Abstract
Background
Quantifying HIV levels in mucosal secretions is essential to study compartmentalized expression of HIV and facilitate development of intervention strategies to prevent disease progression and transmission.
Objectives
To develop a sensitive, reliable, and cost-effective technique to quantify HIV from blood and vaginal secretions that is compatible with efficient implementation in clinical research environments.
Study Design
A sensitive, reliable, internally-controlled real-time reverse transcriptase (RT) PCR assay, which uses the HIV-1 pol gene as a target (Hpol assay) was developed to quantify HIV levels in plasma and genital secretions, and compared to the widely-used Roche Amplicor HIV-1 Monitor assay. In addition, a simplified method of sample collection and processing of genital secretions (self-collection and use of RNAlater with batch processing) was compared to provider collection of samples and immediate processing.
Results
The sensitivity and reliability of HIV levels detected by the assay described herein correlate well with measurements from Roche Amplicor™ HIV-1 Monitor assay for both plasma and vaginal secretions (R2= 0.9179 and R2=0.942, respectively). The Hpol assay reproducibly quantifies a lower limit of 5 HIV-1 RNA copies per reaction, with low-levels of inter-assay and intra-assay variation. Additionally, vaginal viral levels and detection frequency did not differ significantly between the two the collection and processing methods.
Conclusions
The methodologies developed here provide sensitive, reliable, and cost-effective quantification of HIV levels in plasma and mucosal secretions, and are compatible with efficient use in clinical research studies.
Keywords: Human immunodeficiency virus, real-time PCR, genital, plasma, RNAlater
1. Background
Quantifying HIV levels in mucosal secretions is critical to understand viral expression mechanisms and develop suitable intervention strategies for disease progression and transmission (1-5). In clinical research environments, cost and efficiency of analyses favor the use of in-house real-time PCR quantitation assays over commercial assays. Several such assays have been described, but few have been validated to monitor HIV expression in mucosal secretions or have included an internal control to monitor efficiency of RNA recovery, reverse transcription (RT), and inhibition of PCR amplification (6-9). Samples collected from the female genital mucosa are frequently collected during a pelvic examination. To preserve viral RNA integrity, these samples are typically held on ice and processed immediately. This process can be cumbersome and inefficient, especially for large clinical studies.
2. Objectives
We sought to streamline methodologies used to collect genital secretions and evaluate HIV levels in clinical research studies. The objectives of this study were: (1) to develop a cost-effective, internally-controlled, reproducible, and sensitive real-time RT PCR assay to quantify HIV-1, (2) compare viral level analyses of self-collected vaginal secretions with samples collected by providers via pelvic exam, and (3) evaluate the use of RNAlater preservative solution for sample storage and weekly batch processing.
3. Study Design
3.1. Specimen Collection and Processing
Informed consent for sample collection was obtained in accordance with institutional review board of Louisiana State University Health Sciences Center. Peripheral blood and vaginal secretions were collected from women (≥ 18 years) attending an HIV outpatient clinic in New Orleans, LA. HIV-negative subjects were recruited for control samples.
EDTA plasma samples were stored at −80°C in 1mL aliquots. Vaginal secretions were collected using polyester-tipped swabs (Fisher catalog #23-400-111). Before pelvic examination, randomly selected subjects were instructed to self-collect genital secretions and deposit samples in an empty sterile collection tube (15mL conical polypropylene tube, used throughout study). Afterwards, provider-collected samples were obtained from the vaginal vault during pelvic examination and also placed in empty collection tubes. Samples were always collected in the order described.
For immediate processing, vaginal secretion swabs (self and provider collected) were placed in collection and transported to the laboratory on ice. Secretions were eluted in 1mL PBS by vortexing 30 seconds, fractioned by centrifugation (1,500 × g for 10 minutes) and supernatants were stored at −80°C within 3 hours of collection. For batch-processing, provider-collected swabs were immediately placed in collection tubes containing 1 mL of RNAlater (Ambion) preservative solution and stored at 4°C for up to 2 weeks. On a weekly basis, vaginal samples stored in RNAlater were eluted from the swab and fractioned as described above and stored at −80°C.
