Abstract
The ability to degrade extracellular matrix is critical for tumor cells to invade and metastasize. Recent studies show that tumor cells utilize specialized actin-based membrane protrusions termed invadopodia to perform matrix degradation. Invadopodia provide an elegant way for tumor cells to precisely couple focal matrix degradation with directional movement. Here we discuss several key components and regulators of invadopodia that have been uniquely implicated in tumor invasion and metastasis. Furthermore, we discuss existing and new therapeutic opportunities to target invadopodia for anti-metastasis treatment.
Introduction
Metastasis, the spread of tumors cells from a primary tumor to a secondary site, is a complex, multi-step process, and is the main cause of mortality in cancer patients. During metastasis, carcinoma cells invade the surrounding extracellular matrix (ECM), intravasate through endothelium into the systemic circulation, then extravasate again through capillary endothelium, and finally establish secondary tumors at distant sites1. Several key stages of metastasis, including invasion, intravasation, and extravasation, are thought to involve ECM degradation and remodeling. In recent years, actin-rich subcellular protrusions known as invadopodia have been shown to be critical for ECM degradation2. Invadopodia consist of an actin-rich core surrounded by a number of important protein components, including cytoskeletal modulators, adhesion proteins, scaffolding proteins, and signaling molecules3. The central function of invadopodia is to recruit various matrix proteases to cell-ECM focal contacts for matrix degradation.
Unlike other actin-based protrusions such as lamellipodia and filopodia that are present in normal cells, invadopodia are uniquely present in invasive cancer cells and are considered the transformed version of podosomes, which are present in highly invasive normal cells such as macrophages, osteoclasts, and dendritic cells. In-depth reviews have covered all the molecular components of podosomes/invadopodia and their biological functions in development and pathogenesis3. This review focuses on a selective set of invadopodia components and regulators that are relatively unique to invadopodia and are specifically modulated in human cancers (Figure 1, Table 1). Additionally we will discuss the contributions of these invadopodia components to invasion and metastasis, and the therapeutic opportunities to target these components for cancer treatment.
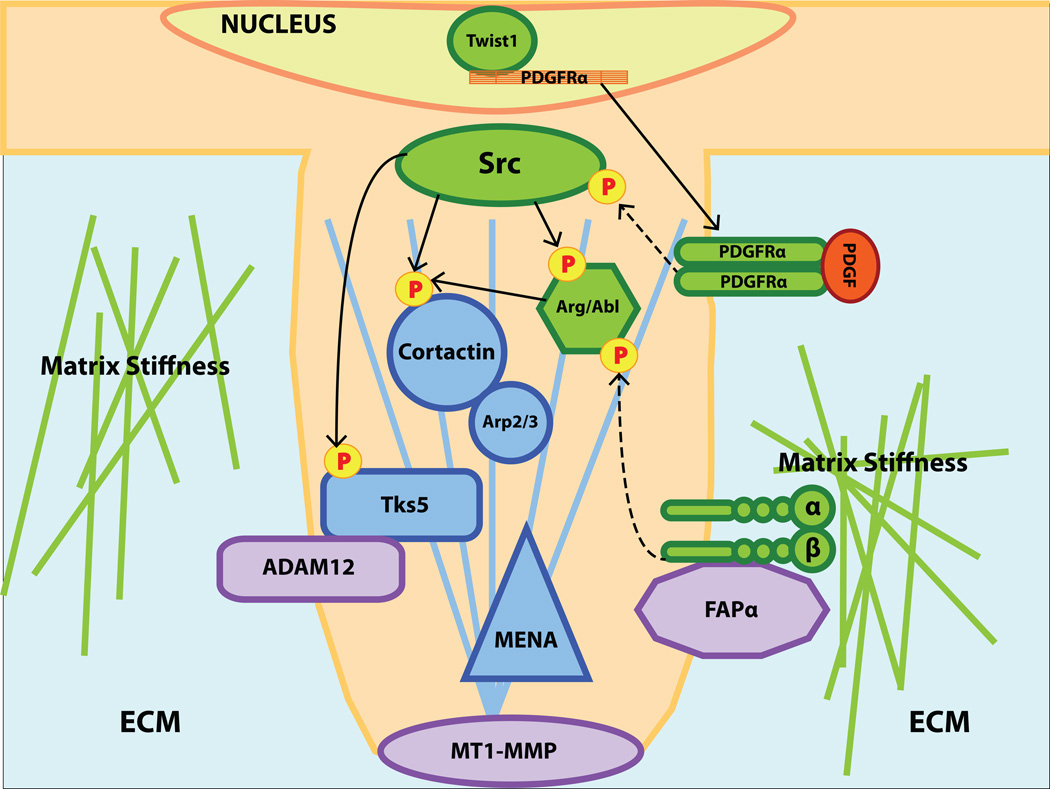
Figure 1. An overview of the invadopodia components and regulators discussed in this review.
Twist1-induced expression of PDGFRα leads to increased Src kinase activity, which serves as a trigger for invadopodia formation. Src-mediated phosphorylation of the structural components cortactin and Tks5 and the Arg/Abl tyrosine kinase promotes invadopodia assembly. Integrin β1 serves as an adhesion mediator between invadopodia and ECM, an activator of Abl/Arg at invadopodia, a sensor of matrix stiffness to regulate invadopodia assembly, and a potential docking site for FAPα. Structural components of invadopodia, which include the actin core, are labeled in blue, proteases are labeled in purple, and regulatory components are labeled in green.
Table 1.
A list of invadopodia components and regulators, their roles in invadopodia, invasion, and metastasis, and their therapeutic potentials.
| Component | Cancer | Invadopodia | Invasion | Metastasis | Therapeutics | |
|---|---|---|---|---|---|---|
| Core Elements | Cortactin | Breast, Head and Neck, Lung, Ovarian, Bladder | 7–11 | 12, 13 | 13, 14 | - |
| MENA | Breast, Pancreatic, Colon, Gastric, Cervical Cancer, and Melanoma | 18 | 17–19, 21 | 18, 20, 21 | - | |
| Tks | Breast, Melanoma | 23, 27–29 | 24, 29 | 24, 30 | - | |
| Proteases | MT1-MMP | Breast, Lung, Squamous Cell, Colorectal, and Melanoma | 8, 40, 42 | 44–46 | 48, 49 | 50–53 |
| ADAM12 | Breast, Prostate, Lung, Brain, Liver, Bone | 25, 57 | 58, 62 | 62 | - | |
| Serine Proteases | Breast, Colon, Ovarian, Melanoma | 65, 66 | - | - | 74, 75, 79 | |
| Regulatory Components | Src | Breast, Colon | 9, 81–84 | 9, 83–86 | 85, 87–89, 91 | 89, 93–95 |
| Abl kinases | Leukemia, Breast | 84, 98 | 97, 99, 100 | 100 | 93, 101, 102 | |
| Integrin | - | 103–106 | 103 | 109 | - | |
| Twist | - | 24 | 24 | 111, 112 | - |
STRUCTRUAL COMPONENTS
As an actin-based structure, invadopodia engage a large number of structural and regulatory proteins that control actin dynamics, such as Arp2/3, Ena/Vasp, and various small GTPases. Here we discuss three players, cortactin, MENA, and Tks proteins that play critical roles at invadopodia and have been implicated in tumor progression. Cortactin and MENA are both key players of actin polymerization and dynamics, therefore their roles in tumor invasion and metastasis go beyond invadopodia to general cell migration and other actin-based cellular processes. In contrast, Tks proteins are known to be more specifically involved in invadopodia formation, therefore its impact on tumor invasion and metastasis is thought to be largely due to their functions at invadopodia.
