Abstract
The distinction between normal right ventricular (RV) trabeculations from abnormal has been difficult. We evaluated whether RV volume and function are related to left ventricular (LV) noncompaction (NC) cardiomyopathy and clinical events. Trabeculations/possible LVNC by cardiac magnetic resonance imaging (cMRI) was retrospectively observed among 105 consecutive cases. We measured LV end-systolic (ES) noncompacted:compacted ratio, RV ejection fraction (EF), RV apical trabecular thickness, and RV end-diastolic (ED) noncompacted:compacted ratio. A control group of 40 subjects was also reviewed to assess the exploratory measures. Comparing those with LV ES noncompacted:compacted ratio ≥ 2, < 2, and the normal control group, adjusted means for RV apical trabecular thickness and RV ED noncompacted:compacted ratio were generated. Logistic regression was used to evaluate the association of composite events traditionally associated with LV NC with RV EF after adjustment for above covariates, cardiovascular risk factors, delayed enhancement, LV EF, and LV ES noncompacted:compacted ratio. Analysis of RV morphology found greater apical trabecular thickness among those with LV ES noncompacted:compacted ratio ≥ 2 as compared with LV ES noncompacted:compacted ratio < 2 or normal control group (31 ± 5 mm vs. 27 ± 2.6 mm vs. 22 ± 4 mm; p = 0.03 and p = 0.003, respectively). There was no difference between the groups in relation to the RV end-diastolic (ED) noncompacted:compacted ratio . Low RV EF and LV ES noncompacted:compacted ratio ≥ 2 had significant association with clinical events in this population even after adjusting for clinical and imaging parameters (p = 0.04 and p < 0.001, respectively). In conclusion, RV dysfunction in a morphologic LVNC population is strongly associated with adverse clinical events. LVNC is associated with increased trabeculations of the RV apex.
Keywords: Noncompaction, Right Ventricle, Heart Failure
Introduction
The most validated criteria for left ventricular (LV) noncompaction (NC) cardiomyopathy were proposed by Jenni et al.1,2 While most reports have focused on the LV, limited studies with echocardiography and cardiac magnetic resonance imaging (cMRI) have described the possibility of right ventricular dysfunction associated with LV trabeculations.3,4 To determine whether RV structure or function is related to potential LVNC, we reviewed 105 cardiac MRI cases where left ventricular trabeculation was noted. We assessed RV size and ejection fraction (EF), as well as ES measures of LVNC. In addition, several exploratory assessments for RV trabeculation were performed. For comparison, a normal control group of 40 subjects was reviewed for comparison to the study group. Finally, we assessed the relationship between RV function and traditional LVNC events. We hypothesized that LVNC would be associated with morphological and functional changes of the RV, and that RVEF would be associated with traditional LVNC clinical events.
Methods
After obtaining institutional review board approval, we retrospectively queried the clinical cMRI database at Wake Forest Baptist Hospital for descriptions of trabeculation or non-compaction. A total of 122 patients had cMRI studies performed between January 2007 and April 2011, who had reports that included these descriptors comprised our study population. Of these cases, 17 were excluded due to the presence of coronary artery disease. Given that 24 subjects met criteria for LVNC, a control group of 40 patients was used to compare RV morphological features. Clinical and demographic data were extracted from the electronic medical record.
