Abstract
We conducted a phase I dose escalation study to determine the maximal tolerated dose of bortezomib that could be combined with standard dose lenalidomide in patients with MDS or AML. Treatment consisted of bortezomib (IV) on Days 1, 4, 8, and 11 and lenalidomide 10 mg daily (PO) days 1–21 in 28 day cycles for up to 9 cycles. 23 patients (14 MDS/CMML, 9 AML) were enrolled. The maximally tested dose of bortezomib, 1.3 mg/m2, was tolerable in this regimen. Responses were seen in patients with MDS and AML. Further testing of this regimen is planned.
Keywords: Bortezomib and lenalidomide in MDS and AML
Introduction
Myelodysplastic syndromes (MDS) are hematopoietic stem cell disorders characterized by cytopenias, dysplasia, and progression to acute myelogenous leukemia (AML). Allogeneic stem cell transplantation (SCT) represents the only potentially curative approach but is possible in only a minority of patients due to the advanced age of presentation, concomitant comorbidities, and limited availability of donor sources. Thus, novel pharmacologic therapies capable of preventing or delaying disease progression are in critical need.
Lenalidomide (Revlimid), an immunomodulatory agent with anti-angiogenic properties, is particularly active in individuals whose MDS harbors alterations in the long arm of chromosome 5 (5q-) [1], but is also active to a lesser degree in individuals with lower risk disease with normal and other karyotypes. [2] However, even in patients with 5q- disease who initially respond to lenalidomide, primitive hematopoietic cells bearing the 5q- deletion persist, leading to resistance to lenalidomide over time. [3] Consequently, novel agents capable of targeting such primitive “MDS stem cells” are required.
One potential molecular approach is to target nuclear factor-kappa B (NF-kB), a master transcription factor involved in cell growth and proliferation. Elevated nuclear NF-kB levels have been identified in primitive bone marrow cells from patients with MDS, suggesting NF-kB as a therapeutic target in MDS. [4] Indeed, bortezomib (Velcade), a potent inhibitor of the proteasome and NF-kB, induces apoptosis in vitro in bone marrow cells from individuals with MDS and AML. [5] In a study of 19 patients, bortezomib was administered at 1.3 mg/m2 on days 1, 4, 8, and 11 every 28 days to 19 patients with IPSS low/intermediate 1 or intermediate-2/high risk MDS. Six of 19 patients received all planned 8 cycles. According to IWG 2006 criteria, 4/19 (21%) achieved erythroid response and 9 (47%) showed stable disease. [6] Similarly, elevated levels of nuclear NF-kB have been identified in primitive AML stem cells, [7, 8] and our previous studies demonstrated that bortezomib may be safely combined with standard anti-AML chemotherapy with encouraging results. [9, 10] In addition, lenalidomide has been demonstrated to downregulate expression of NF-kB in bone marrow from patients with MDS following treatment. [11]
Given the documented activity of both bortezomib and lenalidomide in MDS and AML, and the novel and independent mechanism of action of each agent, we conducted a phase I dose escalation study of bortezomib in combination with standard dose lenalidomide to establish the maximally tolerated dose (MTD) of bortezomib, up to 1.3 mg/m2, that can be safely administered in combination with lenalidomide in patients with MDS and AML.
Materials and Methods
This trial was registered at www.clinicaltrials.gov as NCT00580242. Patients 18 years and older with unequivocal histologic diagnosis of MDS by World Health Organization (WHO) [12] criteria were eligible. The study was open to enrollment at Massachusetts General Hospital and Dana-Farber Cancer Institute from October, 2007 through February, 2012. Patients with primary non-5q deleted myelodysplastic syndrome with IPSS score of ≥ 0.5 and any secondary non-5q deleted MDS were eligible, as were patients who had previously received chemotherapy treatment for their disease provided there was a one-week “washout” period. 5q deletion was considered to be interstitial deletion of chromosome 5 between regions q31 and q34; patients with larger deletions were eligible, as were patients with chronic myelomonocytic leukemia (CMML). Prior radiotherapy treatment must have been completed at least three weeks prior to enrollment. Hydroxyurea was permitted until initiation of protocol treatment. All patients signed a consent form approved by the Institutional Review Board of the Dana Farber/Harvard Cancer Center.