Cortactin
Cortactin is a cytoskeletal protein that when phosphorylated can recruit the Arp2/3 complex to promote invadopodia formation. Cortactin was originally identified as a Src phosphorylation target in Src-transformed chicken embryo fibroblasts4. Src binds to cortactin through direct interaction with the SH2 domain of Src5 and phosphorylates cortactin in v-Src transformed 3T3 fibroblasts4, 6.
Since Src kinase plays an essential role in invadopodia regulation (discussed in detail in a later section), cortactin has been shown to be a key regulator of actin polymerization at invadopodia in response to Src activation. The association of cortactin with invadopodia was first described in MDA-MB-231 cells, in which microinjection of anti-cortactin antibodies reduced their ability to degrade extracellular matrix7. Furthermore, immunoprecipitation of cortactin revealed its presence in invadopodia- enriched membrane fractions7. Finally, immunofluorescence indicated cortactin at actively degrading invadopodia7, 8. Knockdown of cortactin in MDA-MB-231 cells resulted in inhibition of actin/cortactin positive puncta and matrix degradation, suggesting that cortactin is required for invadopodia formation and function8. Cortactin localization at invadopodia coincided with phosphotyrosine puncta, which is consistent with Src regulation of cortactin phosphorylation9. Src activation of cortactin resulted in the localization of Nck1 and N-WASP at invadopodia, in addition to the disengagement of cofilin, all of which are required for Arp2/3-mediated actin polymerization to promote invadopodia formation10, 11.
Since cortactin regulates various actin-based cellular programs, including invadopodia and lamellipodia formation and dynamics, its role in tumor cell invasion and metastasis is well documented. Overexpression of cortactin in NIH3T3 cells resulted in increased invasion in a Matrigel Boyden chamber assay12. Similarly, overexpression of cortactin in MDA-MB-231 cells resulted in increased invasion, which correlated with increased metastatic bone lesions13. In contrast, overexpression of a phosphorylation deficient cortactin inhibited invasion and resulted in minimal bone metastatic lesions13. Similar results were obtained in a hepatocellular carcinoma (HCC) model, where overexpression of wild-type cortactin in the non-metastatic HCC cell line KIM1 increased metastatic incidence without affecting primary tumor growth14.
Cortactin was first implicated in the progression of human cancers through gene amplification at Chromosome 11q in breast and squamous cell carcinomas13, 15, 16. Additionally, high levels of cortactin expression are also observed in human ovarian, bladder, and lung cancer4, 7, 13, 15. Importantly, overexpression of cortactin is associated with poor patient prognosis in breast and head and neck squamous carcinomas, further highlighting a critical role of cortactin in human tumor progression13, 16.
MENA
Mena is a member of the Enabled (Ena)/vasodilator-stimulated phosphoprotein (VASP) family of proteins, which are involved in the regulation of actin polymerization. Mena protein is upregulated in breast, pancreatic, colon, gastric, cervical cancers, and melanoma, with the expression of specific isoforms regulating the invasive properties of breast cancer cells17–19. Due to its role in actin polymerization, Mena is a logical regulator of invadopodia formation. Mena was found to co-localize with cortactin and F-actin at invadopodia18. Additionally, Mena-null MMTV-PyMT mammary tumors exhibited reduced invasion into the surrounding stroma20. In conjunction, Mena-null mice had significantly fewer circulating tumor cells and lung metastases, when compared to control mice, suggesting that Mena expression is necessary for tumor cell intravasation and invasion20.
Interestingly, gene profiling in rat MTLn3 and mouse PyMT breast tumors identified an invasion specific isoform of Mena, called MenaINV, whose expression was correlated with invasive ability17. MenaINV increased invasion of MTLn3 cells into collagen gels, indicating a unique role for MenaINV in carcinoma cell invasion18. Additionally, MtLn3 cells expressing MenaINV exhibited increased membrane protrusions compared to parental MTLn3 cells18. In terms of metastatic progression, MenaINV expression was associated with highly metastatic carcinomas in the PyMT mouse mammary tumor model21. Philippar et al. also observed that overexpression of MenaINV in MTLn3 cells resulted in increased micrometastatic lung formation, despite no effect on primary tumor growth18, again indicating the importance of regulating Mena mRNA splicing in tumor invasion and metastasis20.
Tks Adaptor Proteins
The Tks adaptor proteins Tks4 and Tks5 are named after Tyrosine Kinase Substrate with 4 or 5 SH3 Domains, respectively. Tks proteins only contain SH3 and PH domains for protein-protein and protein-lipid interactions; therefore they are thought to serve as adaptor proteins that recruit other proteins and lipids22 for invadopodia assembly.
The role of Tks5 in invadopodia was first discovered using a Src substrate screening assay, and further characterized based on its localization to invadopodia in Src-transformed fibroblasts23. Tks5 is required for both invadopodia formation and invasion activity in a variety of human cancer cell lines, as knocking down Tks5 reduced matrix degradation activity and invasion24. Likewise, introduction of Tks5 into the human breast epithelial cell line T47D, which lacks endogenous Tks5 expression, promoted invadopodia formation23. Tks5 was shown to bind to ADAM1225, a metalloproteinase associated with invadopodia. Furthermore, Tks5 is also associated with the actin regulatory protein N-WASP26 and involved in the recruitment of AFAPA-110, p190RhoGAP, and cortactin to invadopodia27. Finally, Tks5 binds to p22phox, a part of the NADPH oxidase complex that generates reactive oxygen species (ROS), which facilitates invadopodia assembly and function28.
The family member Tks4 also localizes to invadopodia in Src-transformed cells and is required for invadopodia assembly29. However, Tks4 and Tks5 seem to play non-redundant roles in invadopodia function. Cells lacking Tks4 formed actin puncta resembling invadopodia, but these cells failed to degrade ECM components, even in the presence of high levels of Tks529. This is thought to be due to a crucial role of Tks4 in recruiting MT1-MMP to invadopodia since no MT1-MMP was detected in the rudimentary invadopodia present in Tks4 knockdown cells29.
To test the role of Tks5 in tumor metastasis, Eckert et al. showed that knocking down Tks5 in Ras-transformed human mammary epithelial cells that overexpress Twist1 inhibited both local invasion and the ability of these cells to form lung metastases, while primary tumor formation rates were not altered24. These data strongly indicate that Tks5, and likely its role in invadopodia assembly, are required for the early steps of metastasis. To test whether Tks5 functions during extravasation and metastatic outgrowth, Blouw et al. injected Src-transformed 3T3 cells with Tks5 knockdown into immunocompromised mice via tail vein. While Tks5 knockdown did not significantly affect the number of lung colonies, the metastases derived from the cells with Tks5 knockdown were significantly smaller30. These data suggest that Tks5 could be further required for the expansion of secondary tumors in distant sites.
