Abstract
Fragmentation of rapid eye movement sleep (REMS) is well described in individuals with posttraumatic stress disorder (PTSD) and likely has significant functional consequences. Fear-conditioned rodents may offer an attractive model of the changes in sleep that characterize PTSD. Following fear conditioning (FC), Wistar-Kyoto (WKY) rats, a strain known to be particularly stress-sensitive, have increased REMS fragmentation that can be quantified as a shift in the distribution of REMS episodes towards the more frequent occurrence of sequential REMS (inter-REMS episode interval ≤ 3 min) vs. single REMS (interval > 3 min). The α1 adrenoceptor antagonist prazosin has demonstrated efficacy in normalizing sleep in PTSD. To determine the utility of fear-conditioned WKY rats as a model of sleep disturbances typical of PTSD and as a platform for the development of new treatments, we tested the hypothesis that prazosin would reduce REMS fragmentation in fear-conditioned WKY rats. Sleep parameters and freezing (a standard measure of anxiety in rodents) were quantified at baseline and on days 1, 7, and 14 following FC, with either prazosin (0.01 mg/kg, i.p.) or vehicle injections administered prior to testing in a between-group design. Fear conditioning was achieved by pairing tones with a mild electric foot shock (1.0 mA, 0.5 s). One, 7, and 14 days following FC, prazosin or vehicle was injected, the tone was presented, freezing was measured, and then sleep was recorded from 11 AM to 3 PM. WKY rats given prazosin, compared to those given vehicle, had a lower amount of seq-REMS relative to total REMS time 14 days after FC. They also had a shorter non-REMS latency and fewer non-REMS arousals at baseline and on days 1 and 7 after FC. Thus, in FC rats, prazosin reduced both REMS fragmentation and non-REMS discontinuity.
Keywords: Fear conditioning, Norepinephrine, Posttraumatic stress disorder, REM sleep, Wistar-Kyoto rats
1. Introduction
Posttraumatic stress disorder (PTSD), a prevalent chronic mental disorder caused most often by an uncontrollable exposure to actual or threatened death or serious injury, is characterized by prominent disturbances of sleep, including repetitive, stereotypical nightmares and insomnia. Although there is no consensus regarding the pathophysiology of these disturbances, rapid eye movement sleep (REMS) discontinuity in the early aftermath of psychological trauma has been found to predict the development of PTSD in “at risk” individuals (Mellman et al., 2002). REMS discontinuity also has been reported in established PTSD (Breslau et al., 2004; Habukawa et al., 2007). It is essential to develop an animal model of PTSD sleep disturbances that takes account of these clinical observations.
Stress significantly affects sleep in rodents as well as humans (Pawlyk et al., 2008). REMS, in particular, changes dramatically after fear conditioning (FC) in rats and mice (Pawlyk et al., 2008). While the effects of FC on REMS generally have been assessed using standard measures of REMS macroarchitecture, such as the number and average duration of REMS episodes and the total amount of time spent in REMS, the microarchitecture of REMS also has been investigated (Amici et al., 1994; Zamboni et al., 1999). By partitioning total REMS time into sequential REMS (seq-REMS, inter-REMS episode interval ≤ 3 min) and single REMS (si-REMS, inter-REMS episode interval > 3 min), Amici et al. (1994) and Zamboni et al. (1999) determined that various stressors specifically affect seq-REMS and that episodes of seq-REMS, which are on average of shorter duration than those of si-REMS, occur in clusters. For example, Amici et al. (1994) found that the recovery sleep following exposure to cold stress occurred with an increased number of seq-REMS episodes in rats. This finding suggested that studying REMS microarchitectural changes following FC in rodent models might yield new insights into the mechanisms of stress-induced sleep disturbances that may be particularly relevant to PTSD.
DaSilva et al. (2011) reported that Wistar-Kyoto (WKY) rats, a strain known to be stress-sensitive according to a range of criteria (Paré, 1992; Tejani-Butt et al., 1994), responded to FC with a fragmentation of REMS, defined as a shift in the distribution of REMS episodes towards seq-REMS. This REMS fragmentation contrasted with the responses in FC Wistar and Sprague-Dawley rats, which were characterized by a preponderance of long duration si-REMS episodes (DaSilva et al., 2011; Madan et al., 2008). The heightened disruption of REMS by FC in WKY rats compared to other rat strains suggested to DaSilva et al. (2011) that fear-conditioned WKY rats could serve as an animal model of the sleep disturbances seen in humans with PTSD.
Establishing the construct validity of an animal model depends on drawing parallels to corresponding observations in humans. Although the selective serotonin reuptake inhibitors are widely used in the pharmacotherapy of PTSD, they have shown little or no efficacy for the sleep symptoms, recurrent nightmares and insomnia (Raskind, 2009, Schoenfeld et al., 2012). On the other hand, three placebo-controlled trials of prazosin, a selective α1 adrenoceptor antagonist that blocks noradrenergically mediated suppression of REMS (Makela and Hilakivi, 1986), have found a reduction in nightmares in combat Veterans with PTSD (Raskind et al., 2003;2007; 2013; Raskind, 2009). Furthermore, Taylor et al. (2008) reported that prazosin increased total REMS time and average REMS episode duration in a civilian group with PTSD, suggesting that a normalization of REMS continuity may be essential to the drug’s efficacy. Viewing REMS fragmentation in fear-conditioned WKY rats as a possible animal model of the sleep disturbances in PTSD, we hypothesized that prazosin would reduce REMS disruption induced by FC in WKY rats in a manner analogous to its beneficial effects on sleep in individuals with PTSD.
Individuals with PTSD complain of non-nightmare distressed awakenings as well as nightmares. These awakenings, which may be non-REMS phenomena, are also reported to be reduced by prazosin (Thompson et al., 2008). We therefore predicted that prazosin would reduce arousals from non-REMS in fear-conditioned WKY rats. Freezing, a standard measure of anxiety in animals, served as an independent measure of effective FC in our experiment. Preliminary reports have been published (Gajewski et al., 2011; Laitman et al., 2011a).