3.2. HIV-1 Quantitation Assay
The HIV pol region was selected as the target region for PCR amplification due to its highly conserved nature among clade B HIV genomes (10). To generate a quantitation standard, a 664 base pair region spanning the HIV-1 pol gene was PCR-amplified from HIVHXB2 (nucleotides 2597 to 3261) and cloned into the pCR 2.1 TOPO vector (Invitrogen, CA). The resultant plasmid, pHXB-pol, was serially diluted 10-fold and used as the DNA standard for HIV quantitation. DNA was purified from the cell line ACH-2 (NIH AIDS Research and Reference Reagent Program) to verify quantitation assay accuracy. This cell line contains one copy of HIV proviral DNA per cell. To prepare an HIV RNA template for use as a quantitation standard, a 794 bp transcript was produced from pHXB-pol by T7 RNA polymerase (RiboMax In vitro Transcription System, Promega). The resultant RNA was purified, quantified by spectrophotometry, and serially diluted to contain 107 −101 copies of the pol RNA template. DNA template removal was confirmed by real-time PCR under RT-excluded conditions.
3.3. Exogenous RNA Internal Control
The exogenous internal control RNA was derived from plasmid pMKCP3, containing sequences from brome mosaic virus (BMV) RNA 3, generously provided by Dr. Paul Ahlquist (11). A 2273 base RNA template, ICBMV, was produced by in vitro transcription from pMKCP3 using the RiboMax system (Promega, WI). 300 pg/mL ICBMV RNA was added to each sample in Trizol reagent (Invitrogen) as described below. ICBMV RNA and virion RNA were simultaneously purified and detected in a multiplexed real-time RT PCR assay, utilizing ICBMV -specific and HIVpol-specific primers and probes (Table 1).
Table 1.
Primers and Probes used in Taq-Man Assays
| Primer and Probe Sequences | |
|---|---|
| HpolF | GAAATTTGTACAGAAATGGAAAAGGAA |
| HpolR | TGAGTTCTCTTATTAAGTTCTCTGAAATCTAC |
| HpolProbe | 6FAM—TTGGGCCTGAAATCCATACAATACTCCAGT—TAMRA |
| IC-F | GCTCGCAGAAATCGTTGGA |
| IC-R | CAGCGAGTGGTTCGACAATTAC |
| IC-Probe | VIC—CCGCTAGGGTCCAAC—TAMRA |
Primer and probe sets were designed using Primer Express software, (version 1.0, Applied Biosystems, Foster City, CA), and obtained via custom order from Applied Biosystems.
3.4. HIV- Hpol Real-time RT PCR Multiplex Assay
Virions were pelleted from 1 mL vaginal secretion or plasma aliquots (20,000 × g for 1 hour at 4°C), supernatants were removed, 1mL Trizol reagent containing 300 pg/mL ICBMV was added, and RNA was purified per the manufacturer's protocol. Ten-fold serial dilutions of the Hpol RNA template (diluted in RNAse-free water containing 100μg/mL glycogen) were included in each assay to provide a standard curve for quantitation. The Hpol standards and one-tenth of total RNA from samples were reverse-transcribed in a reaction containing TaqMan PCR Buffer II, 5.0 mM MgCl2, 2.5 μM random hexamers, 25 units MultiScribe reverse transcriptase and incubated for 10 minutes at 25°C, 30 minutes at 48°C, 5 minutes at 95°C, and 5 minutes at 4°C. The PCR master-mix was added to the resultant cDNA by overlay (1X TaqMan Buffer A, 5.5 mM MgCl2, 0.5mM dNTP, 1.25 units AmpliTaq Gold, 450nM of both HpolF and HpolR, and 250nM HpolProbe). When multiplexed, the reaction mixture also contained 125 nM IC-F and IC-R and 70nM IC-Probe.
Amplification was performed using an ABI 7300 through the following: 10 minutes at 95°C prior to 40-45 cycles of 94°C for 15 seconds and 60°C for 1 minute. HIV copy number was determined by plotting the threshold cycle (CT) value of the unknown on the standard curve derived from the Hpol RNA standards. Inhibition was inferred if the CT value for ICBMV was >2.5 units from the reference CT. Two HIV-negative plasma samples, one HIV-positive sample (described below) and two ICBMV only reference samples were included in each assay. HIV levels were reported as copies per mL (plasma) or swab (secretion supernatant). For Roche Amplicor™ HIV-1 Monitor Assay quantitation, viral RNA was purified and quantified using the “ultra-sensitive” method (COBAS, version 1.5), per the manufacturer's recommended protocol (12).