PROTEASES
Given their central function to recruit proteases to cell-matrix contacts for matrix remodeling, invadopodia are shown to contain a large numbers of proteases. The proteases found at invadopodia include metalloproteases (both secreted and membrane-tethered matrix metalloproteinases [MMPs]), the ADAM (A Disintegrin And Metalloproteinase) family members, and membrane-bound serine proteases, all of which have been implicated in cancer progression and metastasis. Past research has focused on developing metalloproteinase inhibitors to suppress ECM degradation and tumor metastasis. Although these inhibitors show promising results in cell culture and tumor xenograft models, numerous metalloproteinase inhibitors have failed in clinical trial31. Further studies indicate that some metalloproteinases could have anti-tumorigenic effects32. Therefore, the strategy of broadly blocking metalloproteinases to abrogate metastasis might not be a viable approach to prevent tumor metastasis. Here we discuss a few proteases that are unique to invadopodia and might be promising new targets in inhibiting tumor invasion and metastasis.
Metalloproteinases
MT1-MMP
MT1-MMP (also known as MMP14), a membrane-anchored metalloproteinase, is considered a central player of invadopodia-mediated ECM degradation. MT1-MMP cleaves33 several substrates in vitro, including ECM components such as fibronectin, type I, II, and III collagen, laminins, vitronectin, and aggrecans34–37. Additionally, MT1-MMP is capable of activating other MMP zymogens: MT1-MMP activates MMP2 by cleaving the N-terminal prodomain of pro-MMP238, and MMP9 is activated through an activation cascade involving MT1-MMP, MMP2, and MMP339. MT1-MMP is shown to be required for the matrix degradation activity of invadopodia. Artym et al. found that cortactin aggregation initiated accumulation of MT1-MMP at invadopodia8. This study also found that while MT1-MMP knockdown moderately impacted the initial stages of invadopodia formation, matrix degradation was strongly suppressed8, indicating that MT1-MMP is essential for functional invadopodia.
MT1-MMP is delivered to invadopodia via multiple routes. Studies from Yu et al. show that N-WASP, which promotes actin nucleation, promotes the delivery of MT1-MMP from late endosomes to invadopodia40, 41. MT1-MMP can also be mobilized by the Rab8-dependent secretory pathway and delivered to collagen-contact sites42. Finally, MT1-MMP can also be internalized by both clathrin- and caveolae-mediated endocytosis3, 43 and this internalization serves to recycle MT1-MMP back to invadopodia when needed.
A key inducer of invadopodia, the Src kinase, has also been shown to directly regulate the delivery of MT1-MMP to invadopodia. Src-mediated phosophorylation of MT1-MMP in its AP2 clathrin adaptor binding domain slows endocytosis of MT1-MMP and increases matrix degradation activity43. In addition, phosphorylation of MT1-MMP by Src at Tyr573 has been shown to be required for tumor cell proliferation, invasion of 3D collagen matrices, and tumor growth in nude mice44, 45. Finally, a recent study found that this phosphorylation was required for mono-ubiquitination of Lys581, which is involved in MT1-MMP trafficking to the cell surface and cellular invasion through collagen matrices46.
An increase in MT1-MMP expression is generally associated with poor prognosis in a wide variety of human cancers, including breast, lung, melanoma, colorectal, and squamous cell carcinomas47. MT1-MMP expression has also been directly linked to metastasis in mouse tumor models. MT1-MMP-deficient mice were bred with MMTV-PyMT mice, and then PyMT-positive mammary glands lacking MT1-MMP were orthotopically transplanted into wild-type mice. While palpable tumors developed faster with MT1-MMP-deficient mammary glands, metastatic spread was reduced by 50%48. Consistent with this study, Perentes et al. injected MDA-MB-231 cells with MT1-MMP knockdown into the mammary fad pad of SCID mice49 and found that MT1-MMP knockdown resulted in a significant decrease in lung metastasis without affecting primary tumor growth49. These results suggest that MT1-MMP is required for metastatic development in vivo.
Since blocking MMP activity has failed in clinical trials as an anti-metastasis therapy, possibly due to the broad spectrum of inhibition and severe toxicities50, 51, new therapeutic strategies aim to target the specific MMPs that contribute to disease progression50. A fully humanized monoclonal antibody (DX-2400, Dyax Corporation) that targets MT1-MMP at its catalytic domain showed great promise in pre-clinical studies. DX-2400 abrogated MMP2 cleavage on tumor and endothelial cells, blocked angiogenesis, and reduced tumor formation and metastasis50, 52. Another humanized antibody targeting the non-catalytic hemopexin domain of MT1-MMP has recently shown promise in inhibiting invasion and angiogenesis in pre-clinical studies53. The therapeutic potential of targeting MT1-MMP to inhibit invadopodia-mediated tumor invasion and metastasis holds great future promise.
ADAM Proteases
The ADAMs are a family of disintegrin and metalloproteinases that are involved in a variety of biological processes, including cell adhesion, migration, proteolysis, myoblast fusion, and fertilization54. Here, we focus on ADAM12 due to its more established presence at invadopodia. ADAM12 has two alternatively spliced variants: ADAM12-L, which consists of pro-, metalloprotease, disintegrin, cysteine-rich, transmembrane, and cytoplasmic domains, and ADAM12-S, which lacks the transmembrane and cytoplasmic domains55.
ADAM12 contributes to invadopodia function at multiple levels, including degrading the ECM, modulating integrin function, and functioning as a sheddase to activate growth factors56. ADAM12 is localized to invadopodia; it binds to the scaffold protein Tks525, and has been found to trigger invadopodia assembly57. The sheddase activity of ADAM12 may contribute to the overall degradation activity of invadopodia. A recent study by Días et al. demonstrated that ADAM12 expression was elevated in a Notch-dependent manner under hypoxic conditions58. ADAM12 promoted the ectodomain shedding of heparin-binding EGF-like growth factor, which in turn induced invadopodia formation and the invasive activity of cancer cells58.
ADAM12 is implicated in a variety of cancers, including breast, prostate, lung, liver, brain, and bone cancers, as well as aggressive fibromatosis59. In human breast cancer patients, Roy et al. showed that ADAM12 is a prognostic marker: urinary levels of ADAM12 increased along with disease stage60. Transgenic mice expressing the ADAM12-S isoform driven by the MMTV-LTR promoter were bred with mice carrying the polyoma middle T (PyMT) oncogene in the mammary gland; PyMT expression in the mammary gland led to rapid formation of mammary carcinomas. Tumors in mice expressing ADAM12-S developed faster than in littermates expressing PyMT alone61. Similarly, ADAM12-S isoform significantly increased the ability of MCF-7 cells to migrate and invade, which led to a higher incidence of local and distant metastases in vivo62. Interestingly, cells expressing a catalytically dead mutant of ADAM12-S failed to promote tumor development, indicating that the proteolytic activity of ADAM12-S is required to promote formation of distant metastases62.
Serine Proteases
Two transmembrane type II serine proteases of the Dipeptidyl-Peptidase (DPP) family, Fibroblast Activation Protein (FAP, FAPα, also known as seprase), and DPP4, have also been associated with invadopodia. Both DPP4 and FAPα contain exopeptidase activity and FAPα also exhibits endopeptidase activity63, 64. Previous studies have shown that FAPα is localized at invadopodia as a complex with DPP465, or alternatively, associated with α3β1 integrin, with the integrin serving as a docking site for FAPα66. The role of FAPα in invadopodia is currently unclear, but some studies suggest that its gelatinase activity may contribute to the overall degradation activity of invadopodia. Christiansen et al. found that FAPα digests collagen I into smaller fragments following initial cleavage by MMP-1, suggesting that FAPα works together with other proteases to cleave partially degraded ECM components67.