2. Methods
2.1. Subjects
Male WKY rats, 8 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). Animals were individually housed for a 1-week acclimation period in a temperature (22 ± 2 °C)- and humidity (45 ± 15%)- controlled animal colony located at the University of Pennsylvania School of Veterinary Medicine. The rats were given adlib access to food and water, except during the 10-min training period. They were maintained on a 12-h light/dark cycle, with lights on at 0700 h. Rats were assigned to either the vehicle group (N=6) or the prazosin group (N=7). All experimental procedures were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.2 Surgical procedure
All surgical procedures were performed under aseptic conditions. A mixture of ketamine (85 mg/kg, i.m.) and xylazine (15 mg/kg, i.m.) was injected to induce anesthesia, which was then maintained with isoflurane (0.25%). An incision was made over the left rib cage to expose the thoracic musculature, and a stainless steel electrode was sutured for recording the electrocardiogram (ECG). The animal's head was then placed in a stereotaxic apparatus and secured using blunt ear bars. A midline incision exposed the frontal and parietal bones and extended over the dorsal cervical musculature. The electroencephalogram (EEG) was recorded between two screws inserted into the skull on opposite sides at a distance of 2 mm from the midline, with one placed 2 mm anterior and the other 2 mm posterior to bregma. One additional screw electrode was implanted into the frontal bone for electrical grounding. Two insulated stainless steel wire electrodes were attached to the dorsal neck muscles for recording the electromyogram (EMG). Leads from the electrodes were routed to a 9-pin connector (Ginder Scientific, Ottawa, Canada) and cemented onto the skull with dental acrylic. Animals were given meloxicam (0.2 mg/kg, i.m.) as an analgesic, prior to surgery and again 24 h post-surgery. Gentamicin (5 mg/kg, s.c.) was diluted in Ringer's solution and given post-surgery as an antibiotic. Animals had a 1-week post-surgery recovery period.
2.3. Experimental procedures
The time line of experimental procedures is illustrated in Figure 1. Following surgery and a 1-wk recovery period, rats were habituated to handling, the injection procedure, and the sleep recording procedure in a recording room for 4 h (11 AM–3 PM) each day over 2 days. The next day (Baseline), animals received either a prazosin (0.01 mg/kg) or saline vehicle injection intraperitoneally and, beginning 15 min later (this delay permitted the measurement of freezing behavior), had a baseline 4-h sleep recording (11 AM–3 PM) in a room designated for sleep recording. This prazosin dose was chosen because, in preliminary experiments, higher doses (0.05 mg/kg, 0.5 mg/kg, and 1.0 mg/kg) disrupted REMS at baseline, i.e., prior to FC, and Pellejero et al. (1984) reported that prazosin doses from 0.125 mg/kg to 1.0 mg/kg suppressed REMS in Wistar rats. One day after the baseline recording (Training Day), rats were brought into a different room, designated for FC, and received 10 presentations of a conditioning stimulus (CS) (tone at 800 Hz, 90 dB, 5 s) co-terminating with a mild electric foot shock (1.0 mA, 0.5 s) at 30-s intervals. No injection was given on the Training Day, and sleep was not recorded. For training, animals were placed in an operant cage (Coulbourn Precision Regulated Animal Shocker Model E13–14; Coulbourn Instruments, Whitehall, PA) located in the training chamber in the training room, and foot shocks (Coulbourn Habitest Model E10-8RF) were transmitted through the grid floor. Tones were produced by a tone generator (Coulbourn Tone/Noise Generator).
Fig. 1.
Timeline of the experimental procedures. F/S - Entry into recording chamber followed by 5-min freezing assessment and 4-h sleep recording; T/F/S - Entry into recording chamber followed by 3 tone presentations, 5-min freezing assessment, and 4-h sleep recording.
One day (Day 1), seven days (Day 7), and 14 days (Day 14) after training, animals were injected intraperitoneally with either a prazosin or vehicle solution in the recording room and then placed in the recording chamber. All injections had a volume of 0.1 ml. Animals then received 3 tone presentations, without foot shock, at 30-s intervals. Fifteen min later (this delay permitted the measurement of freezing behavior), rats were connected to the recording cable for a 4-h sleep recording (11 AM–3 PM). Circadian factors were minimized by conducting each study at the same time each day.
Prazosin solution was made by dissolving prazosin hydrochloride (Sigma-Aldrich) in 33 mM sterile saline, as prazosin cannot be dissolved at this concentration in isotonic saline. The final solution was made daily from a stock solution of prazosin (0.2 mg/ml) by adding an appropriate amount of saline solution to prepare a 0.1 ml volume for injection.
2.4. Sleep recording and signal processing
As in previous studies conducted in this laboratory (DaSilva et al., 2011; Laitman et al., 2011b; Madan et al., 2008; Jha et al., 2005; Pawlyk et al., 2005), we chose to record sleep from 11 AM to 3 PM, a period of the circadian cycle when rats spend most of the time asleep (Dugovic et al., 2000). During sleep recording, rats remained, individually, in their home cage, and the cage was placed in a sound-dampened chamber (1 m3). They were connected to a counter-weighted cable attached to a freely rotating swivel (SL6C, Plastics One, Roanoke, VA). Signals were amplified using a Grass Model 7 polygraph (Grass-Telefactor, West Warwick, RI), digitized using a Power-1401 analog-to-digital converter at a rate of 250 Hz for the EEG and 1000 Hz for the nuchal EMG and the ECG, and stored on computer disk using Spike-2 software (Cambridge Electronics Design, Cambridge, UK). During data acquisition, the EEG was band-pass filtered at 0.3–100 Hz and the EMG and ECG at 30–1000 Hz. Concurrently, animal behavior was recorded via a video camera mounted inside the recording chamber. The ECG signal was acquired during these experiments, but its analysis is outside the scope of the present report.
2.5. Quantification of freezing behavior
Freezing behavior, defined as the absence of all motor activity with the exception of respiratory movements, served as an independent measure of the effectiveness of FC in five rats from the vehicle group and four rats from the prazosin group. It was assessed, at Baseline and again on Days 1, 7, and 14, over a 5-min observation period before the rat was connected to the recording cable. Freezing was calculated as the percent time spent immobile. All scoring was conducted by a person blind to the treatments.
2.6. Sleep data analysis
Somnologica software (Medcare, Buffalo, NY) was used for sleep scoring. EEG and nuchal EMG traces were scored manually in 10-s epochs, employing standard criteria for rats: Waking—low voltage, high frequency EEG, with high and variable EMG activity; non-REMS—high voltage, low frequency EEG and low and steady EMG activity; REMS—low voltage, high frequency EEG, with nuchal muscle atonia punctuated by occasional twitches. Episodes with signal characteristics typical of non-REMS but lasting less than 15 s were not scored as sleep to minimize the inclusion of a drowsy, pre-sleep state in the non-REMS category. Sleep latency (time to the first non-REMS epoch ≥ 15 s), sleep efficiency (total sleep time/total recording time), and total times (min) spent in REMS and non-REMS were measured. REMS was further assessed by partitioning episodes into si-REMS (inter-REMS episode interval > 3 min) and seq-REMS (inter-REMS episode interval ≤ 3 min) (Amici et al., 1994). Using these criteria, total times (min) spent in si-REMS and seq-REMS, as well as the number and mean duration of si-REMS and seq-REMS episodes, were calculated using a program created by Dr. Aaron Pawlyk (2005). The percentages of total REMS time occupied by si-REMS and seq-REMS (si-REMS% and seq-REMS%, respectively), also were determined. Non-REMS fragmentation was assessed by counting the number of arousals during non-REMS. Arousals were defined, as in humans (American Sleep Disorders Association, 1992), as a change in the EEG for a minimum of 3 s with a corresponding change in EMG activity; an example of a brief arousal is provided in Figure 2. All non-REMS arousals except those that occurred within the first or last 10 s of a non-REMS episode were counted. Arousal frequency was calculated as the number of arousals/non-REMS time. Sleep scoring was conducted by a person blind to the treatments.
