Abstract
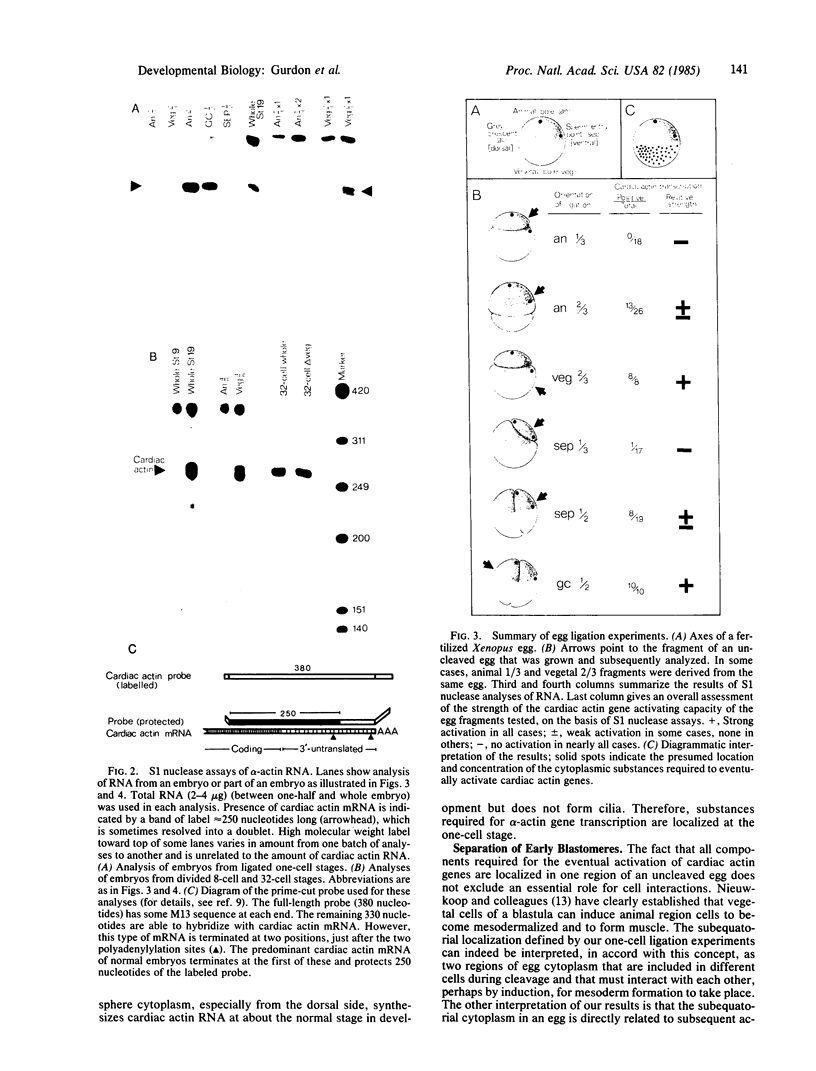
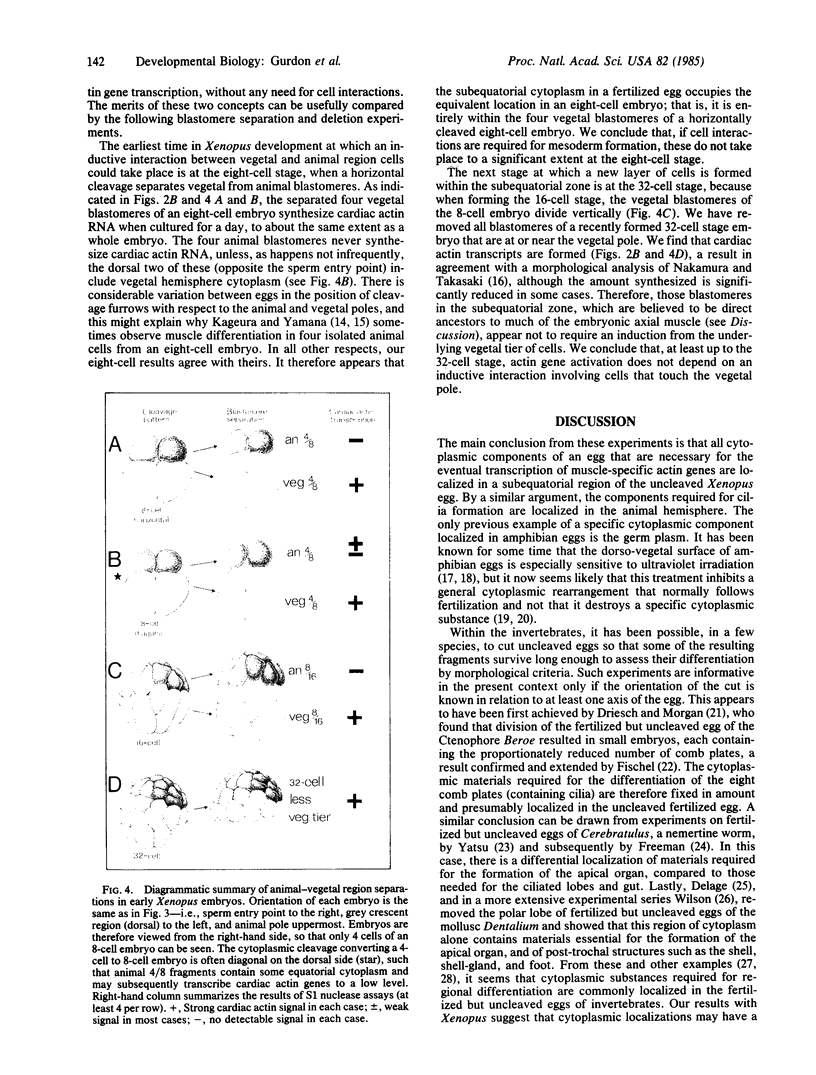
Fertilized Xenopus eggs have been ligated with a hair loop into separate fragments before the first cleavage. The plane of the ligation was varied in relation to the animal-vegetal and dorso-ventral axes. The fragments that contained a nucleus were cultured for 24 hr until controls reached the neurula stage; they were then analyzed by S1 nuclease protection for their content of muscle-specific actin mRNA, using a gene-specific probe. We find that all egg components required for the eventual activation of these actin genes are localized, already at the 1-cell stage, in a region below the equator, and mostly on the dorsal (grey crescent) side. This material subsequently occupies the equivalent position in 8-cell and 32-cell embryos. We interpret our results, in combination with the previous work of others, to mean that mesoderm (including muscle) formation in Amphibia depends both on cytoplasmic substances already localized in the egg as well as on inductive cell interactions during cleavage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eddy E. M. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- Freeman G. The role of cleavage in the localization of developmental potential in the ctenophore Mnemiopsis leidyi. Dev Biol. 1976 Mar;49(1):143–177. doi: 10.1016/0012-1606(76)90264-5. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Gerhart J. C. Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol. 1984 Jul;104(1):117–130. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Grant P., Wacaster J. F. The amphibian gray crescent region--a site of developmental information? Dev Biol. 1972 Jul;28(3):454–471. doi: 10.1016/0012-1606(72)90029-2. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Brennan S., Fairman S., Mohun T. J. Transcription of muscle-specific actin genes in early Xenopus development: nuclear transplantation and cell dissociation. Cell. 1984 Oct;38(3):691–700. doi: 10.1016/0092-8674(84)90264-2. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Kageura H., Yamana K. Pattern regulation in defect embryos of Xenopus laevis. Dev Biol. 1984 Feb;101(2):410–415. doi: 10.1016/0012-1606(84)90155-6. [DOI] [PubMed] [Google Scholar]
- Kageura H., Yamana K. Pattern regulation in isolated halves and blastomeres of early Xenopus laevis. J Embryol Exp Morphol. 1983 Apr;74:221–234. [PubMed] [Google Scholar]
- Malacinski G. M., Benford H., Chung H. M. Association of an ultraviolet irradiation sensitive cytoplasmic localization with the future dorsal side of the amphibian egg. J Exp Zool. 1975 Jan;191(1):97–110. doi: 10.1002/jez.1401910110. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Nakamura O., Takasaki H., Nagata A. Further studies of the prospective fates of blastomeres at the 32-cell stage of Xenopus laevis embryos. Med Biol. 1978 Dec;56(6):355–360. [PubMed] [Google Scholar]
- Nieuwkoop P. D. The organization center of the amphibian embryo: its origin, spatial organization, and morphogenetic action. Adv Morphog. 1973;10:1–39. doi: 10.1016/b978-0-12-028610-2.50005-8. [DOI] [PubMed] [Google Scholar]
- Scharf S. R., Gerhart J. C. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983 Sep;99(1):75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- Scharf S. R., Gerhart J. C. Determination of the dorsal-ventral axis in eggs of Xenopus laevis: complete rescue of uv-impaired eggs by oblique orientation before first cleavage. Dev Biol. 1980 Sep;79(1):181–198. doi: 10.1016/0012-1606(80)90082-2. [DOI] [PubMed] [Google Scholar]



















