Abstract
Primary malignant melanoma arising from the eustachian tube is extremely rare. We report the case of a 63-year-old white man who presented with a 1-month history of left-sided hearing loss and aural fullness. Flexible fiberoptic laryngoscopy detected a blue-purple mass that appeared to arise from the left lateral nasopharynx. Computed tomography demonstrated an enhancing mass arising from an orifice of the left eustachian tube. The tumor was debulked endoscopically and was confirmed to have originated in the left eustachian tube. Histologically, the tumor was made up of heavily pigmented pleomorphic spindle cells with frequent mitoses. The tumor cells were immunohistochemically positive for S-100 protein, HMB-45, Melan-A, and PNL-2. The final diagnosis was a mucosal malignant melanoma. We also performed a nested polymerase chain reaction assay for several genes of interest, including CTLA-4, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, IL-17F, PLZF, Foxp3, RORγt, CD27, and CD70. These genes have been studied mainly in cutaneous melanomas, especially for the development of immunotherapy. But only very limited studies have been done on mucosal melanomas. Our investigation found upregulation of CTLA-4, IL-17A, IL-17C, and IL-17E. Based on our finding of CTLA-4 upregulation, it may be suggested that our patient may have had low antitumor immunity and that he might have benefitted from CTLA-4 blockade. On the other hand, upregulation of IL-17A and IL-17E might reflect increased antitumor immunity, which could suggest that patients with a mucosal melanoma might benefit from immunomodulators associated with the effect of Th17. These genes also have great potential to help melanoma patients obtain tailored treatment, and they can be used as biomarkers for predicting prognosis.
Introduction
Mucosal melanomas represent 1.3% of all cutaneous and noncutaneous melanomas. Approximately 55% of all mucosal melanomas are located in the head and neck region.1 The most common site of origin in the head and neck is the nose and paranasal sinuses, followed by the oral cavity.2
Nasopharyngeal mucosal melanomas are rare. To the best of our knowledge, only 5 cases of primary mucosal melanoma arising in the eustachian tube have been published in the literature (table).3-6 In this report, we describe a new case in a patient who presented with hearing loss. A pigmented mass was found in the left eustachian tube on laryngoscopy. The mass was excised, and histology identified it as a primary malignant melanoma. Abnormal gene expression in CTLA-4 and IL-17 has recently been discovered in cutaneous melanoma, which has led to the development of immunotherapy with IL-2, IL-23, and CTLA-4 blockade.7,8 In the area of mucosal melanoma, gene-expression studies have been very limited.
Table.
Summary of reported cases of primary mucosal melanoma of the eustachian tube
| Author | Age/sex | Side | Positive immunostain | Treatment |
|---|---|---|---|---|
| Lai et al,3 2001 | 58/F | Right | HMB-45 | Surgery |
| Racic et al,4 2004 | 74/F | Left | S-100, HMB-45 | Chemotherapy |
| Baek et al,5 2006 | 62/F | Right | S-100, HMB-45 | Radiation therapy |
| Yang et al,6 2009 | 75/M 35/F |
Left Left |
S-100, HMB-45, vimentin S-100, HMB-45 |
Surgery No data available |
| Wang et al,* 2013 | 63/M | Left | S-100, HMB-45, Melan-A, PNL-2 | Endoscopic surgery plus radiation therapy |
Present case.
Case report
A 63-year-old white man presented to our ENT clinic with a 1-month history of left-sided hearing loss and aural fullness. He denied bleeding, dysphagia, odynophagia, weight loss, and nasal obstruction at the time of presentation, and his medical history was unremarkable. Otoscopic examination detected a violaceous effusion in the left middle ear. Flexible fiberoptic laryngoscopy revealed the presence of a smooth, blue-purple mass that appeared to arise from the left lateral nasopharynx. Physical examination did not find any other lesions throughout the body.