Fig. 2.
Cortical EEG and nuchal EMG traces with an example of a transient arousal during non-REMS. A transient arousal occurs in the framed portion of the record, with the EEG changing to a low voltage/high frequency pattern and a concurrent nuchal EMG activation.
2.7. Statistical analysis
Statistical analysis was performed using SigmaPlot 11 (Systat Software, Inc., Chicago, IL). Mixed models ANOVA was used to analyze the effects of day of test and treatment (prazosin or vehicle) on all parameters. Day was treated as a within subjects factor, and treatment was a between subjects factor. Pairwise post-hoc comparisons were conducted using the Holm-Sidak method to control for type I errors. Measurement variability is characterized by the standard error of the mean (± SEM) throughout the report. Differences were considered significant at P < 0.05.
3. Results
3.1 Effects of prazosin on the EEG and sleep-wake cycling
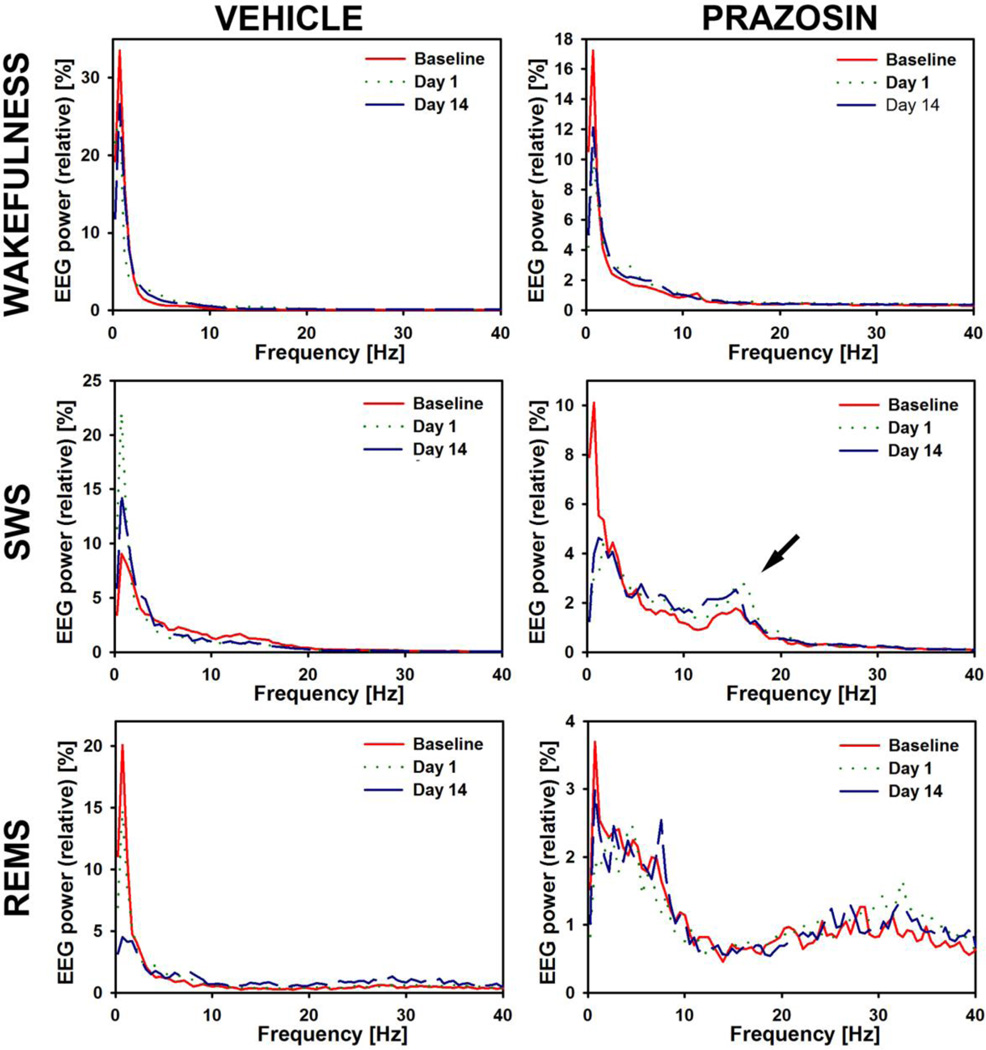
On gross examination, the prazosin-treated rats exhibited normal cycling across different behavioral states, and the general appearance of the cortical EEG was normal. However, power spectral analysis revealed distinct features of cortical activity consistent with those reported previously for rats treated with prazosin (without FC) (Sebban et al., 1999; Berridge and España, 2000). Specifically, when compared to vehicle-treated rats, prazosin-treated rats had reduced EEG power in the delta range (0.5–4.0 Hz) across all sleep-wake states and an increased relative power in the 12–18 Hz range during non-REMS. The latter range includes the sigma band, which shows increased power during non-REMS prior to the transition to REMS (Capitani et al., 2005). Thus, while a detailed analysis of any potential interactions between the effects of prazosin and FC on the cortical EEG was beyond the scope of this study, it is worth noting that the increased cortical 12–18 Hz rhythm during non-REMS is consistent with our finding that prazosin-treated FC rats had a reduced propensity for non-REMS arousals. Figure 3 shows that, within subjects, the power spectra of the cortical EEG, calculated separately for waking, non-REMS, and REMS, maintained their characteristic features across the entire experimental protocol. Figure 3 also compares the typical power spectra in a vehicle-treated and a prazosin-treated rat.
Fig. 3.
