Abstract
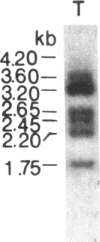
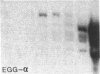
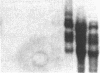
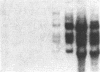
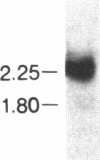
Multiple alpha- and beta-tubulin RNAs were found in the mature unfertilized eggs of the sea urchin Lytechinus pictus. The alpha-tubulin RNAs were polymorphic in number, size, and relative amounts in the eggs of different females. Five to seven different size classes [1.75-4.2 kilobases (kb)] were detected on RNA gel blots. All egg preparations contained variable amounts of 1.8- and 2.25-kb beta-tubulin RNAs, and a few of them contained an additional 2.9-kb beta-tubulin RNA. The total amount of alpha-tubulin RNA did not always parallel that of beta-tubulin RNA. A portion of all of the various alpha- and beta-tubulin RNAs were polyadenylylated. RNase H digestions ruled out the possibility that some of these RNAs represented a single transcript bearing different lengths of 3' poly(A). One class of alpha-tubulin RNAs (2.4-2.65 kb) was reduced to 2 kb by RNase H, suggesting the presence of internal oligo(A) regions. All of the egg beta-tubulin RNAs sedimented as free ribonucleoprotein particles. Only a small portion of the 1.75- to 3.6-kb alpha-tubulin RNAs, but most of the 4.2-kb alpha-tubulin RNA, were found on polysomes before fertilization. In the 30-min embryo, small amounts of each of the various alpha- and beta-tubulin RNAs were recruited onto polysomes. Thus, each of the multiple polymorphic alpha- and beta-tubulin RNAs in the egg represent translationally competent mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Evolution of alpha q- and beta-tubulin genes as inferred by the nucleotide sequences of sea urchin cDNA clones. J Mol Evol. 1983;19(6):397–410. doi: 10.1007/BF02102315. [DOI] [PubMed] [Google Scholar]
- Alexandraki D., Ruderman J. V. Sequence heterogeneity, multiplicity, and genomic organization of alpha- and beta-tubulin genes in sea urchins. Mol Cell Biol. 1981 Dec;1(12):1125–1137. doi: 10.1128/mcb.1.12.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibring T., Baxandall J. Tubulin synthesis in sea urchin embryos. II. Ciliary A tubulin derives from the unfertilized egg. Dev Biol. 1981 Apr 15;83(1):122–126. doi: 10.1016/s0012-1606(81)80014-0. [DOI] [PubMed] [Google Scholar]
- Bibring T., Baxandall J. Tubulin synthesis in sea urchin embryos: almost all tubulin of the first cleavage mitotic apparatus derives from the unfertilized egg. Dev Biol. 1977 Jan;55(1):191–195. doi: 10.1016/0012-1606(77)90330-x. [DOI] [PubMed] [Google Scholar]
- Blobel G. Release, identification, and isolation of messenger RNA from mammalian ribosomes. Proc Natl Acad Sci U S A. 1971 Apr;68(4):832–835. doi: 10.1073/pnas.68.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Cabada M. O., Darnbrough C., Ford P. J., Turner P. C. Differential accumulation of two size classes of poly(A) associated with messenger RNA during oogenesis in Xenopus laevis. Dev Biol. 1977 Jun;57(2):427–439. doi: 10.1016/0012-1606(77)90227-5. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Bruskin A. M., Hardin P. E., Keast M. J., Anstrom J., Tyner A. L., Brandhorst B. P., Klein W. H. Novel proteins belonging to the troponin C superfamily are encoded by a set of mRNAs in sea urchin embryos. Cell. 1984 Mar;36(3):663–671. doi: 10.1016/0092-8674(84)90346-5. [DOI] [PubMed] [Google Scholar]
- Cognetti G., DI Liegro I., Cavarretta F. Studies of protein synthesis during sea urchin oogenesis. II. Synthesis of tubulin. Cell Differ. 1977 Aug;6(2):159–165. doi: 10.1016/0045-6039(77)90037-9. [DOI] [PubMed] [Google Scholar]
- Costantini F. D., Britten R. J., Davidson E. H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980 Sep 11;287(5778):111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain W. R., Jr, Durica D. S., Van Doren K. Actin gene expression in developing sea urchin embryos. Mol Cell Biol. 1981 Aug;1(8):711–720. doi: 10.1128/mcb.1.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Hough-Evans B. R., Britten R. J. Molecular biology of the sea urchin embryo. Science. 1982 Jul 2;217(4554):17–26. doi: 10.1126/science.6178156. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Wilson L. Purification, characterization, and assembly properties of tubulin from unfertilized eggs of the sea urchin Strongylocentrotus purpuratus. Biochemistry. 1983 May 10;22(10):2453–2462. doi: 10.1021/bi00279a023. [DOI] [PubMed] [Google Scholar]