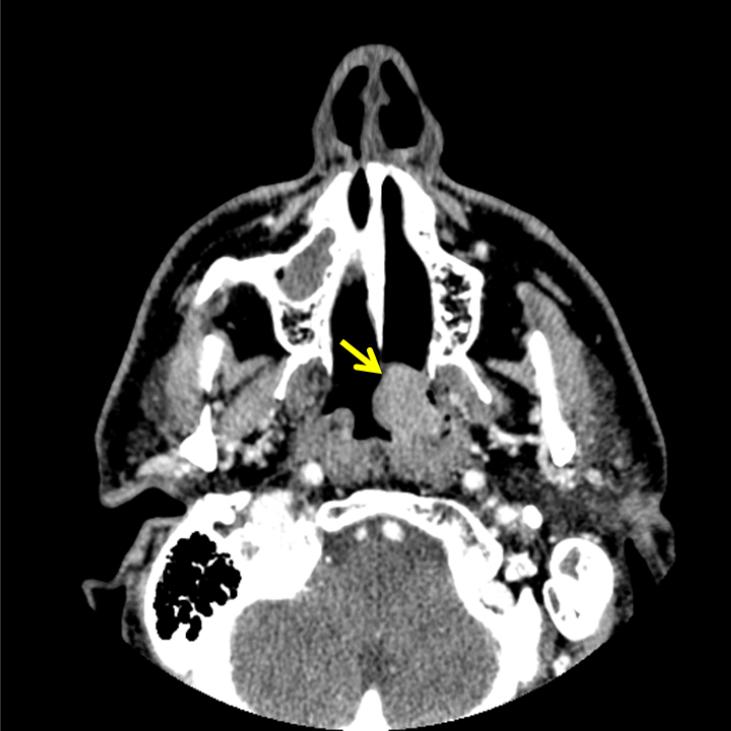
Computed tomography (CT) demonstrated an enhancing polypoid lesion that measured 2.2 × 1.8 × 1.7 cm (figure 1). The lesion had arisen from the mucosa of the left eustachian tube orifice, and it extended into the left choana and barely crossed the midline. The lesion was contiguous with the torus tubarius, and it had effaced the fossa of Rosenmüller. The lesion extended superiorly to the roof of the nasopharynx and inferiorly to the level of the left tonsillar pillar. Positron emission tomography (PET) revealed increased hypermetabolic activity centered in the left lateral nasopharynx, which corresponded to the soft-tissue lesion seen on CT. The standard uptake value (SUV) on PET ranged from 9.5 to 12.2 medially across the midline. The tumor was staged as a localized lesion with no distant metastasis.
Figure 1.
Contrast-enhanced CT shows the enhancing polypoid mass (arrow) involving the left lateral nasopharyngeal wall.
The patient refused surgery for 5 months. He eventually consented to have the lesion excised when he began to experience left-sided nasal obstruction and several episodes of epistaxis. The tumor was debulked endoscopically. Intraoperatively, it was found to have originated in the left eustachian tube. A myringotomy was performed, and a tympanostomy tube was placed. The patient experienced no immediate complications, and he was discharged on the same day.
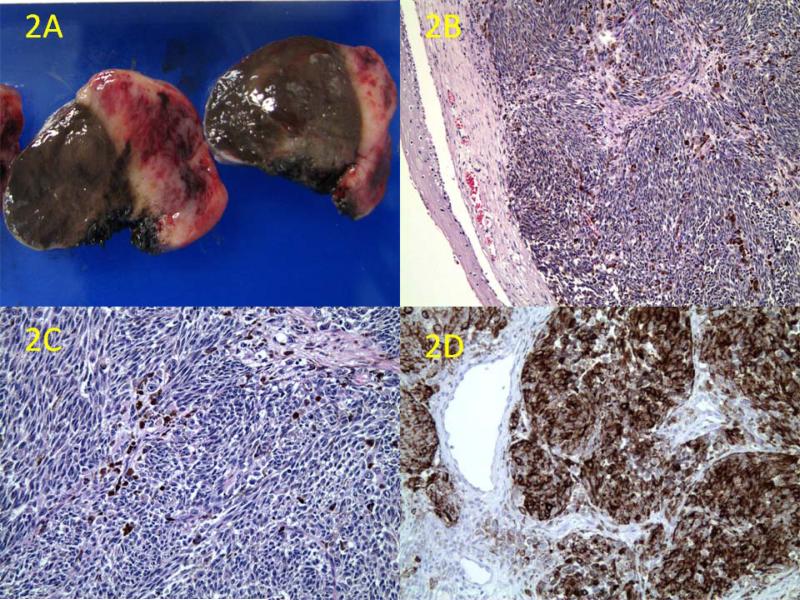
Gross examination of the excised specimen revealed a dark-brown, polypoid, 2.5 × 1.5 × 1.5-cm mucosal mass that was soft and dome-shaped. Cross-sectioning revealed pigmentation with hemorrhagic foci (figure 2, A). The entire specimen was submitted for formalin fixation and paraffin-embedded routine histology. Sections were stained with H&E and immunohistochemistry by standard avidin-biotin immunoperoxidase complex testing (ABC Kit; Dako Corp.; Carpinteria, Calif.). Microscopic examination demonstrated a squamous mucosa with atypical melanocytes (figure 2, B) and a subepithelial hypercellular mass with pleomorphic and heavily pigmented spindle-shaped cells (figure 2, C). Frequent mitoses were identified, and areas of angiolymphatic invasion were noted. Confirmatory immunohistochemical studies revealed that the tumor cells were diffusely positive for S-100 protein, HMB-45 (figure 2, D), Melan-A, and PNL-2. The final diagnosis was a mucosal malignant melanoma.