Stability of the power spectra of the cortical EEG between Baseline and Day 14, and comparison of the characteristic features of cortical power spectra between a vehicle-treated (left) and a prazosin-treated (right) rat. Cortical power in the delta frequency range (0.5–4.0 Hz) is consistently lower in the prazosin-treated rat than in the vehicle-treated rat in all three behavioral states. Also, there is the characteristic increase of relative power in the 12–18 Hz range during non-REMS in the prazosin-treated rat (arrow). For this comparison, cortical power spectra were calculated for continuous 10-min periods of waking prior to the first non-REMS episode in each recording session (top graphs), 2-min periods of the first sufficiently long non-REMS episode in each recording (middle row), and 1-min periods of the first sufficiently long REMS episode in each recording. The graphs show relative powers in 0.4883 Hz bins normalized by the total power of each spectrogram over the 0–50 Hz range.
3.2 Non-REMS macroarchitecture
Prazosin-treated WKY rats had an overall shorter non-REMS latency than the vehicle-treated group (F1,33 = 10.16, P = 0.009), and the differences were significant at Baseline (t = 2.11, P = 0.043), on Day 1 (t = 3.12, P = 0.004), and on Day 7 (t = 2.05, P = 0.048) (Table 1 and Fig. 4A). There was a significant Day-by-Treatment interaction for the average non-REMS episode duration (F3,33 = 3.30, P = 0.032). WKY rats given vehicle had a significantly lower average non-REMS episode duration on Day 1 (t = 3.89, P = 0.001) and on Day 7 (t = 3.48, P = 0.001) when compared to Day 14 (Table 1). In contrast, there was no effect of Day on non-REMS episode duration in the prazosin group. On Day 14, the prazosin group had a shorter non-REMS episode duration when compared to the vehicle group (t = 3.14, P = 0.005) (Table 1). There was no significant change from Baseline in non-REMS episode number or non-REMS amount on any day, nor did any of these measures differ between the treatment groups on any day.
Table 1.
Summary of sleep parameters in vehicle- and prazosin-treated rats across the duration of the experimental protocol.
| Baseline | Day 1 | Day 7 | Day 14 | |||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Prazosin | Vehicle | Prazosin | Vehicle | Prazosin | Vehicle | Prazosin | |
| Non-REMS | ||||||||
| Latency (min) | 21.8±4.9 | 12.2±2.2* | 24.2±6.5 | 9.9±1.2*** | 16.7±1.7 | 7.4±2.3* | 14.7±3.2 | 7.3±1.1 |
| Episode duration (min) | 2.3±0.3 | 1.8±0.1 | 2.0±0.2## | 1.8±0.2 | 2.1±0.2## | 1.9±0.2 | 2.8±0.3 | 1.9±0.2*** |
| Episode no. | 56.2±5.5 | 64.0±4.4 | 59.5±6.5 | 70.7±5.9 | 62.2±6.2 | 68.1±4.9 | 48.8±5.2 | 70.9±6.6 |
| Amount (min) | 120.1±4.0 | 115.2±7.0 | 113.4±7.4 | 120.5±5.2 | 122.8±1.3 | 126.1±4.5 | 129.8±4.9 | 123.2±3.6 |
| Arousals (hour−1) | 16.8±0.6# | 8.4±1.2**** | 10.2±1.2 | 7.8±0.6 | 14.4±1.8# | 7.8±1.2**** | 13.8±1.2 | 7.2±0.6**** |
| REMS | ||||||||
| Latency (min) | 60.4±6.4 | 57.0±11.1 | 68.6±16.1 | 55.0±10.0 | 63.6±8.6 | 67.7±9.8 | 62.5±5.4 | 66.5±6.8 |
| Episode duration (min) | 2.0±0.3 | 1.8±0.2 | 1.6±0.2 | 1.9±0.2 | 1.8± 0.1 | 1.8±0.2 | 1.5±0.1 | 1.9±0.1 |
| Episode no. | 20.8±2.0 | 18.0±2.2 | 21.8±2.1 | 18.6±2.2 | 20.7±2.3 | 16.9±2.3 | 22.2±1.5 | 16.1±0.9* |
| Amount (min) | 38.9±2.2 | 30.5±3.4 | 34.0±2.4 | 33.0±1.4 | 35.9±3.6 | 28.6±3.2 | 33.9±3.3 | 30.1±1.6 |
| Percentage of total sleep | 24.4±0.9 | 21.2±2.8 | 23.1±0.8 | 21.6±0.9 | 22.4±1.6 | 18.5±2.1 | 20.7±1.8 | 19.6±1.0 |
| seq-REMS | ||||||||
| Latency (min) | 90.9±5.0 | 98.5±18.1 | 82.6±14.4 | 110.5±14.0 | 108.6±25.0 | 142.8±19.6 | 85.4±8.2 | 103.4±14.0 |
| Episode duration (min) | 1.8±0.2 | 1.5±0.1 | 1.4±0.1 | 1.4±0.1 | 1.6±0.1 | 1.5±0.1 | 1.5±0.1 | 1.7±0.1 |
| Episode no. | 11.5±1.5 | 8.4±2.1 | 13.2±1.7 | 9.1±2.7 | 12.2±2.7 | 7.7±1.9 | 15.0±2.4 | 7.1±1.0*** |
| Amount (min) | 19.5±2.9 | 11.5±2.3 | 17.4±1.6 | 12.9±4.0 | 18.9±4.0 | 11.6±3.2 | 22.3±3.7 | 12.1±1.7** |
| Percentage of total REMS | 50.0±6.1 | 36.8±5.4 | 53.5±7.6 | 36.8±10.3 | 50.8±6.4 | 37.6±6.5 | 63.9±5.4 | 39.7±5.0*** |
| si-REMS | ||||||||
| Latency (min) | 66.1±10.6 | 60.4±12.6 | 74.5±19.9 | 55.0±10.0 | 75.7±14.2 | 67.7±9.8 | 78.8±12.6 | 70.0±8.9 |
| Episode duration (min) | 2.2±0.3 | 2.0±0.2 | 1.9±0.4 | 2.1±0.2 | 2.0±0.2 | 2.0±0.2 | 1.7±0.1 | 2.0±0.2 |
| Episode no. | 9.3±1.0 | 9.6±0.5 | 8.7±1.2 | 9.4±1.0 | 8.5±0.8 | 9.1±1.1 | 7.2±1.0 | 9.0±0.7 |
| Amount (min) | 19.4±2.7 | 19.0±2.2 | 16.6±3.8 | 20.1±2.8 | 17.1±2.0 | 17.0±1.4 | 11.7±1.4 | 18.0±1.4 |
| Percentage of total REMS | 50.0±6.1 | 63.2±5.4 | 46.5±7.6 | 63.2±10.3 | 49.2±6.4 | 62.5±6.5 | 36.1±5.4 | 60.3±5.0*** |
The means are based on 7 rats treated with prazosin and 6 receiving vehicle (±SEM). Significance levels:
P < 0.05, within Day;
P < 0.025, within Day;
P < 0.02, within Day;
P < 0.002, within Day;
P < 0.006, vs. Day 1;
P < 0.002, vs. Day 14.