3.5. HIV-Positive Control Samples
HIV-positive control samples were prepared using a virus stock (HIVJRFL) obtained from supernatants of infected cultures Frozen aliquots of the stock contained 2.0 × 109 copies/mL as determined by the Hpol. For validation experiments, HIV-negative plasma and vaginal secretion samples were spiked with serial dilutions of HIVJRFL culture aliquots and stored at −80° C. HIV-positive control samples for quantitation assays were prepared by spiking 1mL normal plasma with 5 ×104 copies of HIVJR-FL. For experiments to validate ICBMV inclusion, HIV-negative plasma and vaginal secretion samples were spiked with serial dilutions of HIVJRFL stock aliquots prior to storage at −80° C.
3.6. Statistical Analyses
Linear regression analysis, Yates-corrected chi-square (X2), Fisher's exact test, and Mann-Whitney Wilcoxon sum measures of statistical significance were used for viral level comparisons. For statistical analysis, samples with undetectable levels of HIV (<50 copies) were assigned the value of 35.4 copies/ml or swab (L/√2, where L=limit of detection, 50 copies)(13). Viral copy numbers were log10 transformed for analyses.
4. Results
4.1. Validation of TR-Hpol Real-time RT PCR Assay
The quantitation assay was first validated for DNA amplification. Consistently reproducible measures of sensitivity and accuracy were observed when serial 10-fold dilutions of pHXB-pol were amplified. Assay accuracy was verified by amplifying proviral DNA from ACH-2 cells, wherein there is a single HIV-1 integrated provirus. The measured versus expected HIV provirus copy number correlated well (R2= 0.9973) in ACH-2 DNA samples. For the RNA standard curve, intra- and inter-assay comparisons validated assay reproducibility, with only nominal variation observed (Table 2). Comparisons of observed assay variations with previously published assays indicate that the methods described here meet requirements for reproducible and accurate quantitation(9, 14).
Table 2.
Intra-Assay and Inter-Assay Variation in Amplification of Hpol Standard in 5 Experiments
| Standard Copy Number | CT Value Exp. 1 | % CV Exp.1 | CT Value Exp. 2 | % CV Exp.2 | CT Value Exp. 3 | % CV Exp.3 | CT Value Exp. 4 | % CV Exp.4 | CT Value Exp. 5 | % CV Exp.5 | % Intra-Assay Variation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 37.2 ± 0.02 | 0.5 | 38.1 ± 0.05 | 0.1 | 37.9 ± 0.27 | 0.7 | 38.1 ± 0.22 | 0.6 | 38.3 ± 0.16 | 0.4 | 1.1 |
| 100 | 33.9 ± 0.06 | 0.2 | 34.6 ± 0.19 | 0.6 | 34.5 ± 0.07 | 0.2 | 34.8 ± 0.22 | 0.6 | 34.9 ± 0.07 | 0.2 | 1.5 |
| 1,000 | 30.6 ± 0.31 | 1.0 | 31.3 ± 0.05 | 0.2 | 30.0 ± 0.12 | 0.4 | 31.3 ± 0.08 | 0.2 | 31.5 ± 0.27 | 0.8 | 1.4 |
| 10,000 | 27.7 ± 0.29 | 1.0 | 28.2 ± 0.52 | 2.0 | 27.1 ± 0.03 | 0.1 | 27.6 ± 0.14 | 0.5 | 27.9 ± 0.06 | 0.2 | 1.8 |
| 100,000 | 25.1 ± 0.03 | 0.1 | 24.7 ± 0.01 | 0.2 | 23.6 ± 0.12 | 0.5 | 24.1 ± 0.08 | 0.3 | 24.4 ± 0.18 | 0.7 | 2.1 |