Images were acquired on a 1.5 T (Avanto; Siemens Medical Solutions, Erlangen, Germany) using Steady-State Free Precession (SSFP). Cine images (echo time/repetition time 1.5/3.0 ms, flip angle 60°) were acquired in three long-axis views (i.e., 2 chamber, 3 chamber, and 4 chamber views), planned on short-axis pilots at 60° angles to each other. Multi-slice cine views were also acquired in short axis plane from the base to the apex to visualize all 17 segments according to the American Heart Association recommendation.5 Right ventricular volumes were measured at end-diastole and end-systole (ES) with a modified Simpson’s technique which involved assessing the area of right ventricle per slice multiplied by the slice thickness and summed from base to apex.6
Using short axis cine images, the non-compacted and compacted layers were visually identified, and the papillary muscles were specifically excluded from measurement. The region with the largest non-compacted to compacted ratio was measured at ES using WebPAX (Heart Imaging Technologies, LLC, Durham, NC, USA). Apical short axis views 16–24 mm from the true apical slice were used for all measurements. In accord with previously published standards, individuals were categorized as LVNC if the ES noncompacted:compacted ratio was ≥ 2.1
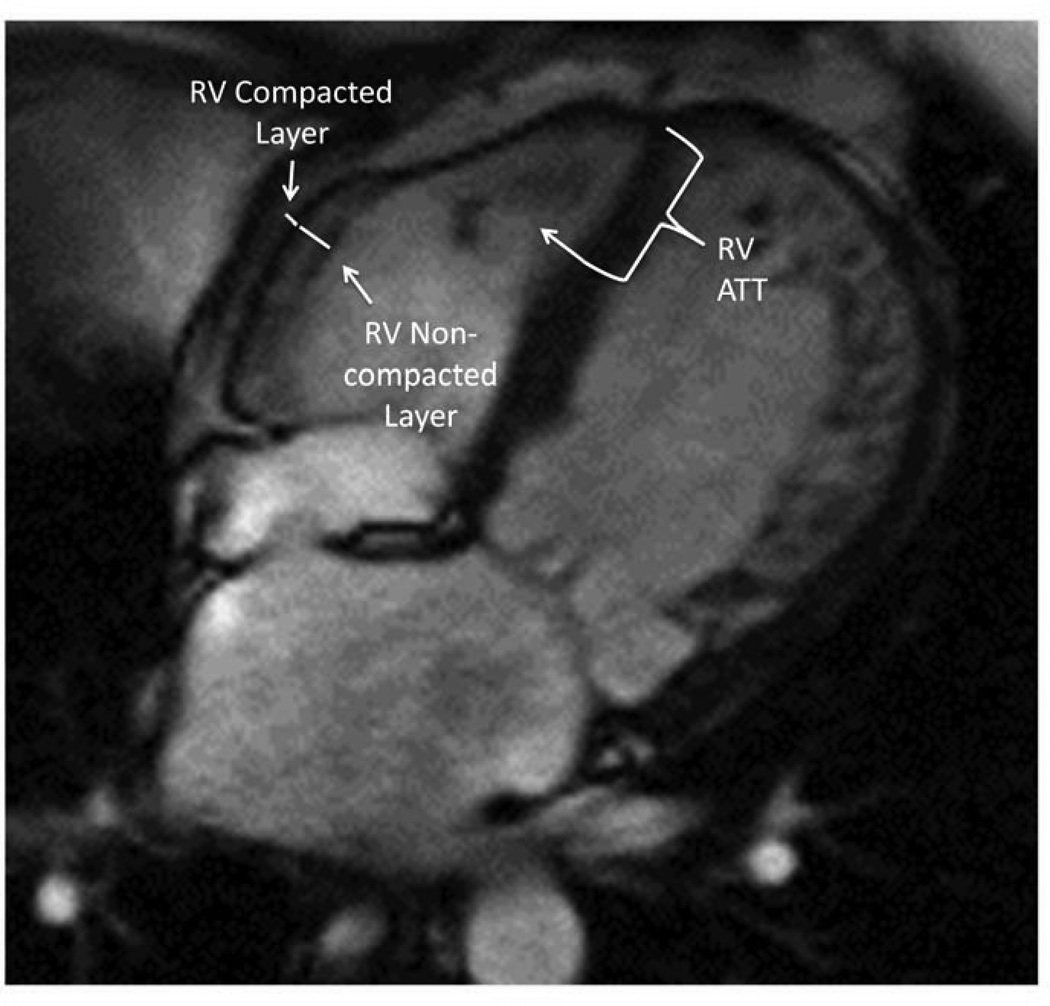
From the 4 chamber SSFP cine images, the right ventricle was evaluated for the presence of apical and lateral wall trabeculations. At ED, the RV apical trabecular thickness was measured from the RV apical insertion point to the trabecular trough (see figure 1). From the same images, the RV lateral wall compacted and noncompacted layers were identified and measured at ED (see figure 1). To avoid over-measuring the apical trabeculations, these measurements were only obtained at the lower mid-level. From these measurements, a right ventricular ED non-compacted:compacted ratio was calculated. An ED measurement was chosen because of the difficulty inherent in measuring the change in right ventricular free wall thickness during the cardiac cycle.
Figure 1.
4 chamber view showing RV apical trabeculations extending at least 1/3 distance up the septal wall in someone with significant LV trabeculations. The white line along the septum is measuring the distance from the RV apical insertion to the nadir of the RV apical trabeculations. The yellow line in the RV free wall is measuring the RV free wall compacted layer. The white line along the RV free wall is measuring the RV non-compacted layer.