Following determination of the MTD for the above cohort, an additional 10 patients were enrolled and treated and, during this phase, the eligibility criteria expanded to include patients with AML, as defined per WHO criteria. Eligible patients included those with relapsed or refractory disease or previously untreated patients who were > 60 years and ineligible for intensive chemotherapy.
Treatment
Patients received bortezomib intravenously on days 1, 4, 8, and 11 at their assigned cohort dose levels, 0.7, 1.0 and 1.3 mg/m2 (Figure 1). Patients also received lenalidomide 10 mg orally once daily on Days 1–21 followed by 7 days off. Each cycle was 28 days in length and patients were eligible to receive up to 9 cycles of treatment. Missed or vomited doses of lenalidomide were not replaced. Patients kept a daily diary of their medication use and time of administration.
Figure 1.
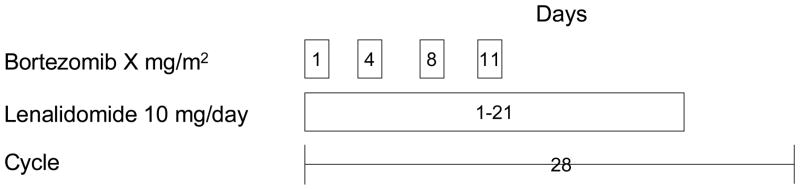
Treatment cycle schema. Up to 9 cycles could be administered on protocol.
Bortezomib dose cohorts consisted of 3–6 patients. The bortezomib dose cohort was advanced if no dose limiting toxicities (DLTs) were observed in the first 3 patients within a cohort in the first cycle of treatment. If a single DLT was experienced in the first 3 patients in a cohort, an additional 3 patients were enrolled and the cohort advanced if < 2 DLTs were experienced in 3–6 patients in a cohort. If ≥ 2 DLTs were experienced within a cohort, the previous dose of bortezomib was declared the MTD. The maximally tested dose of bortezomib was 1.3 mg/m2.
Toxicities were assessed using the CTC V3.0 (Common Terminology Criteria version 3.0). Though toxicities were recorded throughout treatment, a toxicity was only considered a DLTs during the first cycle. DLTs consisted of: drug-induced neutropenia [absolute neutrophil count (ANC) < 250/ul for 5 or more consecutive days], febrile neutropenia with fever > 38.5° C on two occasions with an ANC < 500/ul; thrombocytopenia [platelets < 10,000/ul on more than one occasion despite transfusion support], any grade ≥ 3 non-hematologic toxicity, grade > 2 neurosensory toxicity, and any toxicity newly encountered on day 1 of cycle 2 requiring a dose reduction for cycle two.
Both agents were to be held in instances where febrile neutropenia or hospitalization occurred and where a DLT was experienced. Agents were also held for ≥ grade 3 toxicities and reinitiated at the next lower dose level following resolution of the toxicity to ≤ grade 1. Each cycle required an ANC ≥ 500/ul and platelets ≥ 30,000/ul (growth factors and transfusions permitted), resolution of any lenalidomide-associated rash to ≤ grade 1, and resolution of any other toxicity to ≤ grade 2. Supportive care consisting of antibiotics, blood product transfusions, and myeloid and erythroid growth factors was permitted.
A bone marrow biopsy was required at the end of cycles 3, 6 and 9 and responses were assessed according to IWG 2006[13] criteria for patients with MDS and IWG 2003[14] for those with AML. Patients with progressive disease were removed from protocol therapy.