Fig. 4.
Comparison of selected non-REMS and REMS parameters in prazosin- and vehicle-treated rats. (A) non-REMS latency (± SEM) in rats receiving prazosin (n=7) compared to rats receiving vehicle (n=6). Significance level for between-group comparisons: *P<0.05, ***P<0.02. (B) non-REMS arousal frequency (± SEM) in the same groups of prazosin- and vehicle-treated rats. Significance level for between-group comparisons: ****P<0.002. Significance level for within-group comparisons (Vehicle/Day 1 vs. Baseline and Day 7): #P<0.006. (C) Seq-REMS% (seq-REMS time/total REMS time; ± SEM) in the same groups of prazosin- and vehicle-treated rats. Significance level for the between-group comparison: ***P<0.02.
3.3 Non-REMS microarchitecture
There was a significant Day-by-Treatment interaction for non-REMS arousal frequency (F3,33 = 3.19, P = 0.036) (Fig. 4B). Post-hoc tests revealed a significantly lower arousal frequency in the vehicle group on Day 1 compared to both Baseline (t = 4.70, P = 0.001) and Day 7 (t = 3.00, P = 0.005) (Table 1). There was no effect of Day on the arousal frequency in the prazosin group. The prazosin group had less frequent non-REMS arousals at Baseline (t = 5.09, P = 0.001), on Day 7 (t = 4.11, P = 0.001), and on Day 14 (t = 4.37, P = 0.001) when compared to the vehicle group, and there was a trend toward significance on Day 1 (Table 1).
3.4 REMS macroarchitecture
Compared to the prazosin group, the vehicle group had an overall higher REMS episode number (F1,33 = 7.60, P = 0.019), and post-hoc comparison revealed a significant difference on Day 14 (t = 2.13, P = 0.039) (Table 1). There was no significant change from Baseline in average REMS episode duration, REMS amount, or REMS% on any day, nor did any of these measures differ between treatment groups on any day (Table 1).
3.5 REMS microarchitecture
The prazosin-treated group had fewer seq-REMS episodes when compared to the vehicle group (F1,33 = 8.93; P = 0.012), and the difference also was statistically significant on Day 14 (t = 2.69; P = 0.010) (Table 1). There was no significant change from Baseline in the average seq-REMS episode duration on any day, nor was this measure affected by prazosin. The prazosin group had a decreased seq-REMS amount compared to the vehicle group (F1,33 = 8.34, P = 0.015), with the difference again being significant on Day 14 (t = 2.35, P = 0.024) (Table 1). There was no significant change from Baseline in the average seq-REMS episode duration on any day, nor was this measure affected by prazosin. The prazosin group had an overall lower seq-REMS% (seq-REMS time/total REMS time) when compared to the vehicle group (F1,33 = 8.91, P = 0.012), and the difference was significant on Day 14 (t = 2.49, P = 0.017) (Table 1 and Fig. 4C). This was accompanied by a corresponding significant increase in si-REMS% on Day 14 (t = 2.49, P = 0.017) (Table 1).
3.6 Freezing
Attesting to the effectiveness of the FC procedure, there was a main effect of day on the freezing percentage (F3,20 = 6.32, P = 0.003), which increased between Baseline and Day 1 from 4.4 ± 1.7% to 24 ± 7% in the prazosin group, and from 0.9 ± 0.3% to 46 ± 19% in the vehicle group. Compared to Baseline, the freezing percentage remained significantly higher in both groups on Day 7 (Prazosin: 34 ± 9%; Vehicle: 45 ± 10%) and on Day 14 (Prazosin: 25 ± 5%; Vehicle: 37 ± 14%). With this small number of rats, no main effect of treatment was demonstrated.
4. Discussion
We found an improved REMS consolidation 14 days following FC in prazosin-treated WKY rats when compared to the vehicle-treated group. On Day 14, the number of seq-REMS episodes, the amount of seq-REMS, and the seq-REMS percentage all were decreased in the prazosin compared to the vehicle group. Aside from the greater total number of REMS episodes in the vehicle group, reflecting a larger number of seq-REMS episodes, there were no other REMS macroarchitectural findings. These results support the merits of assessing REMS microarchitecture, and its modulation by potential pharmacologic treatments for PTSD, in FC rodents.
Prazosin is administered chronically in clinical trials, and the dose must be titrated gradually in order to avoid orthostatic hypotension as a side-effect. In Raskind et al. (2013), the behavioral effects of prazosin were first measured after seven weeks of treatment. Our study, which used acute administration (Days 1, 7, and 14) and demonstrated REMS consolidation on Day 14 following FC, suggests that prazosin’s efficacy for reducing the sleep disturbances in PTSD may not depend on receptor and post-receptor changes occurring after chronic administration.
The increase in freezing behavior in both groups on Day 1 shows the effectiveness of the FC protocol. The absence of a significant effect of Day on seq-REMS measures can be explained by the experimental design, which included a presumably stressful intraperitoneal injection prior to each sleep recording, including the Baseline. DaSilva et al. (2011) found that, without any injection of vehicle or drug at any time point, seq-REMS percentage had increased from 29 at Baseline to 54 at Day 14 following FC. In the current study, vehicle-injected rats already had a high seq-REMS percentage, 50, at Baseline, and this had increased to 64 by Day 14. A larger group might reveal statistical significance of this relatively small increase.
The finding that an α1 adrenoceptor antagonist improved REMS continuity in FC rats is consistent with previous observations. We previously reported that, in cats, prazosin partially blocked the inhibition of REMS produced by the NE reuptake-blocking drug desipramine (Ross et al., 1995). In other studies, prazosin prevented the inhibition of REMS caused by noradrenergic stimulation in cats (Hilakivi and Leppavuori, 1984) and increased REMS during the light period in rats (Makela and Hilakivi, 1986). However, given that locus coeruleus cell firing is thought to be near zero during REMS (and postsynaptic noradrenergic “tone” presumably very low or absent), the question arises how an adrenoceptor antagonist could promote REMS continuity. The explanation may be that just before and very early in a REMS episode, following FC, intrasynaptic NE is present, owing, probably, to the few remaining spikes in locus coeruleus neurons (Gottesmann, 2008). Another possible explanation is that an excitatory glutamatergic input from ventrolateral medullary adrenergic (C1) neurons drives noradrenergic locus coeruleus neurons during REMS in fear-conditioned WKY rats (Abbott et al., 2012). Indeed, indirect evidence based on c-Fos immunohistochemistry suggests that C1 neurons, unlike those of the locus coeruleus, do not have reduced firing during REMS (Rukhadze et al., 2008), and direct recordings of their activity show that C1 cells are activated during pharmacologically induced REMS (Stettner et al., 2013).