Delayed enhancement images of the myocardium were reviewed and recorded as being present or absent. If any delayed enhancement was seen, it was categorized as present. If no delayed enhancement was seen, it was categorized as absent. Initially, we attempted to stratify by extent of delayed enhancement, but it provided no additional information above the delayed enhancement being present or absent.
Heart failure (HF) was defined as having a clinical diagnosis of HF by medical record.
The occurrences of death, heart failure readmission, embolic events, and ventricular arrhythmias were collected retrospectively as these have been previously associated with LVNC2. To assess, both the medical chart and the social security death index were reviewed. Ventricular arrhythmias were required to have either a reviewed cardiology consultation for ventricular arrhythmia, Holter monitor or implanted loop recorder that documented the ventricular arrhythmia, or an electrophysiology study documenting VT as source of rhythm disturbance. Heart failure readmissions, death, ventricular arrhythmias, and embolic events were pooled for statistical power and taken to represent a clinical phenotype of LVNC cardiomyopathy.
All cMRI baseline data were presented as mean ± standard deviation. Nominal data were tested using the chi-square test. Continuous data were tested using Student’s T-test. For this analysis, baseline variables are presented for the overall trabeculated population, the normal control group, those with LV ES noncompacted:compacted ratio was ≥ 2, and those < 2. Adjusted means are presented with 95% confidence intervals.
Associations were further evaluated by analysis of covariance (ANCOVA) models. Adjustment was made for age, race, gender, and body surface area. Right ventricular ED volume and right ventricular EF were used as dependent variables to generate adjusted means by ES noncompacted:compacted ratio. The trabeculated population was stratified into those who met LVNC criteria and those who did not. Adjusting for the covariates above, in each group RV EF was compared between those with and without HF.
To describe the RV morphology, the population was divided into those who had LV ES noncompacted:compacted ratio ≥ 2 and those with LV ES noncompacted:compacted ratio < 2. ANCOVA was used to generate adjusted means for the RV apical trabecular thickness and RV free wall noncompacted:compacted ratio in the control group, the ES noncompacted:compacted ratio ≥ 2 group, and the LV noncompacted:compacted ratio < 2 group. Further adjustment was made for age, race, gender, and body surface area.
Categorical data were analyzed using logistic regression. The dependent variable used was combined clinical events associated with LVNC. Covariates used for adjustment included: age, race, gender, body surface area, hypertension, hyperlipidemia, diabetes mellitus, HF, LV EF, the presence of delayed enhancement, LV ES noncompacted:compacted ratio, and RV EF. Further analyses with logistic regression and ROC curve testing were performed to attempt to identify a potential cut-off value of RV apical trabecular thickness to help identify those with LV ES noncompacted:compacted ratio ≥ 2. A p value of <0.05 was considered statistically significant. Correlations were also used to describe unadjusted linear relationships. All statistical analyses were performed using SAS software version 9.1 (SAS Institute, Inc.; Cary, NC). All graphs were produced with GraphPad Software, version 4 (San Diego California USA; www.graphpad.com).
To assess inter- and intra-observer agreement, a subset of cases were randomly selected and interpreted by a second reader blinded to all information (n=20). Inter- and intra-observer agreement was assessed with intraclass correlation analyzing the trabecular trough to apex length, mid RV free wall compacted and noncompacted layer thickness.
Results
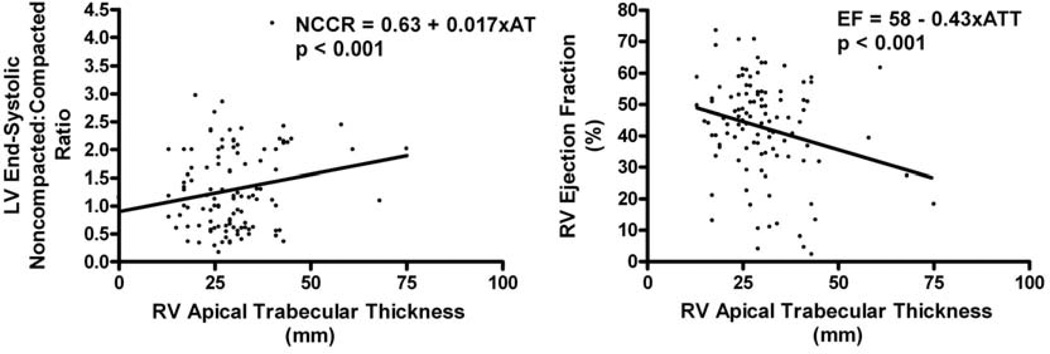
Baseline characteristics are presented in Table 1 first column. The overall population is predominantly Caucasian (69%) with approximately 1/3 having HF. Out of 105 patients, 24 (23%) had an ES noncompacted:compacted ratio ≥ 2. Both the control group and those with LV ES noncompacted:compacted ratio < 2 had higher LV and RV EFs than the LV ES noncompacted:compacted ratio ≥ 2 group. Scatter plots of RV EF and LV ES noncompacted:compacted ratio by RV apical trabecular thickness are provided with simple linear regression analysis (see figure 2).