A key difference between FAPα and DPP4 is that expression of DPP4 is ubiquitous throughout all tissues, whereas that of FAPα is restricted to tissues undergoing wound healing and epithelial cancers64, 68, thus making FAPα a unique player in tumor progression. Indeed, FAPΑα has been shown to be expressed in a variety of aggressive cancers, including breast, colon, and ovarian cancers, and malignant melanoma69. Additionally, genetic deletion of FAPα inhibited tumor growth in a K-ras-driven model of endogenous lung cancer and in a mouse model of colon cancer. Pharmacological inhibition of FAPα also attenuated tumor growth in these mouse models, indicating that FAPα is a promising target for therapeutic intervention70. In human and mouse tumors, FAPα has been shown to be expressed in stromal fibroblasts, carcinoma cells, and immune cells69, 71–73. What remains to be answered is whether and how FAPα in individual cell types contributes to tumor progress and whether the role of FAPα at invadopodia is critical for tumor invasion and metastasis.
Previous attempts to target FAPα for therapeutic intervention have proved to be challenging. In 2003, Phase I/II clinical trials for the humanized FAPα monoclonal antibody Sibrotuzumab failed to demonstrate measurable therapeutic activity in patients with metastatic colorectal cancer,74 with only 2 out of 17 patients having stable disease during the Phase II trial74. However, this antibody has not been shown to block any cellular or protease function of FAPα, which might explain the lack of therapeutic effects. In 2007, a small molecule inhibitor of FAPα, Talabostat, was developed to inhibit the protease activity of FAPα. Again, minimal clinical activity was observed in patients with metastatic colorectal cancer receiving Talabostat alone75, or in metastatic melanoma patients receiving Talabostat in conjunction with cisplatin treatment76. However, the stability of this inhibitor in vivo is thought be extremely poor, thus limiting its effectiveness.
Given these recent setbacks in targeting FAPα, efforts have recently turned to FAPα –mediated immunotherapy. One approach is to develop DNA vaccines to target FAPα, thus eliminating all FAPα-positive cell types in a tumor. Several groups reported that through CD8+ T cell-mediated killing, such therapy successfully suppressed primary tumor cell growth and metastasis of implanted breast and colon tumors without obvious toxicity77, 78. Another approach is to deliver radioisotopes specifically to the tumor site using FAPα antibodies as cargoes. Pre-clinical studies involving two humanized FAPα monoclonal antibodies (ESC11 and ESC14) labeled with the radiolanthanide 177Lu have yielded promising results: both antibodies accumulated in human FAPα-positive xenografts and delayed tumor growth79. Given that FAPα is expressed in various cell types in a tumor, it is important to recognize that the effect of targeting FAPα on tumor progression cannot solely be explained by inhibition of invadopodia. However, these results suggest a unique approach to targeting components of invadopodia in human cancers.
REGULATORY COMPONENTS
Since extracellular matrix is essential for cell survival and proliferation, invadopodia-mediated matrix degradation is a highly regulated process. Understanding the upstream inducing signals of invadopodia formation and function is still in its infancy. A significant numbers of signaling regulators, including EGFR, PDGFR, PI3 kinases, c-Met have been implicated in invadopodia regulation in various cancer cell lines. Since many of them play critical roles in multiple cellular processes in cancer, including cell proliferation and apoptosis, it is difficult to attribute their functional impact on tumor progression specifically to invadopodia. Thereby here we discuss a few key upstream signaling pathways that have been more uniquely implicated in invadopodia function during tumor progression and metastasis.
Phosphorylation via Src and Arg tyrosine kinases
Tyrosine phosphorylation of many core components is critical to trigger invadopodia assembly and function. Especially, two tyrosine kinases, Src and Arg, stand out as essential activators of invadopodia.
Src kinase
The Src kinase is the founding member of the Src family of non-receptor tyrosine kinases80, of which, Src is the only family member that is uniquely linked to invadopodia. The role of Src in invadopodia formation was first described by Chen et al., where Rous sarcoma viral(RSV) transformation of chicken embryonic fibroblasts resulted in actin rosette, or podosome formation. Additionally, RSV mediated cellular transformation correlated with the appearance of pp60Src accumulation at these rosettes81. Similarly, invadopodia formation was enhanced with constitutive expression of active c-Src, as evidenced by co-localization of F-actin and cortactin staining82. In contrast, overexpression of a kinase inactive c-Src and knockdown of c-Src by RNAi showed decreased invadopodia formation and degradation activities9, 83, 84. Closer examination of protrusion formation in MDA-MB-231 cells revealed that overexpressing wild-type Src or constitutively active Src exhibited invadopodia extension into the collagen gel9.
Src kinase has been shown to play a major role in the invasive process. Expression of c-Src in SYF (src−/−, yes−/−, fyn−/−) murine embryonic fibroblasts (MEF) promoted invasion, while RasV-12 failed to do so in a Boyden chamber assay85. Similarly, MDA-MB-231 breast cancer cells treated with Src siRNAs exhibited reduced matrix degradation and invasion through matrix-coated chambers84. Treatment with Src inhibitors, Dasatinib, PP2, or SU6656, in MDA-MB-231 cells reduced invasion through Matrigel, indicating the importance of Src activity for tumor cell invasion into the extracellular matrix (ECM)86.
Although Src was originally isolated as an oncogene, recent studies suggest a more critical role for Src in tumor metastasis85, 87, 88. Specifically, c-Src activity is correlated with increased bone metastases, poor clinical prognosis, and reduced survival for breast and colon carcinoma patients86, 89, 90. Src gene deletion in MMTV-polyoma middle T antigen (PyMT) mammary tumor models resulted in a reduction in circulating tumor cells, despite no defect in primary mammary tumor initiation and proliferation91. Similarly, Src siRNA treated L3.6pl pancreatic cancer cells exhibited a reduction in lung and liver metastasis92. c-Src was required for formation of metastatic lung colony formation by H-RasV-12 expressing SYF (src−/−, yes−/−, fyn−/−) MEF cells, supporting the role of Src in tumor progression85. Likewise, BoM-1833, a bone metastatic derivative of MDA-MB-231 cells, which were injected into recipient mice, showed increased survival and reduced bone metastases upon treatment with Src siRNA89.
Since Src activity plays a prominent role in cancer progression, it becomes an ideal therapeutic target. The Src selective inhibitor, SU6656, has been found to inhibit Src kinase activity, as evidenced by reduced levels of phospho-Y418-Src. SU6656 was also found to significantly reduce invadopodia formation, as well as migration and invasion of the human breast cancer line MDA-MB-23182. KX2–391 is a first-in-class Src selective inhibitor on clinical trial that targets the unique Src substrate binding site93. KX2–391 has shown promising preclinical data, with KX2–391 treatment, in combination with paclitaxel, resulting the regression of pre-established MDA-MB-231 xenograft tumors94. Additionally, KX2–391 treatment led to reduced metastasis formation of MDA-MB-231 tumors the lung and liver94. Phase I trials in patients with solid tumors showed that KX2–391 is well tolerated and demonstrated preliminary antitumor activity, with several patients displayed halted disease progression95.