Results
Patient Characteristics
A total of 23 patients were enrolled, 11 with MDS [1 refractory anemia (RARS), 1 refractory anemia with multilineage dysplasia (RCMD), 1 refractory anemia with excess blasts-1 (RAEB-1), 6 refractory anemia with excess blasts-2 (RAEB-2), and 2 therapy-related (t-MDS)], 2 CMML (1 t-CMML-1, 1 CMML-2], 1 MDS/myeloproliferative neoplasm (MPN) overlap, 9 with AML (Table 1). One of the patients with AML had therapy related disease (t-AML). One patient (with RAEB-2) was removed prior to treatment due to progressive disease prior to starting treatment and was replaced. There were 14 men, and the median age was 73 years (range 54–87). Fourteen patients had received anti-MDS or AML therapy (including hydroxyurea) prior to enrollment, including 3 patients who had previously undergone allogeneic SCT and 3 who had received lenalidomide. Three patients with AML received treatment within 4 weeks of beginning protocol therapy that included intensive reinduction chemotherapy, lenalidomide and a 9th cycle of azacitidine, as did one patient with MDS/MPN, who received dasatinib for hypereosinophilia to which there was no response.
Table 1.
Age, diagnosis, karyotype, and best response.
| Age | Diagnosis | Karyotype | % Bone Marrow Blasts | R-IPSS Score and Category for Primary MDS Patients | Bortezomib Dose (mg/m2) | # cycles | Best Response, Time to Best Response (cycles) | Duration of Best Response, (months) | Prior treatment(s) (excluding GFs) | Prior hypomethylating agent | Proceeded to SCT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 73 | t-MDS | −7 | 2 | 0.7 | 2 | SD, 1 | 4 | For MDS: azacitidine X 5, then lenalidomide For lung cancer: Chemotherapy and XRT | A | ||
| 67 | RAEB-2 | NL | 11 | 6.5, Very high | 0.7 | 7 | mCR, 3 | 6 | Y | ||
| 76 | RAEB-2 | NL | 16 | 5.5, High | 0.7 | 7 | CR, 3 | ||||
| 81 | RAEB-2 | complex | 10 | 8.5, Very high | 1 | 2 | NR | ||||
| 87 | RAEB-1 | −7 | 8 | 1 | 3 | NR | decitabine | D | |||
| 74 | t-MDS | del(20q), −7 | 8 | 1 | 2 | PCyR, 2 | 3 | prior treatment for APML | Y | ||
| 79 | RAEB-2 | del(5q) with additional complex | 11 | 1 | 2 | NR | lenalidomide X 5 years for del5q MDS | ||||
| 75 | MDS/MPN | NL | 4 | 2.5, Low | 1 | 2 | NR | hydroxyurea for HES | |||
| 73 | RAEB-2 | NL | 0* | removed | |||||||
| 67 | RAEB-2 | −Y | 10 | 3, Low | 1 | 1 | NR | Y | |||
| 78 | RCMD | del(5q), del(20q) | 2 | 3, Low | 1.3 | 9 | PCyR, 3 | 3 | |||
| 62 | t-CMML | t(11;19) | 2 | 1.3 | 3 | NR | |||||
| 63 | CMML-2 | NL | 14 | 5, High | 1.3 | 3 | NR | ||||
| 78 | AML | NL (FLT3 ITD+, NPM1 +) | 67 | 1.3 | 1 | NR | |||||
| 58 | AML | NL | 89 | 1.3 | 2 | NR | multiple chemotherapies for AML, SCT | D | |||
| 63 | AML | del(5q) with additional complex | 90 | 1.3 | 3 | NR | decitabine, RIC SCT | D | |||
| 79 | AML | del(12p) | 40 | 1.3 | 1 | NR | hydroxyurea for CMML | ||||
| 64 | RARS | complex | 2 | 1.3 | 9 | CCyR, 3 | 15 (persists) | azacitidine, lenalidomide, thalidomide | A | ||
| 72 | AML | i(11) | 40 | 1.3 | 6 | CR, 3 | 3 | ||||
| 72 | t-AML | −7 | 15 | 1.3 | 2 | NR | For AML: AML induction, azacitidine X 8 For LPL: fludarabine |
A | |||
| 54 | AML | NL | 38 | 1.3 | 1 | NR | AML induction, SCT, azacitidine | A | |||
| 54 | AML | t(3;3) | 60 | 1.3 | 2 | NR | multiple prior inductions | ||||
| 78 | AML | NL | 45 | 1.3 | 1 | NR | decitabine X 8 cycles | D |
= removed prior to start due to rapidly progressive disease, GFs = growth factors, t-MDS = therapy-related MDS, t-AML = therapy-related AML, mCR = marrow complete remission, PCyR = partial cytogenetic response, CCyR = complete cytogenetic response, NR = no response, CR = complete remission, HES = hypereosinophilic syndrome, APML = acute promyelocytic leukemia. A=azacitidine, D=decitabine.