Table 1.
Baseline characteristics: Overall study population listed to the left with breakdown by those with LV ES noncompacted:compacted ratio ≥ 2 vs those with < 2. Normal functioning, non-trabeculated ventricles presented for comparison.
| Overall Trabeculated Population |
Control Group | LV End-Systolic Noncompacted:Compacted Ratio ≥ 2 |
||
|---|---|---|---|---|
| Variable | (n = 105) | (n=40) | No (n=81) |
Yes (n=24) |
| Age (years) | 57 ± 17.5 | 58 ± 13 | 57.8 ± 16.9 | 51 ± 19.5 |
| Black | 31% | 31% | 32% | 30% |
| Female | 52% | 57% | 48% | 58% |
| Body Surface Area (m2) | 2.0 ± 0.4 | 2.01 ± 0.26 | 2.0 ± 0.3 | 1.9 ± 0.2 |
| Diabetes Mellitus -Diagnosed by physician |
17% | 14% | 13% | 30%*,‡ |
| Hypertension -Diagnosed by physician |
56% | 62% | 58% | 50% |
| Hyperlipidemia -Diagnosed by physician |
35% | 42% | 32% | 39% |
| LV Ejection Fraction (%) | 44 ± 16 | 61 ± 12 | 48.5 ± 14.6 | 31 ± 11.7*,‡ |
| RV Ejection Fraction (%) | 42.5 ± 15 | 59 ± 11 | 46 ± 13 | 35 ± 18*,‡ |
| Heart Failure | 20% | 0% | 18% | 30%*,‡ |
| Delayed Enhancement Present | 26% | 0% | 24% | 36%*,‡ |
Mean values presented with standard deviation.
Frequencies presented as percent.
means statistical difference with p < 0.05 in comparison between those with LV ES noncompacted:compacted ratio ≥ 2 vs <2.
means statistical difference with p < 0.05 in comparison between those with LV ES noncompacted:compacted ratio ≥ 2 vs the normal, non-trabeculated control group.
Figure 2.
Scatter plots with linear regression lines and formulas of LV end-systolic non-compacted: compacted ratio and RV ejection fraction by RV apical trabecular thickness, respectively.
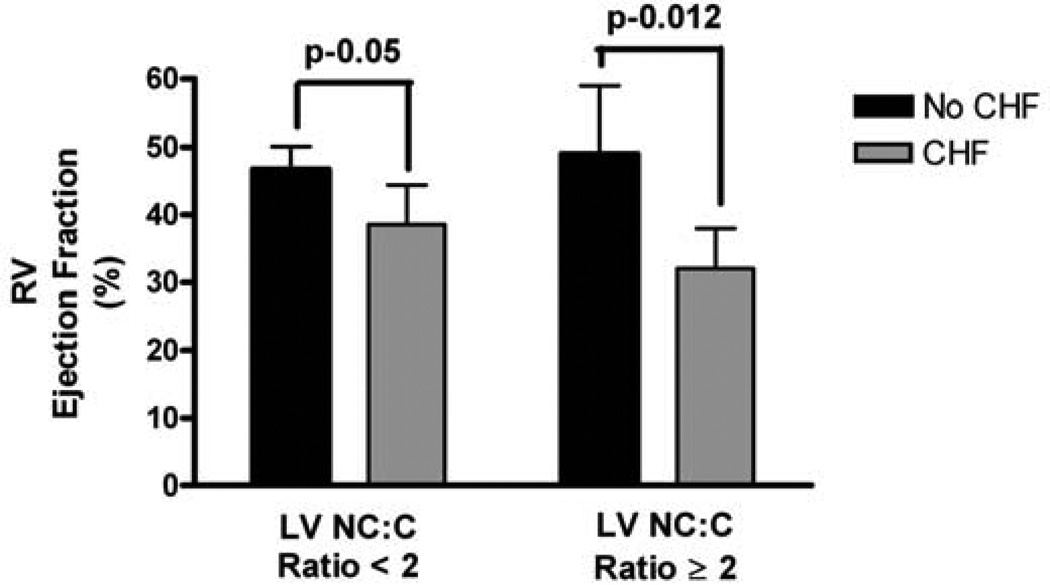
The adjusted right ventricular ED volumes and EFs by LV ES noncompacted:compacted ratio < 2 vs. ≥ 2 are presented in Table 2. Those who had LV ES noncompacted:compacted ratio ≥ 2 demonstrated statistically lower RVEF (35 ± 6% vs. 45 ± 3.2%; p = 0.0018) and had higher volumes (171 ± 18 ml vs. 147 ± 10 ml; p = 0.017). For those with LV ES noncompacted:compacted ratio ≥ 2, the adjusted RV EF was lower for those with HF than those without HF (30 ± 6% vs. 50 ± 12%; p = 0.012). The same pattern was seen in those with ES noncompacted:compacted ratio < 2 (38 ± 6% vs. 44 ± 3.5%, respectively; p = 0.05; see figure 3).