| 1,000,000 | 21.5 ± 0.57 | 3.0 | 21.1 ± 0.00 | 0 | 20.1 ± 0.08 | 0.3 | 20.4 ± 0.08 | 0.4 | 20.9 ± 0.21 | 1.0 | 2.5 |
| 10,000,000 | 17.7 ± 0.29 | 2.0 | 17.9 ± 0.09 | 0.5 | 16.7 ± 0.03 | 0.2 | 17.0 ± 0.11 | 0.6 | 17.5 ± 0.11 | 0.6 | 2.2 |
| Assay Values | Slope: −3.31 Y-intercept: 40.12 Corr. Coeff: 0.991 |
Slope: −3.30 Y-intercept: 40.95 Corr. Coeff: 0.997 |
Slope: −3.36 Y-intercept: 39.26 Corr. Coeff: 0.991 |
Slope: −3.39 Y-intercept: 40.04 Corr. Coeff: 0.996 |
Slope: −3.38 Y-intercept: 39.11 Corr. Coeff: 0.998 |
||||||
CT Value – mean threshold cycle at which respective standard copy number is detected ± standard deviation; %CV – inter-assay percent coefficient of variation between replicates; Corr. Coeff – correlation coefficient (degree to which individual standard copy number data points fit the slope of the standard curve, exact fit = 1.00); CT values representative of triplicate analyses from 5 independent standard curves.
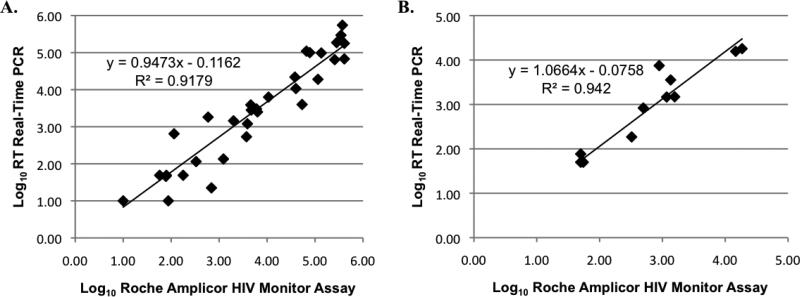
To further validate the Hpol assay, HIV levels from 36 plasma and 20 vaginal samples were quantified by the Roche Amplicor HIV-1 Monitor Assay (15) alongside the Hpol real-time RT PCR assay. Matched HIV RNA levels from each assay were highly similar for plasma and vaginal HIV levels (Figures 1A and 1B). In addition, there was no significant difference in the frequency of detection with either method or sample type. Reagents utilized in the real-time assay are commercially available from several sources, resulting in a significant reduction in reagent cost as compared to the commercial quantitation kits.
Figure 1. Comparison of Plasma and Vaginal HIV Levels Derived from the Roche Amplicor System and the Hpol Real-time RT PCR Assay.
Comparisons were conducted from duplicate aliquots of a single 1 ml sample of plasma and provider-collected vaginal secretion supernatants; 500 ul of each sample were analyzed in the tandem assays. A. Comparison of Log10 value from plasma samples (n=36). HIV RNA was detected in 30 plasma samples using the real-time RT PCR assay and 31 samples using the Roche Amplicor system. B. Comparison of Log10 HIV levels from vaginal secretion supernatant samples (n=20). HIV RNA was detected in all vaginal secretion supernatant samples analyzed.
To evaluate the sensitivity of the Hpol assay, aliquots HIVJR-FL culture supernatant (2.0 × 109 copies/mL as determined by independent quantitation) were serially diluted to contain an estimated 100, 50, 30, and 10 copies/mL and spiked into HIV-1 negative samples. Because 1/10 of total RNA is evaluated in each reaction, this test evaluated the ability of the Hpol assay to detect 1, 3, 5, and 10 copies of HIV-1 RNA per reaction. The results of this analysis (Table 3) indicate the limit of accurate quantitation using the Hpol assay is 5 copies of HIV-1 RNA per reaction, translating to 50 copies per mL/sample. By evaluating sample RNA in duplicate, the probability of not detecting ≥5 viral copies in at least one replicate is < 0.1% .
Table 3.