Toxicities
Among the 6 patients treated with bortezomib at a dose of 1.0 mg/m2, there was 1 DLT observed, grade 4 neutropenia. There were no DLTs observed at doses of 0.7 or 1.3 mg/m2. Hematologic toxicities possibly, probably, and definitely attributed to the study treatment in any cycle included neutropenia (4 grade 1, 1 grade 2, 2 grade 3, and 4 grade 4) and thrombocytopenia (1 grade 1, 3 grade 2, 11 grade 3, and 7 grade 4). First instances of hematologic toxicities occurred within the first 2 cycles of treatment. In patients with MDS, grade 4 neutropenia and thrombocytopenia were more commonly though not exclusively observed in patients with low initial ANC and platelets levels, less than 1000/mcl and 50,000/mcl, respectively. Because all patients with AML had low initial ANC and platelet levels, it could not be determined if the hematologic toxicity was related to the treatment or the disease. Grade ≥ 3 toxicities possibly, probably, and definitely attributed to study treatment are presented in Table 2. Two fatal grade 5 toxicities occurred, one involving pneumonia and the other hypotensive sepsis. There were 4 instances of grade 4 infection and 1 each of grade 4 renal insufficiency, hypotension, and sensory neuropathy. Regarding neurotoxicities, there were 5 patients with grade 1, and 1 patient with grade 2 sensory neuropathy, and 3 patients with grade 1 motor neuropathy. Of the 23 patients on study, 17 required hospitalization during the course of treatment, primarily for treatment of febrile neutropenia.
Table 2.
Grade ≥ 3 non-hematologic toxicities which were possibly, probably, and definitely attributed to the study treatment. (n=number of patients)
| Toxicity | Grade | ||
|---|---|---|---|
| 3 | 4 | 5 | |
| Hypoxia | 3 | 0 | 0 |
| Infection | 0 | 4 | 2 |
| Rash/Desquamation | 1 | 0 | 0 |
| Chest Pain | 1 | 0 | 0 |
| Dizziness | 1 | 0 | 0 |
| Pneumonitis/pulmonary infiltrates | 1 | 0 | 0 |
| Upper respiratory | 1 | 0 | 0 |
| Renal insufficiency | 0 | 1 | 0 |
| Hypotension | 0 | 1 | 0 |
| Sensory neuropathy | 5 | 1 | 0 |
| Motor neuropathy | 3 | 0 | 0 |
Responses
Responses according to type of disease, karyotype, and dose of bortezomib are presented in Table 1. Among the 13 patients with MDS and CMML, there was 1 CR (RAEB-2), 1 mCR (marrow CR, defined as < 5% BM blasts and ≥ 50% reduction in blasts from baseline) (RAEB-2), 1 complete CyR (RARS), and 2 partial CyRs (defined as at least 50% reduction of the chromosomal abnormality) (t-MDS, RCMD). Among 9 patients with AML, there was 1 CR. A patient with t-MDS had stable disease for a period of 4 months following 2 cycles of therapy. There were no hematologic improvements in patients with MDS according to IWG criteria separate from the responses described.
All 3 CyRs in MDS patients were achieved by the 3-month bone marrow biopsy, as was the CR in the patient with AML. The patient with RAEB-2 achieved a mCR based on a bone marrow biopsy done at the 6-month time-point. Three patients on study proceeded to allogeneic SCT. One of these patients, with a diagnosis of RAEB-2, received one cycle of therapy on study and proceeded to SCT despite an increase in marrow blasts to 15% from a pretreatment value of 10%.