Where in the brain prazosin can act to maintain REMS continuity in the aftermath of fearful tone presentations is uncertain. Pal and Mallick (2006) observed that both prazosin and the β adrenoceptor antagonist propranolol injected into the rat pedunculopontine tegmentum increased REMS amount, and they inferred that NE in the pedunculopontine tegmentum tonically inhibits REMS. The α1 adrenoceptor agonist phenylephrine has been reported to activate dorsal raphe serotonergic neurons, which, under normal conditions, are silent during REMS (Brown et al., 2002). Therefore, prazosin acting in the dorsal raphe nucleus might be expected to promote REMS.
Observing that NE release in the amygdala was highest during active waking and lowest during REMS, Park (2002) suggested that NE acts in the amygdala to enhance waking and inhibit REMS. Pathways from the amygdala to brainstem REMS-generating regions have been well described. The basolateral nucleus of the amygdala (BLA) projects to the central nucleus of the amygdala (CNA) and through CNA to the brainstem, as well as the bed nucleus of the stria terminalis (BNST) (Amaral et al., 1992). Alpha1 adrenoceptors, the most abundant adrenoceptors in the central nervous system, exist in three different isoforms, α1A, α1B, and α1D, and prazosin is an antagonist of each. The expression of mRNA encoding the α1A receptor was found to be high in the central part of the lateral division of the CNA and lower in the medial division. There was moderate expression in the BLA and less expression in the lateral nucleus of the amygdala (LA). The expression of α1B adrenoceptor mRNA was low in the CNA, low to moderate in the BNST, and high in the dorsolateral division of the LA. Alpha1D mRNA expression was high in the LA and moderate in the ventral BLA (Day et al., 1997).
Forebrain regions other than the amygdala also participate in REMS regulation (Morrison and Reiner, 1985). The preoptic area, in particular the extended ventrolateral preoptic nucleus (VLPO), plays a role in the homeostatic control of REMS, and the extended VLPO projects to brainstem regions that regulate REMS (Semba, 2011). VLPO neurons in the rat are inhibited by NE (Gallopin et al., 2000), suggesting that REMS consolidation by prazosin might depend on the VLPO.
Possible sites of prazosin’s action in preventing REMS fragmentation also are suggested by the stress literature. The rat hippocampus, like that of humans, receives a heavy noradrenergic innervation, and three different unconditioned aversive stimuli were found to produce an upregulation of α1D adrenoceptor mRNA in the dentate gyrus, peaking about 90 min after stressor onset (Campeau et al., 2010). Given the prominent role of the hippocampus in memory, it is possible that, in the current experiment, prazosin interfered with tone-induced aversive memory retrieval and downstream effects on sleep. Likewise, there is evidence that NE acting in the LA and the BNST promotes the expression of fear-potentiated startle in the rat (Schulz et al., 2002; Schweimer et al., 2005), suggesting that prazosin could act in the amygdala and extended amygdala to block fear retrieval and consequent REMS fragmentation. Blocking α1 adrenoceptor function in the dorsal raphe nucleus has been shown to reduce a FC waking behavior in rats (Grahn et al., 2002).
There were no effects of day or drug on non-REMS amount or non-REMS%. Supporting the rationale for measuring non-REMS microarchitecture in FC rodents and its modulation by drug treatment, we found that WKY rats given prazosin compared to those given vehicle had less frequent non-REMS arousals on all recording days except Day 1, when there was a trend toward significance. Possible sites of prazosin’s action that may be responsible for this effect include the nucleus basalis of the basal forebrain, a major relay in the pathway from the ascending reticular activating system that produces cortical activation (Fort et al., 1998); in the guinea pig, both cholinergic and non-cholinergic neurons in this region are excited by NE, predominantly via α1 adrenoceptors (Fort et al., 1998). The paraventricular nucleus of the thalamus, a member of the dorsal group of midline and intralaminar thalamic nuclei, might also be involved; it is thought to play a role in setting behavioral state during stress or fear, and it receives strong noradrenergic input (Van der Werf et al., 2002).
Largely on the basis of observations in the waking state, it has been suggested that noradrenergic hyper-responsiveness is essential to the pathophysiology of PTSD (Southwick et al., 1999). However, increased noradrenergic activity may also occur during sleep in individuals with PTSD (Mellman et al., 1995;2004), and, given the inhibitory action of NE on sleep-promoting mechanisms, it can be hypothesized that abnormal NE release disrupts sleep in individuals with PTSD. The one polysomnographic study of prazosin in humans with PTSD reported on REMS and non-REMS macroarchitecture only (Taylor et al., 2008). In a small sample of individuals with chronic PTSD regularly receiving prazosin compared to placebo at bedtime for three weeks, active drug increased total REMS time and average REMS episode duration, as well as total sleep time (Taylor et al., 2008). Our rodent model is not strictly analogous as the rats had their sleep studied only up to 14 days after FC and they received prazosin at Baseline and only on the three test days (Days 1, 7, and 14), not daily. Nevertheless, Taylor et al.’s (2008) finding of an increase in REMS episode duration suggests a relation to our observation of an increase in si-REMS% in prazosin-treated compared to vehicle-treated rats on Day 14, as si-REMS episodes are on average longer than seq-REMS episodes.
The hypothesis that prazosin’s effectiveness for reducing the nightmare disturbance in PTSD depends on its increasing REM sleep continuity should be considered in the context of other findings regarding PTSD pharmacotherapy. Among psychotropic medications, the selective serotonin reuptake inhibitors are most commonly used for the treatment of PTSD. However, they do not consistently improve sleep quality and reduce nightmares (Schoenfeld et al., 2012), and, unlike prazosin, these drugs suppress REM sleep (Qureshi and Lee-Chiong Jr., 2004). Likewise, the tricyclic antidepressant drugs, which inhibit REM sleep, have limited effectiveness in managing the sleep disturbances in PTSD (Schoenfeld et al., 2012). Benzodiazepines and non-benzodiazepine drugs that act at the benzodiazepine receptor also have a suppressant effect on REM sleep (Tobler et al., 2001); there have not been sufficient studies to assess their effects on sleep in PTSD. Interestingly, the antidepressant bupropion, which was reported to increase REM sleep in depressed men (Nofzinger et al., 1995), did improve sleep in one placebo-controlled study of individuals with PTSD (Becker et al., 2007).