Sensitivity of Detection with Hpol Quantitation Assay
| Input Amount | % Detection |
|---|---|
| 1 copy | 56%* |
| 3 copies | 73% |
| 5 copies | 97% |
| 10 copies | 100% |
Hpol RNA templates were diluted to the estimated copy number and detection frequencies determined from analysis of 6 replicates at each dilution in 5 independent quantitation assays (total of 30 replicates per dilution).
55.6% detection fits expected Poisson probability of one copy detection rate (22); observed versus expected rates of detection were not statistically different (Yates-corrected X2 1.461, p > 0.20)
4.2. Validation of the Internal Control (ICBMV)
To confirm that addition of ICBMV RNA did not interfere with the sensitivity and accuracy of HIV quantification, serial 10-fold dilutions of HIVJRFL (to a limiting dilution) were spiked into HIV-negative plasma and vaginal secretion supernatant samples. For both types of samples, HIV levels quantified in the presence and absence of ICBMV RNA were highly similar (R2= 0.8693 and R2=0.8154 for plasma and vaginal secretions, respectively). No significant difference in detection of HIV-1 RNA was observed between the ICBMV inclusive and exclusive groups for both plasma and vaginal secretions as well (p=0.66 and p=0.93, plasma and vaginal secretions, respectively, Mann-Whitney Wilcoxon rank-sum). At limiting dilutions (estimated at 1-10 copies) of HIVJRFL per sample, detection sensitivity was also not significantly different between the two groups in both sample types.
4.3. Validation of Vaginal Secretion Collection and Processing Protocols
To compare sample collection protocols, vaginal secretions were obtained from 54 subjects by self-collection and a medical provider via pelvic examination. Additionally, immediate processing of samples was compared with a protocol utilizing RNAlater preservative solution and weekly batch processing (n=43 subjects). HIV detection frequencies in both the provider vs. self-swab and immediate processing vs. RNAlater solution comparisons were not significantly different in either comparison (Table 4). Samples stored in RNAlater were not negatively impacted by the preservative solution, as four additional positive samples were detected in this sample set that were undetected in the immediate processing group. Similarly, a marginally higher frequency of HIV detection was observed in self-collected samples compared to provider-collected. HIV RNA was detected in vaginal samples from 15 subjects. HIV was detected in seven samples by both collection protocols, while self-collection identified an additional six positive samples not found in provider-collected swabs. Provider collected samples identified two HIV-positive specimens that were not detected in self-collected samples.
Table 4.
Detection Frequencies in Methodology Comparisons
| Methodology | % Detection Vaginal HIV | p Value† |
|---|---|---|
| Self-Swab Collection | 24.2% (13/54) | 0.47 |
| Provider Collection | 16.7% (9/54) | |
| RNAlater Solution | 26.8% (12/43) | 0.44 |
| Immediate Processing | 19.5% (8/43) |
Yates-corrected chi-square test
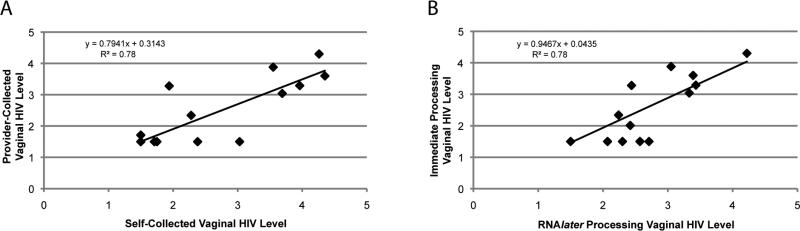
Levels of vaginal HIV were compared between provider or self-swab collection and RNAlater or immediate processing, and in both sets of analyses, levels obtained were not significantly different (Table 5). Direct comparisons by linear regression yielded correlation coefficients of 0.78 for both collection and processing methods (Figure 2). Although these correlation coefficients are lower than that observed in comparison of the Hpol assay with the Roche Amplicor assay, it is emphasized that this difference reflects the inherent variability associated with duplicate, independently-collected and processed samples from the same subject. These R2 values, with which approximately 80% of variation within each comparison is explained by the linear relationship, indicate the validity of self-swab and batch processing methods as compared to provider collection and immediate processing.
Table 5.