Of patients who achieved a CyR on this study, all occurred by the first assessment at 3 months. A patient who had undergone extensive treatment for APML and then developed t-MDS, was able to achieve a PCyR and proceed to undergo allogeneic SCT. Another patient with RARS and complex karyotype achieved a CCyR after 3 cycles that has been sustained for 15 months. Notably, this patient had previously been treated with lenalidomide alone without response (as well as thalidomide and azacitidine subsequently), suggesting that the addition of bortezomib may have contributed to her response. A 72 year old patient with previously untreated AML achieved a CR to this regimen. None of the responding patients had an abnormality of chromosome 5, and only one of the eight patients previously treated with a hypomethylating agent responded to protocol therapy.
Of 14 patients with non-AML disease, 3 patients previously received a hypomethylating agent; one patient had stable disease and one achieved a CCyR. Of 9 patients with AML, 5 previously received a hypomethylating agent; none of these patients responded to the protocol therapy.
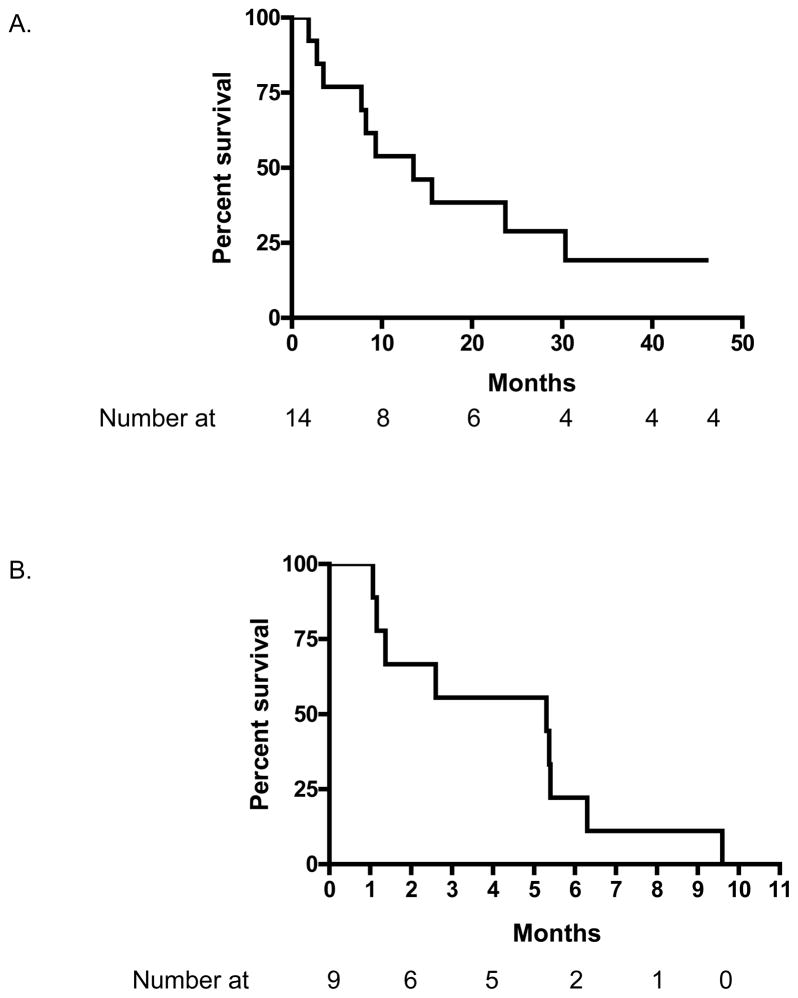
The median survival in months for patients with MDS and CMML was 13.5 months (95% CI 8.3–25.1) and with AML was 5.3 (95% CI 2.0–6.5) (Figure 2).
Figure 2.
Survival of patients with MDS and CMML (A) and those with AML (B).
Discussion
We conducted a phase I dose-escalation study of bortezomib in combination with lenalidomide in patients with MDS and AML. The maximally tested dose of bortezomib, 1.3 mg/m2 on days 1, 4, 8, and 11, in combination with lenalidomide at 10 mg/day on days 1–21 of a 28 day cycle, was well-tolerated.