Abl/Arg
Similar to Src kinase, the Abl family of non-receptor tyrosine kinases, which includes c-Abl and the Abl-related gene (Arg/Abl2), plays an important role in tumor progression in human leukemia, non-small cell lung cancer, breast cancer, melanoma, and pancreatic cancer96. Specifically, c-Abl and Arg kinases activities have been shown to correlate with poorly differentiated and highly invasive breast cancer lines97. The role of Abl/Arg kinase was initially hypothesized in invadopodia formation due to previously known activation of Abl by Src kinases. More specifically, PDGF and EGF stimulation of fibroblast and breast cancer cells resulted in c-Abl activity through Src and Fyn phosphorylation97, 98. Treatment of various highly invasive breast cancer cell lines with Src inhibitor SU6656 reduced c-Abl and Arg activity97. Src activation of Arg is required for cortactin phosphorylation and actin polymerization at invadopodia, as knockdown of Arg in MDA-MB-231 cells resulted in no cortactin phosphorylation and reduced F-actin barbed end generation84.
The Abl family of non-receptor tyrosine kinases has also been localized directly to the invadopodia structure. YFP-tagged wildtype and constitutively active Arg co-localized with cortactin positive invadopodia, in Src expressing NIH3T3 cells, while kinase-inactive Arg expression disrupted invadopodia formation99. Similarly, immunofluorescence staining of Arg in MDA-MB-231 cells showed co-localization with Tks5 positive invadopodia84.
Abl and Arg kinases also play a significant role in tumor invasion and metastasis. Knockdown of Abl and Arg reduced the ability of MDA-MB-231, and its metastatic derivatives, to degrade extracellular matrix and invade97, 99. Inhibition of Abl kinase activity with STI571, an Abl/Arg inhibitor, reduced invasion of MDA-MB-435S breast cancer cells in a Matrigel invasion assay97. MDA-MB-231 cells, expressing Arg and Abl shRNA constructs, showed fewer circulating tumor cells in vivo compared to control tumor-bearing mice100. Similarly, Gil-Henn et al. showed that STI571-treated mice showed fewer circulating tumor cells than control mice bearing MDA-MB-231 tumors100. These results indicate that Abl kinase activity is required for tumor cell intravasation.
A number of tyrosine kinase inhibitors with dual specificities toward Src and Abl family tyrosine kinases, including Dasatinib, Saracatinib, and Bosutinib, have been developed and showed promising activities against tumor invasion and metastasis in several solid tumors preclinical studies. Specifically, these inhibitors were found to significantly reduce invadopodia formation, as well as migration and invasion of the human breast cancer line MDA-MB-23186, 101, 102. Additionally, treatment of mice with Dasatinib led to reduced formation of bone metastases by BoM-1833 cells89. Similar results have been observed in pancreatic tumors, with Dasatinib treatment leading to a reduction in primary tumor growth and metastasis formation by L3.6pl cells92. However, these inhibitors have shown limited activity in monotherapy trials93. It is important to note, however, that completed clinical trials studying the efficacy of Src/Abl inhibitors have been conducted in unselected cancer patients. Many ongoing trials using biomarkers (such as cortactin phosphorylation) to pre-select patients who are more likely to benefit from Src/Abl inhibition, hold promise for the future success of Src/Able inhibitors in cancer treatment93.
Integrin-mediated signaling
Given that integrins are the key connection between cell protrusions and the surrounding extracellular matrix, it is not surprising that integrins play important roles in invadopodia regulation. Integrin clustering at invadopodia was first described in Rous sarcoma viral transformation of chicken embryonic fibroblasts (RSVCEF). RSVCEFs cultured with fibronectin coated beads exhibited invadopodia formation, which was associated with β1 staining103. Interestingly, fibronectin positive vesicles were found to co-stain with β1 integrin; further validating the functional role of invadopodia in matrix degradation103. In contrast, murine embryonic fibroblasts overexpressing Src and depleted for β1 integrin exhibit reduced rosette formation104. In addition, treatment of SCC61 squamous cell carcinoma cells with an integrin blocking peptide or a β1 integrin blocking antibody led to a reduction in actively degrading invadopodia per cell105. Lastly, knocking down β1 integrin in MDA-MB-231 and MTLn3 mammary adenomacarcinoma cells led to a reduction in mature invadopodia formation106.
How integrins regulate invadopodia function has not been clearly elucidated. Various studies indicate that integrins promote invadopodia maturation by serving as a docking station for various proteases and/or activating Arg kinase for actin stabilization. Laminin peptide activation of β1 integrin in LOX human melanoma cells led to increased invadopodia-mediated degradation107 due to increased seprase/FAPΑα recruitment to invadopodia via binding to β1 integrin7, 107. More recently, β1 integrin has been found to activate Arg kinase. FRET-based experiments point to direct interaction between β1 integrin and Arg at Tks5 positive invadopodia106.
Changes in integrin-mediated adhesion signaling complexes are known to play an important role in tumor cell proliferation, migration and survival108. Specifically, loss of β1 integrin in MMTV-driven Erb2 breast tumor mice reduced Y416 c-Src phosphorylation and metastatic lesion formation in the lungs109. Similarly, tail vein injection of MDA-MB-435 breast cancer cells, expressing a constitutively active mutant αvβ3 exhibited enhanced metastatic lung colonization109. To date, it is unclear which integrin subunits are the predominant forms required for invadopodia function. Since the focus of current integrin-targeted therapies has been on their anti-angiogenic properties, their potential as an anti-metastatic treatment via invadopodia inhibition requires identifying and targeting invadopodia-specific integrins in the near future.
Transcriptional regulation by EMT-inducing factors
To detach from the primary tumor and invade through the surrounding tissue, carcinoma cells need to first break down cell-cell junctions, become more motile, remodel cell-matrix adhesion sites, and invade through the ECM. A developmental program termed Epithelial-Mesenchymal Transition (EMT) enables tumor cells to obtain such properties. During EMT, cells need to coordinate the dissociation of cell-cell adhesion and the breakthrough of basement membrane to accomplish this complex morphogenetic event1. Recent studies indicate a critical role of invadopodia-mediated ECM degradation during EMT.
The EMT program is orchestrated by a group of transcription factors, all of which have been implicated in tumor invasion and metastasis110. Specifically, the bHLH transcription factor Twist1 was shown to play critical roles in tumor metastasis in both breast tumor xenografts and in mouse skin tumor models111, 112. Eckert et al. found that Twist1 was required for ECM invasion by inducing the formation of invadopodia in human and mouse breast tumor cells24. Twist1 was found to directly induce expression of PDGFRα, which then activates Src kinase to promote invadopodia formation24. Furthermore, blocking invadopodia formation by knocking down PDGFRα or Tks5 abolished the ability of Twist1 to promote tumor metastasis in mice. This study not only uncovers Twist1 as a novel upstream regulator of invadopodia formation, but also provides a direct link between invadopodia and metastasis.
Another potent inducer of EMT is Transforming Growth Factor beta (TGFβ)1. TGFβ promotes EMT via activation of extensive intracellular signaling that involves Smad proteins, ERK, and Jagged/Notch signaling, among others113. Of particular interest in the context of invadopodia, Eckert et al., showed that knocking down Twist1 blocked the ability of TGFβ to induce PDGFRα and invadopodia formation24. In addition, Pignatelli et al. also demonstrated that Hic-5, a focal adhesion adaptor protein induced by TGFβ, localized to invadopodia in TGFβ-treated MCF-10A cells. They showed that Hic-5 was phosphorylated by Src kinase upon TGFβ stimulation and this phosphorylation was required for TGFβ-induced invadopodia formation and invasion, thereby further emphasizing the role of EMT-inducing genes in invadopodia regulation114.