Table 2.
ANCOVA analysis to generate adjusted means with 95% confidence intervals.
| Measure | Right Ventricular End- Diastolic Volume (ml) |
p-Value | Right Ventricular Ejection Fraction (%) |
p-Value |
|---|---|---|---|---|
| ES NC:C Ratio ≥ 2 |
171 ± 18 | 0.017 | 35 ± 6 | 0.0018 |
| ES NC:C Ratio < 2 |
147 ± 10 | 45 ± 3.2 |
Adjusted for age, race, gender, BSA. (ES = End-Systolic; NC:C = Noncompacted-to-Compacted)
Figure 3.
Adjusted means for RV ejection fraction by presence of HF stratified by those who have LV end-systolic noncompacted:compacted ratio. ANCOVA adjusted for age, race, gender, body surface area.
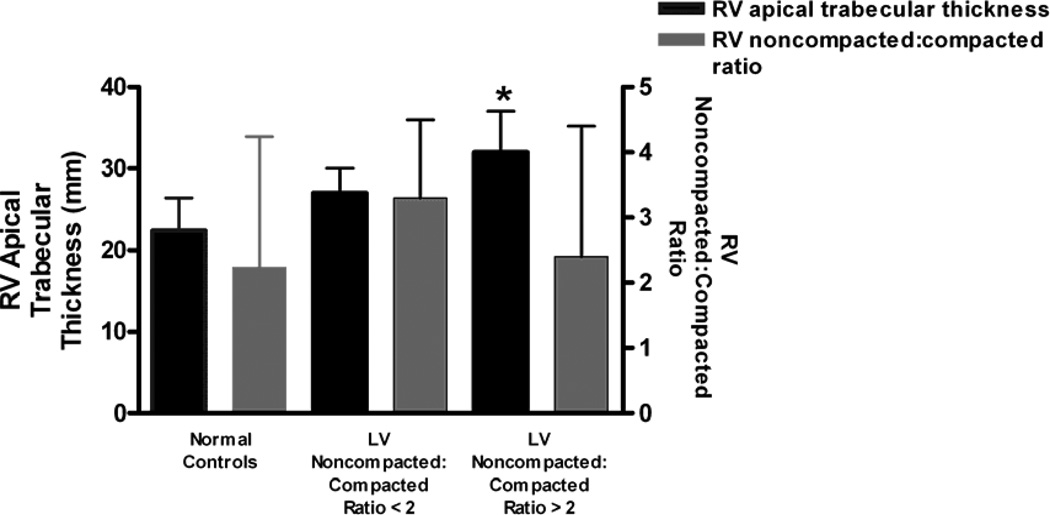
Analyzing RV morphology using ANCOVA (see figure 4), those with LV ES noncompacted:compacted ratio ≥ 2 had thicker apical trabecular thickness than those with LV ES noncompacted:compacted ratio < 2 or the normal control group (31 ± 5 mm vs. 27 ± 2.6 mm vs. 22 ± 4 mm; p = 0.03 and p = 0.003, respectively). There was no meaningful difference in RV free wall noncompacted:compacted ratio between LV ES noncompacted:compacted ratio ≥ 2, LV ES noncompacted:compacted ratio < 2, or the control group (2.4 ± 2 vs. 3.3 ± 1.4 vs. 2.2 ± 1.8, respectively; overall p > 0.5).