As expected, all 22 evaluable patients experienced thrombocytopenia, a known side effect of both bortezomib and lenalidomide. However, there were no CNS or other hemorrhagic events. Myelosuppression of various grades was observed in 11 patients, again expected given the association between lenalidomide and myelosuppression. While 9 patients experienced grade 1 or 2 neuropathy, there were no instances of ≥ 3 neuropathy. The absence of grade ≥ 3 peripheral sensory neuropathy is similar to an initial study in patients with advanced multiple myeloma treated with similar doses of lenalidomide and bortezomib, typically for 10 cycles. [15] In contrast, in a subsequent study of bortezomib with higher dose lenalidomide (15–25 mg daily, median 8 cycles) in newly diagnosed patients with myeloma, grade 3 and 4 neuropathic pain was seen in 32% and 3% of patients, respectively, and grade 3 and 4 motor neuropathy was seen in 18% and 2% of patients, respectively. [16]
Our results raise the possibility that the combination of bortezomib and lenalidomide is active in patients with MDS and AML. Of the 3 patients with MDS treated at 0.7 mg/m2 bortezomib, 2 achieved CRs. However, of the MDS patients treated at 1.0 mg/m2 bortezomib, the best response was SD in 1 patient, whereas at a dose of 1.3 mg/m2, there were 2 mCRs. Thus, there was no discernable dose-response effect in this limited number of patients.
Though we observed a relatively low overall response rate, this study included both pretreated patients and those with advanced disease. A similarly low rate of response has been observed with low doses of single-agent lenalidomide. [17] However, increased responses have been observed using higher doses of lenalidomide - such as 50 mg daily in patients with AML. [18, 19] Consequently, further testing of this regimen is planned in patients with AML using a higher dose of lenalidomide.
References
- 1.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 2.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 3.Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, Mead AJ, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 2010;363:1025–37. doi: 10.1056/NEJMoa0912228. [DOI] [PubMed] [Google Scholar]
- 4.Braun T, Carvalho G, Coquelle A, Vozenin MC, Lepelley P, Hirsch F, et al. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood. 2006;107:1156–65. doi: 10.1182/blood-2005-05-1989. [DOI] [PubMed] [Google Scholar]
- 5.Liesveld JL, Rosell KE, Bechelli J, Lu C, Messina P, Mulford D, et al. Proteasome inhibition in myelodysplastic syndromes and acute myelogenous leukemia cell lines. Cancer Invest. 2011;29:439–50. doi: 10.3109/07357907.2011.590567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alimena G, Breccia M, Musto P, Cilloni D, D’Auria F, Latagliata R, et al. Erythroid response and decrease of WT1 expression after proteasome inhibition by bortezomib in myelodysplastic syndromes. Leuk Res. 2011;35:504–7. doi: 10.1016/j.leukres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 8.Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99:16220–5. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attar EC, De Angelo DJ, Supko JG, D’Amato F, Zahrieh D, Sirulnik A, et al. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14:1446–54. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 10.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, Deangelo DJ, et al. Bortezomib Added to Daunorubicin and Cytarabine During Induction Therapy and to Intermediate-Dose Cytarabine for Consolidation in Patients With Previously Untreated Acute Myeloid Leukemia Age 60 to 75 Years: CALGB (Alliance) Study 10502. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliva EN, Cuzzola M, Aloe Spiriti MA, Poloni A, Lagana C, Rigolino C, et al. Biological activity of lenalidomide in myelodysplastic syndromes with del5q: results of gene expression profiling from a multicenter phase II study. Ann Hematol. 2013;92:25–32. doi: 10.1007/s00277-012-1569-0. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. In: WHO Classification of Tumours of Haematopioetic and Lymphoid Tissues. Bosman F, Jaffe E, Lakhani S, Ohgaki H, editors. Lyon: International Agency for Research on Cancer (IARC); 2008. [Google Scholar]
- 13.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:5713–9. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Kantarjian H, Estrov Z, Faderl S, Ravandi F, Rey K, et al. A Phase II Study of Lenalidomide Alone in Relapsed/Refractory Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndromes With Chromosome 5 Abnormalities. Clinical lymphoma, myeloma & leukemia. 2012;12:341–4. doi: 10.1016/j.clml.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117:1828–33. doi: 10.1182/blood-2010-07-297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, et al. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010;28:4919–25. doi: 10.1200/JCO.2010.30.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]