REMS fragmentation has been described in humans with PTSD (Breslau et al., 2004; Habukawa et al., 2007). Because fear-conditioned WKY rats, but not identically treated Wistar rats, showed an increase in seq-REMS following fearful reminders, DaSilva et al. (2011) proposed fear-conditioned WKY rats as an animal model of the sleep disturbance in PTSD. Morilak et al. (2005) have suggested that genetic factors contributing to noradrenergic reactivity might help to explain stress vulnerability. Although, compared to Sprague-Dawley rats, WKY rats displayed diminished noradrenergic responsiveness to an acute stressor, WKY rats repeatedly exposed to the cold showed a robust noradrenergic response to acute immobilization stress; furthermore, administration into the BNST of an α1 adrenoceptor antagonist reduced the hypothalamo-pituitary-adrenal axis response to acute stress in cold-sensitized vs. non-sensitized WKY rats, but not Sprague-Dawley rats (Morilak et al., 2005). It might be hypothesized that WKY rats, with low baseline NE release, have compensatory elevated postsynaptic α1 adrenoceptor function that underlies heightened, prazosin-sensitive noradrenergic reactivity following FC.
Non-nightmare distressed awakenings occur commonly in PTSD and are also reduced by prazosin (Thompson et al., 2008). Although the physiological substrate(s) of these awakenings remains unknown, many likely occur from non-REMS because they are less often associated with dream reports (Dement et al., 1957). The observation that prazosin decreased arousals from non-REMS in fear-conditioned WKY rats adds to the validity of FC in this rat strain as an animal model of the sleep disturbances in PTSD.
Given Mellman et al.’s (2002) finding that REMS fragmentation within a month following an accidental injury predicted the development of PTSD within two months, sleep discontinuity could have particular functional significance in the early aftermath of trauma. Indeed, there is considerable evidence that REMS is important in the adaptive processing of fearful stimuli during associative learning in animals (Datta, 2000; Mavanji and Datta, 2003; Walker et al., 2009). Thus, we propose that REMS fragmentation in recently fear-conditioned WKY rats may bear particular relation to that seen in traumatized humans at heightened risk for developing PTSD.
5. Conclusions
There is considerable evidence that REMS discontinuity characterizes sleep in individuals with chronic PTSD and that, in the early aftermath of trauma, it serves as a predictor of who will go on to develop PTSD (Mellman et al., 2002; Breslau et al., 2004; Habukawa et al., 2007). Fear conditioning protocols have been used to model PTSD in rodents. Different from other rat strains, fear-conditioned WKY rats show, upon exposure to fearful reminders 14 days post-FC, REMS fragmentation, defined as a shift in the distribution of REMS episodes to seq-REMS (Amici et al., 1994; Zamboni et al., 1999; DaSilva et al., 2011). This led DaSilva et al. (2011) to propose fear-conditioned WKY rats as an animal model of the sleep disturbances in PTSD. In clinical studies, the α1 adrenoceptor antagonist prazosin has been shown to improve the subjective sleep disturbances in PTSD, perhaps in part by promoting REMS continuity (Raskind et al., 2003; 2007; 2013; Taylor et al., 2008). Fourteen days following FC, WKY rats administered prazosin compared to vehicle immediately prior to being presented with a fearful tone reminder showed less REMS fragmentation, as well as fewer arousals from non-REMS. These observations add to the construct validity of fear-conditioned WKY rats as an animal model of the PTSD sleep disturbances. Where in the brain systemically administered prazosin might act to consolidate sleep requires further investigation. The results of such studies should shed light on the brain mechanisms of the sleep disturbances in PTSD.
Research Highlights.
REMS discontinuity characterizes sleep in individuals with PTSD.
WKY rats show REMS fragmentation after FC.
Prazosin improves sleep in PTSD, possibly by promoting sleep continuity.
Prazosin reduced REMS, as well as non-REMS, disruption in fear-conditioned WKY rats.
Fear-conditioned WKY rats may provide an animal model of sleep in PTSD.
ACKNOWLEDGMENTS
The study was supported by NIH grant RO1-MH072897. The authors thank Dr. Philip R. Gehrman for his assistance with statistical analysis.
Abbreviations
- BLA
basolateral nucleus of the amygdala
- BNST
bed nucleus of the stria terminalis
- CNA
central nucleus of the amygdala
- CS
conditioning stimulus
- ECG
electrocardiogram
- EEG
electroencephalogram
- EMG
electromyogram
- FC
fear-conditioned or fear conditioning
- LA
lateral nucleus of the amygdala
- NE
norepinephrine
- PTSD
posttraumatic stress disorder
- REMS
rapid eye movement sleep
- seq-REMS
sequential rapid eye movement sleep
- si-REMS
single rapid eye movement sleep
- VLPO
ventrolateral preoptic nucleus
- WKY
Wistar-Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views presented in this article do not represent those of the Department of Veterans Affairs or of the US Government.