Matrix stiffness and mechano-regulation
Recent evidence has implicated matrix stiffness in tumor progression and increased incidence in metastasis, through increased collagen deposition115–117. Several elegant studies show that increasing matrix stiffness without altering biochemical components of extracellular matrix (ECM) can induce a malignant phenotype, suggesting that mechanical force exerted by stiff ECM could play a critical role in tumor invasion and metastasis118. In mice, Lysyl oxidase-mediated collagen crosslinking stiffens tumor ECM and promotes breast tumor progression119. Furthermore, inhibition of lysyl oxidase (LOX) blocks tumor invasion and eliminates metastasis formation from orthotopically grown breast tumors33.
Indeed, ECM rigidity is indicated in invadopodia regulation. Specifically, Alexander et al. noted that CA1d breast cancer cells plated on increasing concentrations of gelatin showed increased invadopodia formation and ECM degradation120. Interestingly, two studies showed that both CA1d and 804G bladder cancer cells exhibited increased invadopodia formation when placed on matrix substrates with increasing mechanical rigidity without changing their biochemical components121. Furthermore, these studies showed that invadopodia-mediated ECM degradation only increased when placed on surfaces within the kPa range, which corresponds to the stiffness in tumors121. While it is not well understood how matrix rigidity regulates invadopodia formation, this study suggests that activation of p130Cas and FAK via myosin II, which acts as mechanosensors that transmit mechanical signals from ECM, could play a prominent role120. Given the effects of increasing matrix stiffness on invadopodia functions, it is possible that mechanical forces generated by rigid tumor matrix stiffness could modulate invadopodia function to impact tumor progression and metastasis.
The role of invadopodia in tumor invasion and metastasis
The critical role of invadopodia in ECM degradation explains why the ability to form invadopodia largely correlates with the invasive and metastatic potential of tumor cells122. Suppressing invadopodia formation by inhibiting Src, Twist1, or Tks5 have been convincingly shown to inhibit tumor metastasis in various tumor models. Invadopodia could play critical roles during three steps of the metastatic process: invasion into the surrounding stroma, intravasation into the vasculature, and extravasation (Figure 2). Direct in vivo visualization and assessment of invadopodia formation during the metastatic process has proven to be challenging due to the limited numbers of invadopodia-specific markers in a 3D microenvironment. Furthermore, it remains unclear whether actin-based protrusions observed in 2D culture, including filopodia and invadopodia, share similar components or merge into one structure in 3D; thus confusing the issue of defining invadopodia in vivo.
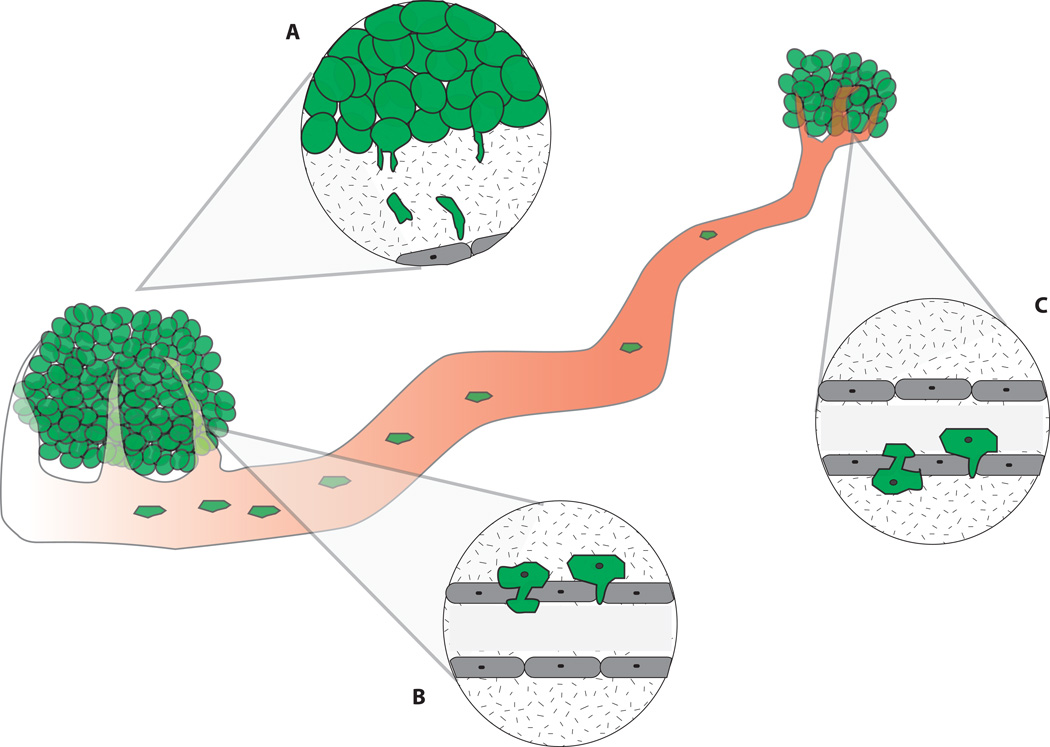
Figure 2. The role of invadopodia in metastasis progression.
Tumor metastasis takes place in a series of steps: invasion into the surrounding stroma(A), intravasation into the vasculature(B), extravasation out of the vasculature(C), and colonization at distant sites. Given that invadopodia function to degrade ECM, invadopodia are thought to play critical roles during various steps of metastasis (A–C).
New imaging techniques have made it possible to begin to identify invadopodia-like protrusions in vivo (Figure 2). Using 3D time-lapse imaging, Gligorijevic et al. (2012) observed protrusion formation by MTLn3 rat mammary adenocarcinoma xenograft tumors growing in the mammary fat pad of SCID mice. These protrusions were positive for cortactin and proteolytic activity, as evidenced by cleaved collagen 3/4 staining, indicating the presence of invadopodia in vivo123. Looking specifically at the intravasation, Gligorijevic et al. used the photoconvertible Dendra2 protein to trace tumor cell intravasation in vivo. Control MTLn3 primary tumor cells were shown to disappear from the imaging area due to dissemination of cells into the blood stream. In contrast, knocking down N-WASP resulted in no visible dissemination123. Yamaguchi, H. et al (2005) observed similar invadopodia-like protrusions during tumor cell intravasation using intravital imaging. GFP expressing MTLn3 cells revealed invadopodia-like protrusions extending into the blood vessel wall124. These invadopodia-like protrusions were shown to help tumor cells to penetrate the ECM surrounding the blood vessel walls and to squeeze through the endothelial barrier124. Together, these data strongly support the notion that invadopodia are required for tumor cell intravasation (Figure 2).
Assessment of invadopodia formation during the extravasation process is more limited; however, protrusive structures have been identified by actively extravasating tumor cells125, 126. Specifically, time course observations of intra-meseneric vein injection of GFP-positive rat tongue carcinoma cells reveal clusters of tumor cells in the sinusoids125. Over time, the authors noted focal loss of basement membrane at sinusoidal areas where extravasation was taking place125. The loss of basement membrane indicates potential sites of active ECM degradation by invadopodia. Protrusive structures have also been identified by actively extravasating MDA-MB-435 cells expressing Twist1 upon injection into the vasculature of zebrafish126. Direct imaging of tumor cells in the vasculature revealed that Twist1-overexpressing cells display large rounded protrusions126, suggesting that invadopodia-like protrusions by extravasating tumor cells contribute to ECM degradation and breaking through the endothelium barrier (Figure 2).
