Abstract
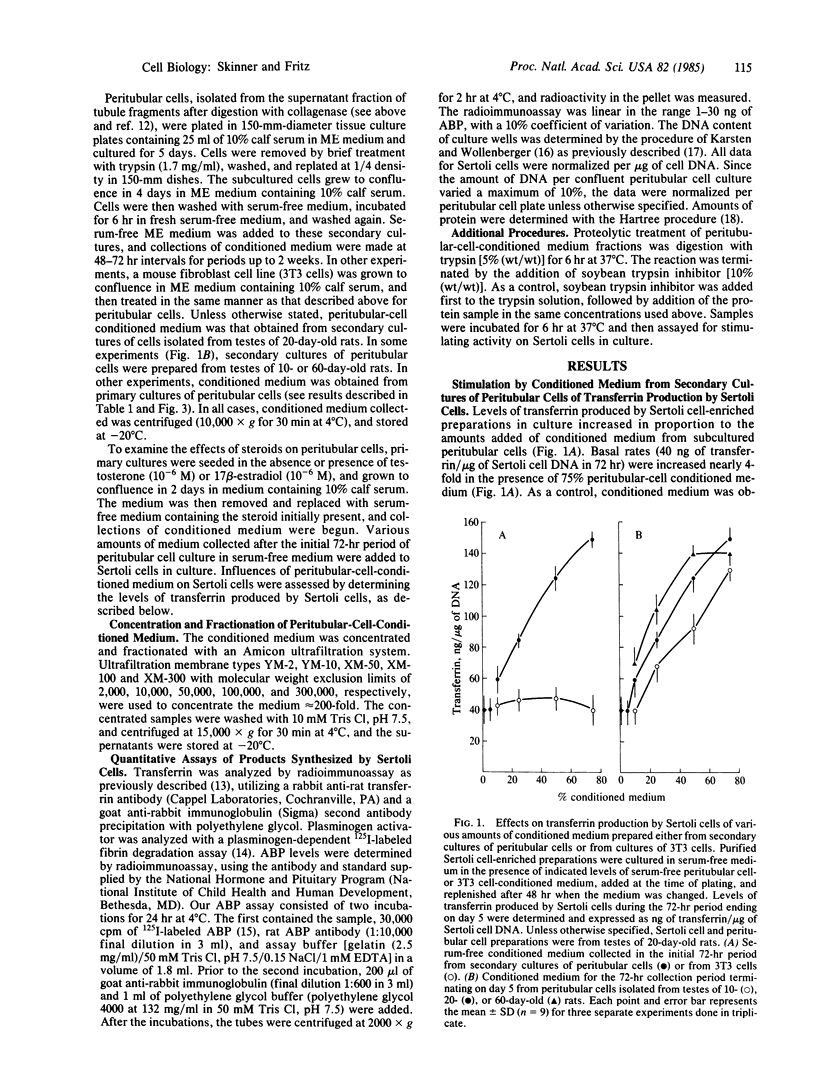
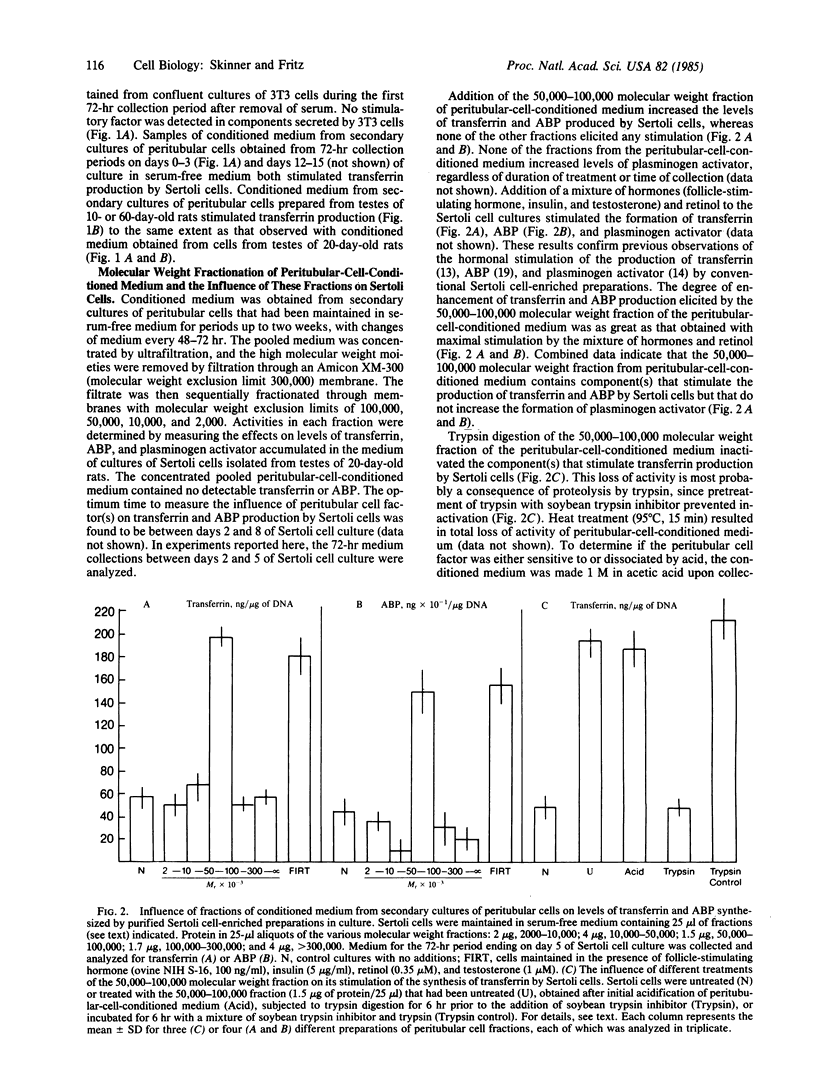
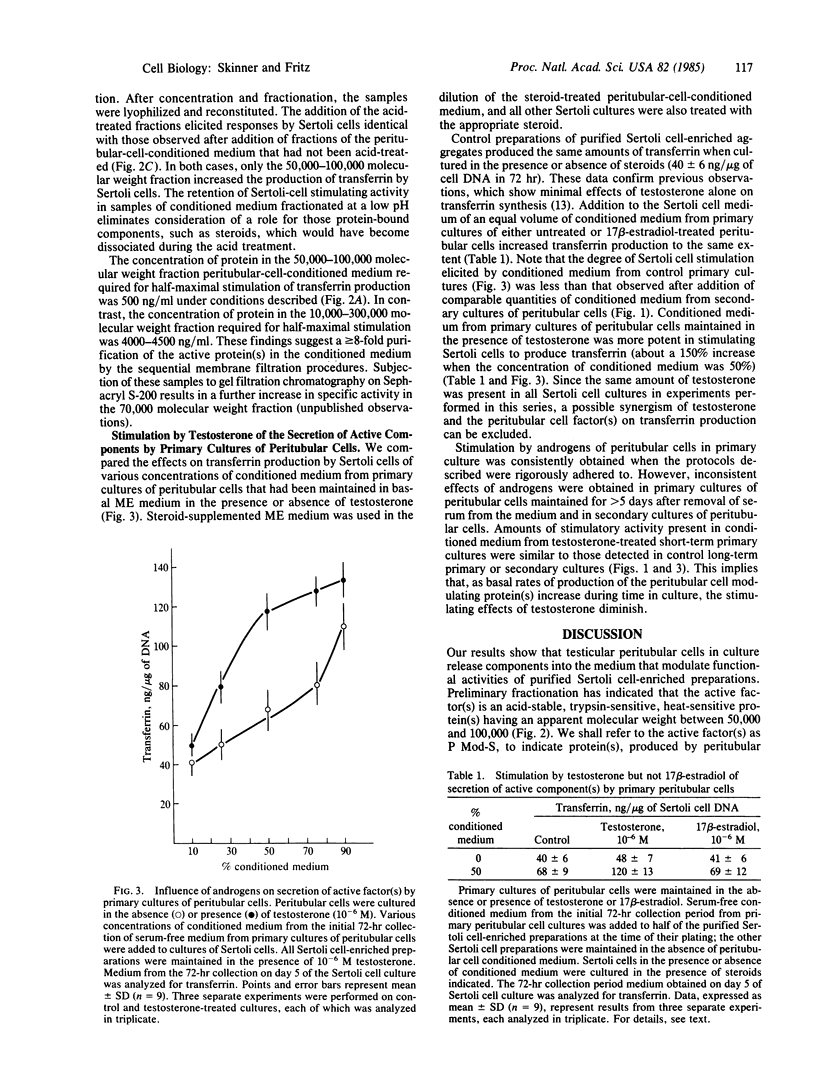
Peritubular cells of the seminiferous tubule synthesize component(s) that stimulate Sertoli cells in culture to increase the production of androgen-binding protein and testicular transferrin. The active peritubular cell component(s) are trypsin-sensitive, heat-sensitive, acid-stable molecule(s) having a molecular weight between 50,000 and 100,000. These specific factors(s) are referred to as P Mod-S to designate protein(s), produced by peritubular cells (P), that modulate the functions of Sertoli cells (S). The degree of stimulation by P Mod-S is comparable to that obtained by maximal hormonal stimulation of the synthesis of ABP and transferrin by Sertoli cells. Levels of P Mod-S secreted into the medium by primary cultures of peritubular cells are increased in the presence of testosterone. Comparable concentrations of 17 beta-estradiol do not stimulate peritubular cells to synthesize P Mod-S. Data are interpreted to indicate that androgens act on testicular peritubular cells to increase the formation of P Mod-S and that P Mod-S may modulate the properties of adjacent Sertoli cells. Findings are discussed in relation to the nature of mesenchymal-epithelial cell interactions in the seminiferous tubule and to the possible role of P Mod-S as a mediator of androgen actions of Sertoli cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972 Jan;52(1):198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Chung L. W., Shannon J. M., Taguchi O., Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res. 1983;39:559–598. doi: 10.1016/b978-0-12-571139-5.50018-5. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Roller N. F., Fritz I. B. Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol. 1975 Jul;3(1):57–70. doi: 10.1016/0303-7207(75)90031-3. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Dürnberger H., Kratochwil K. Specificity of tissue interaction and origin of mesenchymal cells in the androgen response of the embryonic mammary gland. Cell. 1980 Feb;19(2):465–471. doi: 10.1016/0092-8674(80)90521-8. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Rommerts F. G., Louis B. G., Dorrington J. H. Regulation by FSH and dibutyryl cyclic AMP of the formation of androgen-binding protein in Sertoli cell-enriched cultures. J Reprod Fertil. 1976 Jan;46(1):17–24. doi: 10.1530/jrf.0.0460017. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Gunsalus G. L., Musto N. A., Bardin W. Immunoassay of androgen binding protein in blood: a new approach for study of the seminiferous tubule. Science. 1978 Apr 7;200(4337):65–66. doi: 10.1126/science.635573. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Effect of androgens and antiandrogens on the development of the myoid cells of the rat seminiferous tubules. Z Zellforsch Mikrosk Anat. 1972;131(3):299–308. doi: 10.1007/BF00582853. [DOI] [PubMed] [Google Scholar]
- Hutson J. C., Stocco D. M. Peritubular cell influence on the efficiency of androgen-binding protein secretion by Sertoli cells in culture. Endocrinology. 1981 Apr;108(4):1362–1368. doi: 10.1210/endo-108-4-1362. [DOI] [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Improvements in the ethidium bromide method for direct fluorometric estimation of DNA and RNA in cell and tissue homogenates. Anal Biochem. 1977 Feb;77(2):464–470. doi: 10.1016/0003-2697(77)90259-7. [DOI] [PubMed] [Google Scholar]
- Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969 Jul;20(1):46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Smith F. E., Fritz I. B. Secretion of plasminogen activator by Sertoli cell enriched cultures. Mol Cell Endocrinol. 1977 Dec;9(2):227–236. doi: 10.1016/0303-7207(77)90124-1. [DOI] [PubMed] [Google Scholar]
- Louis B. G., Fritz I. B. Follicle-stimulating hormone and testosterone independently increase the production of androgen-binding protein by Sertoli cells in culture. Endocrinology. 1979 Feb;104(2):454–461. doi: 10.1210/endo-104-2-454. [DOI] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977 Mar;148(3):313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Sar M., Stumpf W. E., McLean W. S., Smith A. A., Hansson V., Nayfeh S. N., French F. S. Localization of androgen target cells in the rat testis: autoradiographic studies. Curr Top Mol Endocrinol. 1975;2:311–319. doi: 10.1007/978-1-4613-4440-7_22. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Secretion of testicular transferrin by cultured Sertoli cells is regulated by hormones and retinoids. Biol Reprod. 1982 Aug;27(1):211–221. doi: 10.1095/biolreprod27.1.211. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Interactions of sertoli cells with myoid cells in vitro. Biol Reprod. 1980 Aug;23(1):207–217. doi: 10.1093/biolreprod/23.1.207. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Skinner M. K., Fritz I. B. Fibronectin synthesis is a marker for peritubular cell contaminants in Sertoli cell-enriched cultures. Biol Reprod. 1984 Feb;30(1):199–211. doi: 10.1095/biolreprod30.1.199. [DOI] [PubMed] [Google Scholar]
- Verhoeven G. Androgen receptor in cultured interstitial cells derived from immature rat testis. J Steroid Biochem. 1980 May;13(5):469–474. doi: 10.1016/0022-4731(80)90200-9. [DOI] [PubMed] [Google Scholar]