- Duncan R., Humphreys T. Multiple oligo(A) tracts associated with inactive sea urchin maternal mRNA sequences. Dev Biol. 1981 Dec;88(2):211–219. doi: 10.1016/0012-1606(81)90165-2. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Gross K. W., Jacobs-Lorena M., Baglioni C., Gross P. R. Cell-free translation of maternal messenger RNA from sea urchin eggs. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2614–2618. doi: 10.1073/pnas.70.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig E. M., Thiele D. J., Leibowitz M. J. Saccharomyces cerevisiae killer virus transcripts contain template-coded polyadenylate tracts. Mol Cell Biol. 1984 Jan;4(1):101–109. doi: 10.1128/mcb.4.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane R. E. Induction of either contractile or structural actin-based gels in sea urchin egg cytoplasmic extract. J Cell Biol. 1980 Sep;86(3):803–809. doi: 10.1083/jcb.86.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn O., Wilt F. H. Chromatin proteins of sea urchin embryos: dual origin from an oogenetic reservoir and new synthesis. Dev Biol. 1981 Jul 30;85(2):416–424. doi: 10.1016/0012-1606(81)90273-6. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Shott R. J., Rose S. J., 3rd, Thomas T. L., Britten R. J., Davidson E. H. Sea urchin actin gene subtypes. Gene number, linkage and evolution. J Mol Biol. 1984 Jan 15;172(2):149–176. doi: 10.1016/s0022-2836(84)80035-2. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Spudich J. A. Purification and properties of soluble actin from sea urchin eggs. J Biochem. 1980 Mar;87(3):785–802. doi: 10.1093/oxfordjournals.jbchem.a132808. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Noronha J. M., Sheys G. H., Buchanan J. M. Induction of a reductive pathway for deoxyribonucleotide synthesis during early embryogenesis of the sea urchin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2006–2010. doi: 10.1073/pnas.69.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer T. A., Asnes C. F., Wilson L. Properties of tubulin in unfertilized sea urchin eggs. Quantitation and characterization by the colchicine-binding reaction. J Cell Biol. 1976 Jun;69(3):599–607. doi: 10.1083/jcb.69.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poccia D., Salik J., Krystal G. Transitions in histone variants of the male pronucleus following fertilization and evidence for a maternal store of cleavage-stage histones in the sera urchin egg. Dev Biol. 1981 Mar;82(2):287–296. doi: 10.1016/0012-1606(81)90452-8. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony J. W., Flytzanis C. N., Britten R. J., Davidson E. H. Interspersed sequence organization and developmental representation of cloned poly(A) RNAs from sea urchin eggs. J Mol Biol. 1983 Jun 25;167(2):361–389. doi: 10.1016/s0022-2836(83)80340-4. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Morris P. W. DNA polymerase alpha and beta in the California urchin. Nucleic Acids Res. 1978 Oct;5(10):3945–3957. doi: 10.1093/nar/5.10.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Colot H. V., Selvig S. E., Gross P. R. Oogenetic origin of messenger RNA for embryonic synthesis of microtubule proteins. Nature. 1972 Jan 28;235(5335):211–214. doi: 10.1038/235211a0. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Greenhouse G., Gross K. W., Gross P. R. Synthesis and storage of microtubule proteins by sea urchin embryos. J Cell Biol. 1971 Aug;50(2):516–527. doi: 10.1083/jcb.50.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Primary structural differences among tubulin subunits from flagella, cilia, and the cytoplasm. Biochemistry. 1978 Jul 11;17(14):2882–2891. doi: 10.1021/bi00607a029. [DOI] [PubMed] [Google Scholar]
- Suprenant K. A., Rebhun L. I. Assembly of unfertilized sea urchin egg tubulin at physiological temperatures. J Biol Chem. 1983 Apr 10;258(7):4518–4525. [PubMed] [Google Scholar]
- Vournakis J. N., Efstratiadis A., Kafatos F. C. Electrophoretic patterns of deadenylylated chorion and globin mRNAs. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2959–2963. doi: 10.1073/pnas.72.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Showman R. M., Klein W. H., Raff R. A. Delayed recruitment of maternal histone H3 mRNA in sea urchin embryos. Nature. 1981 Jul 30;292(5822):477–478. doi: 10.1038/292477a0. [DOI] [PubMed] [Google Scholar]
- Young E. M., Raff R. A. Messenger ribonucleoprotein particles in developing sea urchin embryos. Dev Biol. 1979 Sep;72(1):24–40. doi: 10.1016/0012-1606(79)90095-2. [DOI] [PubMed] [Google Scholar]