REFERENCES
- Abbott SB, Kanbar R, Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats. J Physiol (Lond) 2012;590:2897–2915. doi: 10.1113/jphysiol.2012.232157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Price J, Pitkanen A, Carmichael S. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss, Inc.; 1992. [Google Scholar]
- American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- Amici R, Zamboni G, Perez E, Jones CA, Toni I, Culin F, et al. Pattern of desynchronized sleep during deprivation and recovery induced in the rat by changes in ambient temperature. J Sleep Res. 1994;3:250–256. doi: 10.1111/j.1365-2869.1994.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Becker ME, Hertzberg MA, Moore SD, Dennis MF, Bukenya DS, Beckham JC. A placebo-controlled trial of bupropion SR in the treatment of chronic posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27:193–197. doi: 10.1097/JCP.0b013e318032eaed. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA. Synergistic sedative effects of noradrenergic α1- and β-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psych. 2004;61:508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Kryskow EM, Masini CV, Babb JA, Sasse SK, et al. Stress rapidly increases alpha 1d adrenergic receptor mRNA in the rat dentate gyrus. Brain Res. 2010;1323:109–118. doi: 10.1016/j.brainres.2010.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani P, Cerri M, Amici R, Baracchi F, Jones CA, Luppi M, et al. Changes in EEG activity and hypothalamic temperature as indices for non-REM sleep to REM sleep transitions. Neurosci Lett. 2005;383:182–187. doi: 10.1016/j.neulet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- DaSilva JK, Lei Y, Madan V, Mann GL, Ross RJ, Tejani-Butt S, et al. Fear conditioning fragments REM sleep in stress-sensitive Wistar-Kyoto, but not Wistar, rats. Prog Neuropsychopharmacol Biol Psych. 2011;35:67–73. doi: 10.1016/j.pnpbp.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. EEG Clin Neurophysiol. 1957;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11:627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Serafin M, Muhlethaler M, Jones BE. Pharmacological characterization and differentiation of non-cholinergic nucleus basalis neurons in vitro. Neuroreport. 1998;9:61–65. doi: 10.1097/00001756-199801050-00013. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gajewski ND, Laitman BM, Mann GL, Kubin L, Ross RJ, Morrison AR. α-1 adrenoceptor antagonist reduces non-REM sleep (NREMS) arousals in fear-conditioned Wistar-Kyoto rats (WKY) Sleep. 2011;34(Suppl):A74. (Abstr. #0209). [Google Scholar]
- Gottesmann C. Noradrenaline involvement in basic and higher integrated REM sleep processes. Prog Neurobiol. 2008;85:237–272. doi: 10.1016/j.pneurobio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O'Connor KA, Deak T, Sparks PD, et al. Blockade of alpha1 adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behav Brain Res. 2002;134:387–392. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol Psych. 2007;62:1179–1182. doi: 10.1016/j.biopsych.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hilakivi I, Leppavuori A. Effects of methoxamine, and alpha-1 adrenoceptor agonist, and prazosin, an alpha-1 antagonist, on the stages of the sleep-waking cycle in the cat. Acta Physiol Scand. 1984;120:363–372. doi: 10.1111/j.1748-1716.1984.tb07396.x. [DOI] [PubMed] [Google Scholar]
- Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–1080. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- Laitman BM, Gajewski ND, Mann GL, Kubin L, Ross RJ, Morrison AR. α-1 adrenoceptor antagonist prazosin reduces REM sleep (REMS) fragmentation and non-REM sleep (NREMS) latency in fear-conditioned Wistar-Kyoto rats (WKY) Sleep. 2011a;34(Suppl):A67. (Abstr. #0186). [Google Scholar]
- Laitman BM, DaSilva JK, Ross RJ, Tejani-Butt S, Morrison AR. Reduced gamma range activity at REM sleep onset and termination in fear-conditioned Wistar-Kyoto rats. Neurosci Lett. 2011b;493:14–17. doi: 10.1016/j.neulet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Brennan FX, Mann GL, Horbal AA, Dunn GA, Ross RJ, et al. Long-term effect of cued fear conditioning on REM sleep microarchitecture in rats. Sleep. 2008;31:497–503. doi: 10.1093/sleep/31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela JP, Hilakivi IT. Effect of alpha-adrenoceptor blockade on sleep and wakefulness in the rat. Pharmacol Biochem Behav. 1986;24:613–616. doi: 10.1016/0091-3057(86)90566-6. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–370. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psych. 2002;159:1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psych. 2004;55:953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psych. 1995;38:174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psych. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morrison AR, Reiner PB. A dissection of paradoxical sleep. In: McGinty DJ, Drucker-Colin R, Morrison AR, Parmeggiani PL, editors. Brain Mechanisms of Sleep. New York: Raven Press; 1985. pp. 97–110. [Google Scholar]
- Nofzinger EA, Reynolds CF, III, Thase ME, Frank E, Jennings JR, Fasiczka AL, et al. REM sleep enhancement by bupropion in depressed men. Am J Psychiatry. 1995;152:274–276. doi: 10.1176/ajp.152.2.274. [DOI] [PubMed] [Google Scholar]
- Pal D, Mallick BN. Role of noradrenergic and GABA-ergic inputs in pedunculopontine tegmentum for regulation of rapid eye movement sleep in rats. Neuropharmacology. 2006;51:1–11. doi: 10.1016/j.neuropharm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Paré WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–1056. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Park SP. In vivo microdialysis measures of extracellular norepinephrine in the rat amygdala during sleep-wakefulness. J Korean Med Sci. 2002;17:395–399. doi: 10.3346/jkms.2002.17.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psych. 2005;57:268–277. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellejero T, Monti JM, Baglietto J, Jantos H, Pazos S, Cichevski V, et al. Effects of methoxamine and alpha-adrenoceptor antagonists, prazosin and yohimbine, on the sleep-wake cycle of the rat. Sleep. 1984;7:365–372. doi: 10.1093/sleep/7.4.365. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Lee-Chiong T., Jr Medications and their effects on sleep. Med Clin North Am. 2004;88:751–766. doi: 10.1016/j.mcna.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psych. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;AiA:1–8. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Raskind MA. Pharmacologic treatment of PTSD. In: Shiromani PJ, LeDoux JE, Keane TM, editors. Post-Traumatic Stress Disorder: Basic Science and Clinical Practice. New York: Humana; 2009. pp. 337–362. [Google Scholar]
- Ross RJ, Gresch PJ, Ball WA, Sanford LD, Morrison AR. REM sleep inhibition by desipramine: evidence for an alpha-1 adrenergic mechanism. Brain Res. 1995;701:129–134. doi: 10.1016/0006-8993(95)00984-x. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following rapid eye movement sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience. 2008;152:208–222. doi: 10.1016/j.neuroscience.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld FB, DeViva JC, Manber R. Treatment of sleep disturbances in posttraumatic stress disorder: A review. J Rehabil Res Dev. 2012;49:729–752. doi: 10.1682/jrrd.2011.09.0164. [DOI] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Schnitzler HU. Clonidine injections into the lateral nucleus of the amygdala block acquisition and expression of fear-potentiated startle. Eur J Neurosci. 2002;15:151–157. doi: 10.1046/j.0953-816x.2001.01831.x. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Sebban C, Zhang XQ, Tesolin-Decros B, Millan MJ, Spedding M. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br J Pharmacol. 1999;128:1045–1054. doi: 10.1038/sj.bjp.0702894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K. Preoptic and basal forebrain modulation of REM sleep. In: Mallick BN, Pandi-Perumal SR, McCarley RW, Morrison AR, editors. Rapid Eye Movement Sleep: Regulation and Function. New York: Cambridge University Press; 2011. [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psych. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Lei Y, Benincasa Herr K, Kubin L. Evidence that adrenergic ventrolateral medullary cells are activated whereas precerebellar lateral reticular nucleus neurons are suppressed during REM sleep. PLoS ONE. 2013;8:e62410. doi: 10.1371/journal.pone.0062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psych. 2008;63:629–632. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt SM, Paré WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Thompson CE, Taylor FB, McFall ME, Barnes RF, Raskind MA. Nonnightmare distressed awakenings in veterans with posttraumatic stress disorder: response to prazosin. J Trauma Stress. 2008;21:417–420. doi: 10.1002/jts.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: Role of the α1 GABAA receptor subtype. Proc Natl Acad Sci USA. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G, Perez E, Amici R, Jones CA, Parmeggiani PL. Control of REM sleep: an aspect of the regulation of physiological homeostasis. Arch Ital Biol. 1999;137:249–262. [PubMed] [Google Scholar]