Figure 4.
Adjusted means for RV apical trabecular thickness and RV free wall noncompacted:compacted ratio by the normal control group, LV end-systolic noncompacted:compacted ratio < 2, and LV end-systolic noncompacted:compacted ratio ≥ 2. An asterisk (*) denotes a p < 0.05 difference in comparison with the control group. (NC:C Ratio = noncompacted:compacted ratio)
Using logistic regression, after adjustment for covariates previously listed, the RV EF and the LV ES noncompacted:compacted ratio continued to have a significant association with clinical events associated with LVNC (p = 0.04 and p < 0.001, respectively). The LV EF had no clear relationship (p = 0.2), and the presence of delayed enhancement was not significantly related to the LVNC events (p > 0.2). From the ROC curve analysis to determine a cut-point value of RV apical trabecular thickness to identify potential LVNC, the AUC was 0.67, which suggests only a fair ability to identify potential LVNC. If one were to take an RV apical trabecular thickness ≥ 40 mm as indicative of potential LVNC, it would yield a specificity of 91.2 % and a sensitivity of 38.7%. However, given the limited AUC, it is difficult to confidently identify a clinically useful cut-point at this time.
The inter-observer agreement on apical trabecular thickness, mid RV free wall noncompacted layer, and RV compacted layer by intraclass correlation were 0.86, 0.88, and 0.81, respectively. The intra-observer agreement on apical trabecular thickness, mid RV free wall noncompacted layer, and RV compacted layer by intraclass correlation were 0.93, 0.9, and 0.85, respectively.
Discussion
This study presents several novel observations concerning the relationship between RV morphology and function among individuals noted to have increased LV trabeculations by cMRI. First, individuals with LV ES noncompacted:compacted ratio ≥ 2 had higher RV ED volumes and lower RV EFs. Second, those with LVNC had increased RV apical trabecular thickness; a relationship which was further correlated with the severity of LV trabeculation. Third, among those with trabeculated left ventricles, markedly reduced right ventricular systolic function was associated with higher clinical events traditionally associated with LVNC, even after adjustment for LV EF and presence of delayed enhancement.
Historically, the right ventricle has served as a marker of advanced LV disease and clinical events. Right ventricular dysfunction is associated with decreased exercise capacity in those with left-sided failure.7 However, even in moderate HF, right ventricular function serves as an independent predictor of survival.8 Among patients with myocarditis, right ventricular dysfunction has been associated with a higher risk of adverse outcomes,9 and among those with idiopathic dilated cardiomyopathy, right ventricular dysfunction portends increased mortality.10 Further, those with biventricular failure have worse outcomes than those with only left-sided dysfunction.11
