice. Therefore 100% of SERTs at the plasma membrane of Slc6a4+/− mice have high capacity uptake, independently of Itgb3. As integrin αvβ3 may modulate SERT levels at the plasma membrane (Carneiro et al. 2008), Itgb3+/− mice may have reduced plasma membrane SERT expression in both low- and high-capacity uptake populations, and thus the resulting 5-HT reuptake observed is significantly reduced. While highly speculative, this interpretation is supported by the data observed in hippocampal preparations, where Itgb3 heterozygozity has no effect in SERT uptake and Slc6a4+/− mice show elevated SERT activity. Interestingly, these data suggest a protective effect of Slc6a4 heterozygozity in the context of Itgb3 heterozygozity. As both genes are highly polymorphic in humans, it is possible that this genetic interaction may play an important role in conferring risk for neuropsychiatric disorders.
4. Conclusions
Our studies reveal a surprising genetic interaction between Slc6a4 and Itgb3 in the modulation of 5-HT uptake in midbrain and cortical synapses. In accordance to previous studies, deletion or loss of function in Itgb3 reduces SERT uptake capacity in some brain areas, whereas Slc6a4 heterozygozity seems to increase or has no effect in SERT uptake. These effects suggest an important role of integrin αvβ3 in the midbrain. Future studies will reveal whether these alterations are sufficient to modify 5-HT signaling.
Highlights.
Epistatic interaction between Slc6a4 and Itgb3 in the modulation of the serotonin system.
Slc6a4 heterozygosity dictates SERT expression in midbrain synapses.
Midbrain and cortical serotonin uptake was significantly reduced in Itgb3−/+ mice.
A significant Slc6a4 x Itgb3 genetic interaction drives SERT Vmax in the midbrain.
Integrin αvβ3 is involved in the regulation of SERT in cortex and midbrain.
Acknowledgments
We thank Dennis Murphy and Richard Hynes for generating the Slc6a4 and Itgb3 lines used in this paper. We thank Jeremy Veenstra-Vanderweele and Randy D. Blakely for many helpful discussions. This work was supported by NIMH grant 090256-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bengel D, Johren O, Andrews AM, Heils A, Mossner R, Sanvitto GL, Saavedra JM, Lesch KP, Murphy DL. Cellular localization and expression of the serotonin transporter in mouse brain. Brain Res. 1997;778:338–45. doi: 10.1016/s0006-8993(97)01080-9. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–52. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–62. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional GxG and GxE differences in health and disease. Curr Opin Pharmacol. 2011;11:3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–79. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94:821–30. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Velayudhan A, Sunitha TA, Balachander S, Reddy JY, Khanna S. A study of platelet serotonin receptor in mania. Biol Psychiatry. 1999;45:1059–62. doi: 10.1016/s0006-3223(98)00239-x. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105:19520–5. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Veenstra-Vanderweele J, Newman DL, Kim SJ, Dytch H, McPeek MS, Cheng S, Ober C, Cook EH, Jr, Abney M. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet. 2004;12:949–54. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P, Winkler D, Brannath W, Fischer MB, Bondy B, Kasper S, Singer EA. Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology. 2008;33:1503–13. doi: 10.1038/sj.npp.1301560. [DOI] [PubMed] [Google Scholar]