Future directions and therapeutic implications
As discussed, recent progresses suggest an essential role of invadopodia in tumor invasion and metastasis. The specific presence of invadopodia in invasive tumor cells and their unique ability to precisely coordinate localized ECM degradation with cell movement make them ideal targets for anti-metastasis therapies.
A number of critical issues need to be resolved to put invadopodia at the forefront of tumor metastasis research and treatment. First, although actin assembly and elongation during invadopodia initiation have been extensively studied, it remains unclear what and how matrix degrading enzymes are recruited to invadopodia to perform their functions. Since broad inhibition of MMPs has not been successful in blocking metastasis, the strategy to inhibit protease recruitment could be a promising new route to specifically targeting ECM degradation and tumor invasion. Second, it remains unclear whether and how the molecular components and regulation of invadopodia and other actin-based membrane protrusions are different. Since many invadopodia components play critical roles in various cellular processes, such as proliferation, apoptosis, and migration, it is difficult to attribute all their observed activities on tumor progression to invadopodia function. Understanding such differences would further solidify the unique role of invadopodia in tumor metastasis in vivo, and lead to more specific targeting therapies against invadopodia in tumors without affecting normal cellular functions.
To move invadopodia inhibitors into therapeutic applications against tumor metastasis, we first need to determine how to apply invadopodia inhibitors for cancer treatment. As discussed, the main function of invadopodia in tumors is to promote matrix degradation and tumor invasion, but not to regulate cell proliferation or survival. Therefore, the main utility of invadopodia inhibitors should be to prevent primary and secondary metastasis occurrences, instead of inhibiting the growth of established primary tumors and metastases. Invadopodia inhibitors could be beneficial in preventing new metastasis development in a number of metastasis-prone cancer patient groups. Using breast cancer as an example, a group of cancer patients who have already developed limited metastatic diseases, such as a single brain metastasis, may use invadopodia inhibitors to prevent secondary metastasis lesions in the brain. Also patients that have presented lymph node positivity could benefit from invadopodia inhibitors to prevent distant metastasis development. Furthermore, recent gene expression profiling and biomarker studies make it possible to predict long-term metastasis occurrence and survival outcome in early-stage breast cancer patients. Invadopodia inhibitors, in combination with traditional chemotherapies, could potentially reduce metastasis development in the selected high-risk patient population based on such molecular profiling.
Another pressing issue that faces the entire metastasis field, including the invadopodia research, is how to develop proper clinical trials to test anti-metastatic agents, such as invadopodia inhibitors. As discussed above, invadopodia mainly function to promote matrix degradation, thus perturbation of their functions in tumors have little or no effect on tumor proliferation. Unfortunately, the current clinical trial system requires all anti-cancer agents to show efficacy in phase II by shrinking established primary tumors and/or distant metastases in patients before moving to phase III trials and regulatory approval. Only after approval can these agents be tested in metastasis prevention trials for more early-stage cancer patients. Since invadopodia-specific inhibitors are unlikely to shrink existing tumors and metastases, these inhibitors would fail in current phase II clinical trials even though they might be potent to prevent new metastasis occurrence. A stimulating article by Dr. Patricia Steeg (Nature 2012) has proposed that the rate of new metastasis occurrence in metastasis high-risk patients as a more appropriate end point for metastasis prevention trials127. Given the unique role of invadopodia in tumor invasion and metastasis, invadopodia inhibitors need to be tested in better-designed metastasis prevention trails to explore their full potentials in combating tumor metastasis.
Acknowledgement
We apologize to the many researchers in this field whose work we were unable to cite due to space restrictions. Our research on tumor metastasis is supported by grants from National Institutes of Health 1DP2OD002420, National Cancer Institute 1RO1CA168689, American Cancer Society grant RSG-09-282-01-CSM, The Hartwell Foundation, and DOD Breast Cancer Program W81XWH-13-1-0132 to J.Y., by the NIH IRACDA training grant 5K12GM068524 to H.P, and by the NIH Pre-doctoral Training grant in Pharmaceutical Science 5T32GM007752 to N.P.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 5.Okamura H, Resh MD. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J Biol Chem. 1995;270:26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 7.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 8.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 9.Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, et al. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006;312:1240–1253. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Tondravi M, Liu J, Smith E, Haudenschild CC, Kaczmarek M, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–6911. [PubMed] [Google Scholar]
- 14.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, et al. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–636. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 16.Weaver AM. Cortactin in tumor invasiveness. Cancer Lett. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, Wang W, et al. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis. 2009;26:153–159. doi: 10.1007/s10585-008-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertler F, Condeelis J. Metastasis: tumor cells becoming MENAcing. Trends Cell Biol. 2011;21:81–90. doi: 10.1016/j.tcb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, et al. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 2010;12:R101. doi: 10.1186/bcr2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011;124:2120–2131. doi: 10.1242/jcs.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtneidge SA, Azucena EF, Pass I, Seals DF, Tesfay L. The SRC substrate Tks5, podosomes (invadopodia), and cancer cell invasion. Cold Spring Harb Symp Quant Biol. 2005;70:167–171. doi: 10.1101/sqb.2005.70.014. [DOI] [PubMed] [Google Scholar]
- 23.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, et al. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 26.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crimaldi L, Courtneidge SA, Gimona M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res. 2009;315:2581–2592. doi: 10.1016/j.yexcr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 32.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 33.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 35.d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Fosang AJ, Last K, Fujii Y, Seiki M, Okada Y. Membrane-type 1 MMP (MMP-14) cleaves at three sites in the aggrecan interglobular domain. FEBS Lett. 1998;430:186–190. doi: 10.1016/s0014-5793(98)00667-x. [DOI] [PubMed] [Google Scholar]
- 37.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 39.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun. 2003;308:386–395. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, et al. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol. 2012;199:527–544. doi: 10.1083/jcb.201203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol. 2008;18:926–931. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 42.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 44.Nyalendo C, Beaulieu E, Sartelet H, Michaud M, Fontaine N, Gingras D, et al. Impaired tyrosine phosphorylation of membrane type 1-matrix metalloproteinase reduces tumor cell proliferation in three-dimensional matrices and abrogates tumor growth in mice. Carcinogenesis. 2008;29:1655–1664. doi: 10.1093/carcin/bgn159. [DOI] [PubMed] [Google Scholar]
- 45.Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, et al. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- 46.Eisenach PA, de Sampaio PC, Murphy G, Roghi C. Membrane type 1 matrix metalloproteinase (MT1-MMP) ubiquitination at Lys581 increases cellular invasion through type I collagen. J Biol Chem. 2012;287:11533–11545. doi: 10.1074/jbc.M111.306340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 48.Szabova L, Chrysovergis K, Yamada SS, Holmbeck K. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease. Oncogene. 2008;27:3274–3281. doi: 10.1038/sj.onc.1210982. [DOI] [PubMed] [Google Scholar]
- 49.Perentes JY, Kirkpatrick ND, Nagano S, Smith EY, Shaver CM, Sgroi D, et al. Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors. Cancer Res. 2011;71:4527–4538. doi: 10.1158/0008-5472.CAN-10-4376. [DOI] [PubMed] [Google Scholar]