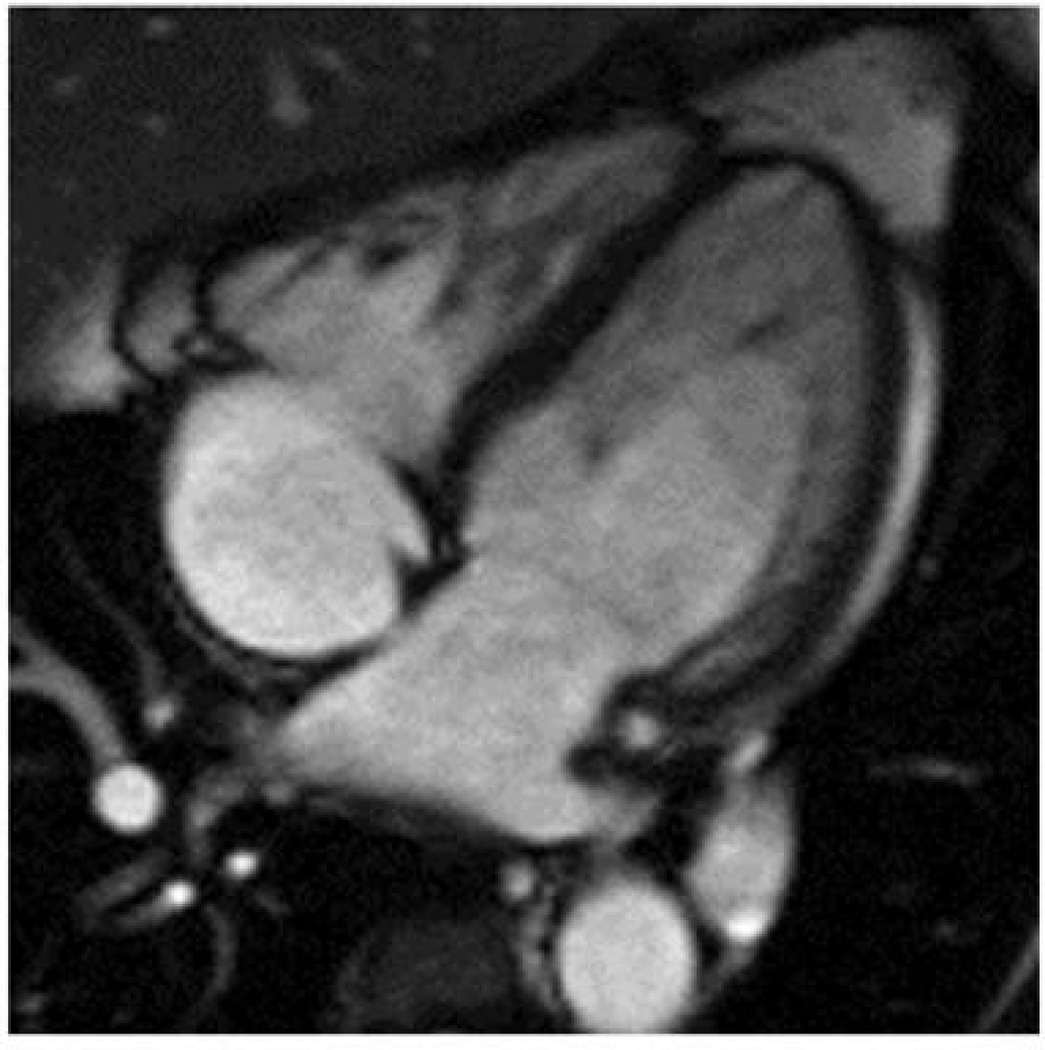
LVNC is often associated with severe left ventricular systolic dysfunction, 1, 12 as reflected by original reports which were isolated to populations that had refractory heart failure and subsequently died or required heart transplantation.1, 12 Increasing awareness of LVNC has been mediated by advances in imaging technologies, particular cMRI, and it is likely that only a fraction of morphologic descriptions of LV trabeculation represent LVNC. We have recently demonstrated that cMRI assessments of LV ESNCCR ≥ 2 among patients with morphologic description of trabeculation are strongly associated with clinical events commonly associated with LVNC (heart failure, death, ventricular arrhythmias, and embolic events).2 Limited data are available regarding RV structure and function among this population of patients and its influence on clinical outcome. Previous case series have described echocardiographic Doppler surrogates of RV function to be altered in LVNC. 3 Similarly, a small case series of 14 patients evaluated with cardiac MRI have noted severe RV systolic dysfunction among those diagnosed with LVNC.4 Our study extends these observations and demonstrates increased RV EDV, decreased RV EF, and increased RV apical trabecular thickness among those who meet ES criteria for LVNC (see figure 5 for example). Further, after separating this population into those with LV ES noncompacted:compacted ratio ≥ 2 vs. < 2, those with lower RV EF had a higher incidence of HF and traditional LVNC events. . Therefore, decreased RV EF by cMRI may help to segregate risk in this population of patients. .Furthermore, we demonstrated that a decreasing RV EF is independently associated with experiencing clinical events associated with LVNC, even after adjustment for the LV ES noncompacted:compacted ratio and LV EF.
Figure 5.
Example of RV apical trabecular thickness extending to over half the distance between the apex and base.
Originally, NC was a term used to describe a pathological process mainly seen in the left ventricle. The criteria proposed by Jenni and colleagues did not include RV size or function,1, 13 nor did criteria proposed by Chin et al.12 These investigations did report that there were often increased trabeclations seen in the RV apex of those diagnosed with LVNC.13,14 Small case reports have suggested potential RVNC by morphology,15 but no one has proposed measurements or cut-points to definitively diagnosis it. It is also unclear if RVNC can occur or has clinical significance in the absence of LVNC.16,17 To further complicate matters, the normal right ventricle often has significantly more trabeculations than the left ventricle, which makes separating normal from pathological somewhat challenging. Given the difficulty in measuring the thin RV wall, this would further complicate efforts at quantification if one were to assess it as it is done in the LV.