Comparisons of Viral Levels Obtained with Methods of Processing and Collection
| Methodology | Mean Vaginal Viral Level (copies/swab) | Range Vaginal Viral Level (copies/ swab) | Comparison* |
|---|---|---|---|
| RNAlater processing+ | 651.4 | <50 – 19,952 | p=0.52 |
| Immediate processing | 884.4 | <50 – 16,595 | |
| Self-swab collection# | 4840.7 | <50 – 222,387 | p=0.53 |
| Provider collection | 3896.0 | <50 – 191,953 |
comparison of duplicate samples collected by a provider from 43 subjects
comparison of samples collected from 54 subjects; self-collected samples were collected approximately 30 minutes prior to provider-collected specimens
Mann Whitney Wilcoxon Rank Sum; Variability in levels is expected due to independent samplings and virus quantitation near the level of detection.
Median vaginal HIV RNA value for all methodologies was 50 copies per swab.
Figure 2. Comparison of Collection and Processing Methodologies.
A. Self-collected vs. Provider Collected Vaginal secretion samples. Comparisons of HIV levels detected from two independent vaginal swabs collected from the same participant (n=54), within a 30 minute time span, with immediate processing for storage. B. RNAlater vs. Immediate Processing Methodologies. Comparisons of HIV levels detected from duplicate samples collected simultaneously from subjects (n=43) by a provider during a pelvic exam and processed by different methodologies as described. .
5. Discussion
We have described the development and use of an internally-controlled, reliable, efficient, and sensitive methodology to quantify HIV-1 RNA in plasma and vaginal secretions. The assay described here represents a significant improvement over previously described real-time RT-PCR quantitation assays for HIV (8, 14, 16-20), as the inclusion of an internal RNA standard allows monitoring of RNA extraction efficiency, reverse transcription, and inhibition of PCR amplification. Additionally, vaginal viral levels obtained with the described Hpol assay are statistically indistinguishable from those obtained with the Roche Amplicor Monitor assay. The Hpol assay can be used to quantify HIV-1 RNA in other mucosal secretions; its use to quantify HIV-1 levels in cervical secretions has been validated (data not shown) and pilot studies have been conducted in saliva. A commercially-available mixture of BMV RNAs has previously been used as an internal control for HIV-2 quantitation(21). We have significantly improved this methodology by transcribing a molecular clone of a BMV RNA segment in vitro, eliminating variability in the commercial RNA mixture and providing a standard RNA template. Finally, our results validate the use of self-collection of genital secretions and an RNAlater batch processing protocol. This protocol facilitates sampling in the absence of a pelvic examination, allows sampling at clinic and non-clinic sites, and permits sample collection independent of laboratory scheduling and immediate processing. These methods also represent a significant reduction in cost due to the use of widely available reagents and reduced personnel time required for batch processing. The combined methodologies provide significantly improved tools with which HIV-1 expression can be comprehensively examined in multiple compartments with accuracy and sensitivity. They will permit more frequent sampling, and thereby augment longitudinal clinical research studies in settings where the cost of commercially available assays is prohibitive and laboratory facilities are limited.
Acknowledgements
We would like to gratefully acknowledge Patricia Kissinger, PhD, Norine Schmidt, Mary Meyaski-Schluter, NP, and Rebecca Clark, MD for their help with this study, and we especially thank the participants from New Orleans HIV Outpatient clinic. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIVJR-FL from Dr. Irvin Y.S. Chen and HIV-1 Infected Cell Line (ACH-2) from Dr. Thomas Folks.
Funding: This study was supported by grants from the Health Excellence Fund and South Louisiana Institute for Infectious Disease Research, both sponsored by the Louisiana Board of Regents, and the LSUHSC Translational Research Initiative.
Abbreviations
- HIV/HIV-1
human immunodeficiency virus/ human immunodeficiency virus type 1
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- RNA
ribonucleic acid
- DNA
deoxyribonucleic acid
- EDTA
ethylenediaminetetraacetic acid
- PBS
phosphate buffered solution
- BMV
brome mosaic virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The authors have no conflicts of interest.
Declared ethical approval: Informed consent for sample collection was obtained in accordance with the Institutional Review Board of Louisiana State University Health Sciences Center.