- 50.Devy L, Dransfield DT. New Strategies for the Next Generation of Matrix-Metalloproteinase Inhibitors: Selectively Targeting Membrane-Anchored MMPs with Therapeutic Antibodies. Biochem Res Int. 2011;2011:191670. doi: 10.1155/2011/191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 52.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 53.Basu B, Correa de Sampaio P, Mohammed H, Fogarasi M, Corrie P, Watkins NA, et al. Inhibition of MT1-MMP activity using functional antibody fragments selected against its hemopexin domain. Int J Biochem Cell Biol. 2012;44:393–403. doi: 10.1016/j.biocel.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 55.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM. Cellular roles of ADAM12 in health and disease. Int J Biochem Cell Biol. 2008;40:1685–1702. doi: 10.1016/j.biocel.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Moss ML, Lambert MH. Shedding of membrane proteins by ADAM family proteases. Essays Biochem. 2002;38:141–153. doi: 10.1042/bse0380141. [DOI] [PubMed] [Google Scholar]
- 57.Albrechtsen R, Stautz D, Sanjay A, Kveiborg M, Wewer UM. Extracellular engagement of ADAM12 induces clusters of invadopodia with localized ectodomain shedding activity. Exp Cell Res. 2011;317:195–209. doi: 10.1016/j.yexcr.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201:279–292. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handbook of proteolytic enzymes, volumes 1, 2 and 3. Anticancer Res. (Third edition) 33:2345. [Google Scholar]
- 60.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279:51323–51330. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 61.Kveiborg M, Frohlich C, Albrechtsen R, Tischler V, Dietrich N, Holck P, et al. A role for ADAM12 in breast tumor progression and stromal cell apoptosis. Cancer Res. 2005;65:4754–4761. doi: 10.1158/0008-5472.CAN-05-0262. [DOI] [PubMed] [Google Scholar]
- 62.Roy R, Rodig S, Bielenberg D, Zurakowski D, Moses MA. ADAM12 transmembrane and secreted isoforms promote breast tumor growth: a distinct role for ADAM12-S protein in tumor metastasis. J Biol Chem. 2011;286:20758–20768. doi: 10.1074/jbc.M110.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meadows SA, Edosada CY, Mayeda M, Tran T, Quan C, Raab H, et al. Ala657 and conserved active site residues promote fibroblast activation protein endopeptidase activity via distinct mechanisms of transition state stabilization. Biochemistry. 2007;46:4598–4605. doi: 10.1021/bi062227y. [DOI] [PubMed] [Google Scholar]
- 64.O'Brien P, O'Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784:1130–1145. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 2006;66:4652–4661. doi: 10.1158/0008-5472.CAN-05-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller SC, Ghersi G, Akiyama SK, Sang QX, Howard L, Pineiro-Sanchez M, et al. A novel protease-docking function of integrin at invadopodia. J Biol Chem. 1999;274:24947–24952. doi: 10.1074/jbc.274.35.24947. [DOI] [PubMed] [Google Scholar]
- 67.Christiansen VJ, Jackson KW, Lee KN, McKee PA. Effect of fibroblast activation protein and alpha2-antiplasmin cleaving enzyme on collagen types I, III, and IV. Arch Biochem Biophys. 2007;457:177–186. doi: 10.1016/j.abb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busek P, Malik R, Sedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol. 2004;36:408–421. doi: 10.1016/s1357-2725(03)00262-0. [DOI] [PubMed] [Google Scholar]
- 69.Chen D, Kennedy A, Wang JY, Zeng W, Zhao Q, Pearl M, et al. Activation of EDTA-resistant gelatinases in malignant human tumors. Cancer Res. 2006;66:9977–9985. doi: 10.1158/0008-5472.CAN-06-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pure E. The road to integrative cancer therapies: emergence of a tumor-associated fibroblast protease as a potential therapeutic target in cancer. Expert Opin Ther Targets. 2009;13:967–973. doi: 10.1517/14728220903103841. [DOI] [PubMed] [Google Scholar]
- 72.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 73.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 74.Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jager D, Renner C, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 75.Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 76.Eager RM, Cunningham CC, Senzer NN, Stephenson J, Jr, Anthony SP, O'Day SJ, et al. Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. BMC Cancer. 2009;9:263. doi: 10.1186/1471-2407-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 78.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischer E, Chaitanya K, Wuest T, Wadle A, Scott AM, van den Broek M, et al. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin Cancer Res. 2012;18:6208–6218. doi: 10.1158/1078-0432.CCR-12-0644. [DOI] [PubMed] [Google Scholar]
- 80.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 81.Chen WT, Chen JM, Parsons SJ, Parsons JT. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- 82.Balzer EM, Whipple RA, Thompson K, Boggs AE, Slovic J, Cho EH, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29:6402–6408. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelley LC, Ammer AG, Hayes KE, Martin KH, Machida K, Jia L, et al. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci. 2010;123:3923–3932. doi: 10.1242/jcs.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan PC, Chen HC. p120RasGAP-mediated activation of c-Src is critical for oncogenic Ras to induce tumor invasion. Cancer Res. 2012;72:2405–2415. doi: 10.1158/0008-5472.CAN-11-3078. [DOI] [PubMed] [Google Scholar]
- 86.Sanchez-Bailon MP, Calcabrini A, Gomez-Dominguez D, Morte B, Martin-Forero E, Gomez-Lopez G, et al. Src kinases catalytic activity regulates proliferation, migration and invasiveness of MDA-MB-231 breast cancer cells. Cell Signal. 2012;24:1276–1286. doi: 10.1016/j.cellsig.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 87.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 88.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 91.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, et al. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151–156. doi: 10.1073/pnas.0808703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2010;16:566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor alpha positive breast cancer. Breast Cancer Res Treat. 2012;132:391–409. doi: 10.1007/s10549-011-1513-3. [DOI] [PubMed] [Google Scholar]
- 95.Naing A, Cohen R, Dy GK, Hong DS, Dyster L, Hangauer DG, et al. A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Invest New Drugs. 2013;31:967–973. doi: 10.1007/s10637-013-9929-8. [DOI] [PubMed] [Google Scholar]
- 96.Lin J, Arlinghaus R. Activated c-Abl tyrosine kinase in malignant solid tumors. Oncogene. 2008;27:4385–4391. doi: 10.1038/onc.2008.86. [DOI] [PubMed] [Google Scholar]
- 97.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 98.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 2010;285:40201–40211. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gil-Henn H, Patsialou A, Wang Y, Warren MS, Condeelis JS, Koleske AJ. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene. 2013 doi: 10.1038/onc.2012.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 2007;67:1580–1588. doi: 10.1158/0008-5472.CAN-06-2027. [DOI] [PubMed] [Google Scholar]
- 102.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16:3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 103.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99(Pt 2):213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 104.Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, et al. beta1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–4119. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biol Open. 2012;1:711–722. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, et al. beta1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol Biol Cell. 2013 doi: 10.1091/mbc.E12-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- 108.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huck L, Pontier SM, Zuo DM, Muller WJ. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A. 2010;107:15559–15564. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 114.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-beta-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–437. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adh Migr. 2009;3:288–292. doi: 10.4161/cam.3.3.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 118.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 119.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 120.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, et al. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys J. 2011;100:573–582. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91:902–907. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 123.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–734. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 125.Ito S, Nakanishi H, Ikehara Y, Kato T, Kasai Y, Ito K, et al. Real-time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. Int J Cancer. 2001;93:212–217. doi: 10.1002/ijc.1318. [DOI] [PubMed] [Google Scholar]
- 126.Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Steeg PS. Perspective: The right trials. Nature. 2012;485:S58–S59. doi: 10.1038/485S58a. [DOI] [PubMed] [Google Scholar]