As part of this study, we made efforts to identify patterns of trabeculation of the right ventricle that may aid in the diagnosis of LVNC. Overall, most patients have trabeculations in the right ventricular apex. However, our study demonstrated a correlation between RV apical trabecular thickness and degree of LV trabeculation (see figure 2 example). Additional studies should be conducted to to determine whether a cut-point for RV apical trabecular thickness can be used to evaluate LVNC.
The limitations associated with the present study include those that are inherent in any retrospective database analysis. As such, our results are primarily hypothesis-generating, and ideally, they will need to be validated by a more prospective approach. However, given the rarity of LVNC a multi-institutional database may help validate our observations. Importantly, since the MRI laboratory at Wake Forest Baptist Hospital is a tertiary care referral center, some degree of referral bias may have enriched the study population. Third, while rigorous effort were undertaken to identify clinical events, our population may have suffered from under reporting since events were not defined and collected prospectively.
Acknowledgments
Funding source: NIH T32-HL091824 (Michael E. Hall, MD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None
Conflicts of Interest: None
References
- 1.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction. J Am Coll Cardiol. 2000;36:493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 2.Stacey RB, Andersen MM, St.Clair SM, Hundley WG, Thohan V. Comparison of Systolic and Diastolic Criteria for Isolated Left Ventricular Noncompaction in Cardiac MRI. JACC Imaging. 2013;6(9):931–940. doi: 10.1016/j.jcmg.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Peters F, Khandheria BK, dos SC, Matioda H, Maharaj N, Libhaber E, Marndoo F, Essop MR. Isolated left ventricular noncompaction in sub-Saharan Africa: a clinical and echocardiographic perspective. Circ Cardiovasc Imaging. 2012;5:187–193. doi: 10.1161/CIRCIMAGING.111.966937. [DOI] [PubMed] [Google Scholar]
- 4.Leung SW, Elayi CS, Charnigo RJ, Jr, Syed MA. Clinical significance of right ventricular dysfunction in left ventricular non-compaction cardiomyopathy. Int J Cardiovasc Imaging. 2012;28:1123–1131. doi: 10.1007/s10554-011-9925-z. [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 6.Nijveldt R, Germans T, McCann GP, Beek AM, van Rossum AC. Semi-quantitative assessment of right ventricular function in comparison to a 3D volumetric approach: a cardiovascular magnetic resonance study. Eur Radiol. 2008;18:2399–2405. doi: 10.1007/s00330-008-1017-7. [DOI] [PubMed] [Google Scholar]
- 7.Baker BJ, Wilen MM, Boyd CM, Dinh H, Franciosa JA. Relation of right ventricular ejection fraction to exercise capacity in chronic left ventricular failure. Am J Cardiol. 1984;54:596–599. doi: 10.1016/0002-9149(84)90256-x. [DOI] [PubMed] [Google Scholar]
- 8.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure . J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 9.Mendes LA, Dec GW, Picard MH, Palacios IF, Newell J, Davidoff R. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–307. doi: 10.1016/0002-8703(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 10.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JF, Webber JD, Sutton LL, Chesoni S, Curry CL. Discordance in degree of right and left ventricular dilation in patients with dilated cardiomyopathy: recognition and clinical implications. J Am Coll Cardiol. 1993;21:649–654. doi: 10.1016/0735-1097(93)90097-k. [DOI] [PubMed] [Google Scholar]
- 12.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 13.Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 14.Bleyl SB, Mumford BR, Brown-Harrison MC, et al. Xq28-linked noncompaction of the left ventricular myocardium: prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet. 1997;72:257–265. [PubMed] [Google Scholar]
- 15.Ying ZQ, Xu G, Chen S, Ma J, You XD. Cerebral infarction in an adult patient with right ventricular hypertrabeculation/noncompaction. Int J Cardiol. 2008;127:e150–e151. doi: 10.1016/j.ijcard.2007.04.148. [DOI] [PubMed] [Google Scholar]
- 16.Alehan D, Dogan OF. Right ventricular noncompaction in a neonate with complex congenital heart disease. Cardiol Young. 2005;15:434–436. doi: 10.1017/S1047951105000910. [DOI] [PubMed] [Google Scholar]
- 17.Dogan R, Dogan OF, Oc M, Duman U, Ozkutlu S, Celiker A. Noncompaction of ventricular myocardium in a patient with congenitally corrected transposition of the great arteries treated surgically: case report. Heart Surg Forum. 2005;8:E110–E113. doi: 10.1532/hsf98.20041142. [DOI] [PubMed] [Google Scholar]