References
- 1.Kovacs A, Wasserman SS, Burns D, Wright DJ, Cohn J, Landay A, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358(9293):1593–601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 2.Philpott S, Burger H, Tsoukas C, Foley B, Anastos K, Kitchen C, et al. Human immunodeficiency virus type 1 genomic RNA sequences in the female genital tract and blood: compartmentalization and intrapatient recombination. J Virol. 2005;79(1):353–63. doi: 10.1128/JVI.79.1.353-363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. Aids. 2001;15(7):837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180(1):93–8. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 6.Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177(2):320–30. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 7.Fiscus SA, Brambilla D, Coombs RW, Yen-Lieberman B, Bremer J, Kovacs A, et al. Multicenter evaluation of methods to quantitate human immunodeficiency virus type 1 RNA in seminal plasma. J Clin Microbiol. 2000;38(6):2348–53. doi: 10.1128/jcm.38.6.2348-2353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benki S, McClelland RS, Emery S, Baeten JM, Richardson BA, Lavreys L, et al. Quantification of genital human immunodeficiency virus type 1 (HIV-1) DNA in specimens from women with low plasma HIV-1 RNA levels typical of HIV-1 nontransmitters. J Clin Microbiol. 2006;44(12):4357–62. doi: 10.1128/JCM.01481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, et al. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000;16(13):1247–57. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch M, Curran J. In: Human Immunodeficiency Viruses. Fields Virology. 3rd ed. Fields BN DMK, Howley PM, editors. Lippincott-Williams; Philadelphia: 1996. pp. 1953–1976. [Google Scholar]
- 11.Krol MA, Olson NH, Tate J, Johnson JE, Baker TS, Ahlquist P. RNA-controlled polymorphism in the in vivo assembly of 180-subunit and 120-subunit virions from a single capsid protein. Proc Natl Acad Sci U S A. 1999;96(24):13650–5. doi: 10.1073/pnas.96.24.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AMPLICOR/COBAS HIV-1 Monitor Test v1.5 Package Insert. Roche Molecular Diagnostics; [Google Scholar]
- 13.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990;5(1):46–51. [Google Scholar]
- 14.Sun R, Ku J, Jayakar H, Kuo JC, Brambilla D, Herman S, et al. Ultrasensitive reverse transcription-PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36(10):2964–9. doi: 10.1128/jcm.36.10.2964-2969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissinger P, Amedee A, Clark RA, Dumestre J, Theall KP, Myers L, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36(1):11–6. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe M, Klett C, Wieland E, Gille S, Landt O. Two-step real-time PCR quantification of all subtypes of human immunodeficiency virus type 1 by an in-house method using locked nucleic acid-based probes. Folia Med (Plovdiv) 2008;50(3):5–13. [PubMed] [Google Scholar]
- 17.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Promso S, Srichunrusami C, Utid K, Lulitanond V, Pairoj W, Chantratita W. Quantitative detection of human immunodeficiency virus type 1 (HIV-1) viral load by real-time RT-PCR assay using self-quenched fluorogenic primers. Southeast Asian J Trop Med Public Health. 2006;37(3):477–87. [PubMed] [Google Scholar]
- 19.Yamamoto N, Tanaka C, Wu Y, Chang MO, Inagaki Y, Saito Y, et al. Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes. 2006;32(1):105–13. doi: 10.1007/s11262-005-5851-2. [DOI] [PubMed] [Google Scholar]
- 20.Zazzi M, Romano L, Catucci M, Venturi G, De Milito A, Valensin PE. Clinical evaluation of an in-house reverse transcription-competitive PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1999;37(2):333–8. doi: 10.1128/jcm.37.2.333-338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferns RB, Garson JA. Development and evaluation of a real-time RT-PCR assay for quantification of cell-free human immunodeficiency virus type 2 using a Brome Mosaic Virus internal control. J Virol Methods. 2006;135(1):102–8. doi: 10.1016/j.jviromet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Lockey C, Otto E, Long Z. Real-time fluorescence detection of a single DNA molecule. Biotechniques. 1998;24(5):744–6. doi: 10.2144/98245bm09. [DOI] [PubMed] [Google Scholar]