Figure 2.
A: Cross-section examination of the tumor reveals dark-brown pigmentation in the mucosa and tumor parenchyma with areas of hemorrhage and necrosis. B: Microscopic section shows the hypercellular tumor partially covered by squamous mucosa, along with areas of pigmentation (H&E, original magnification ×50). C: The tumor is composed of heavily pigmented pleomorphic spindle cells with mitotic figures (H&E, original magnification ×100). D: Immunohistochemistry shows diffuse positivity for HMB-45 (original magnification ×100)
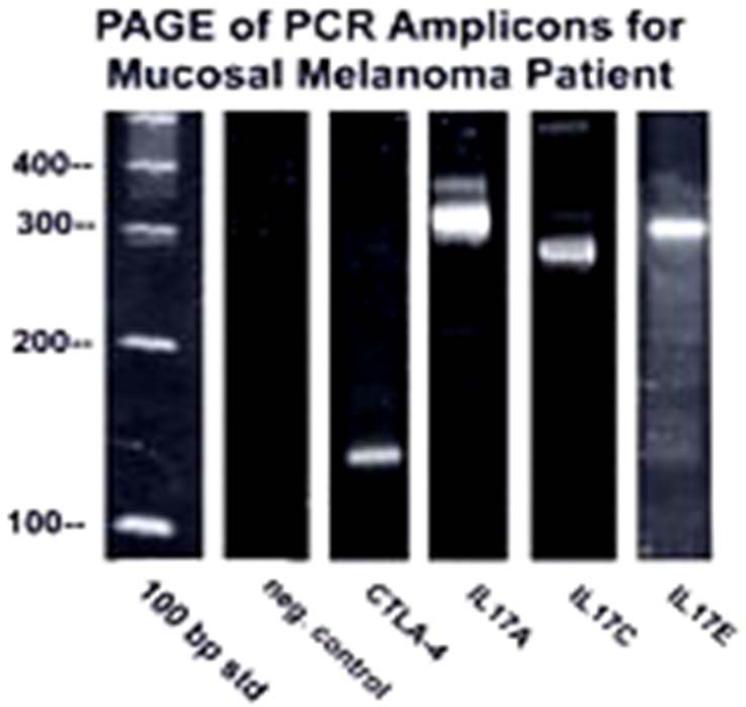
Formalin-fixed paraffin tumor tissue was analyzed for the genes CTLA-4, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, IL-17F, PLZF, Foxp3, RORγt, CD27, and CD70. These genes were selected because they are applicable for (delete in) immunomodulators used to treat advanced melanoma. RNA was extracted with TRIzol after deparaffinization with xylene, and first-strand cDNA synthesis was performed. Polymerase chain reaction (PCR) testing for hGAPDH was used as an internal control to normalize samples. Nested PCR testing for these genes was performed. Agarose gel electrophoresis of the PCR amplicons showed that CTLA-4, IL-17A, IL-17C, and IL-17E were upregulated (figure 3).
Figure 3.
Image shows the agarose gel electrophoresis of nested PCR amplicons. Appropriately sized bands were detected for CTLA-4, IL-17A, IL-17C, and IL-17E. No detectable bands were found for IL-17B, IL-17D, IL-17F, PLZF, Foxp3, RORγt, CD27, CD70, and the negative control (no template DNA). The 100-bp ladder is shown in the left lane.
Postoperatively, the patient underwent a 3-month course of radiation therapy without any major complications, although he did experience left-sided otorrhea and a mild decrease in hearing. At 8 months of follow-up, he exhibited no evidence of recurrence or metastasis. Seven months later, however, imaging detected a lesion on his liver, which was treated with chemotherapy. He subsequently experienced a local recurrence of the nasopharyngeal mucosal melanoma, but at the most recent follow-up, he was alive. (Checked his record that lost of follow up the patient since 6/5/08. Therefore, we know he survived for at least 8 months after operation.)
Discussion
Mucosal melanoma represents 1.7% of all melanoma cases and 6.3% of all head and neck melanoma cases.9 Mucosal melanomas can be found throughout the sinonasal area, including the turbinates, maxillae, ethmoid sinuses, nasal walls, septa, cribriform plates, and premaxillary area.2 Most patients with mucosal melanomas are between 50 and 80 years of age. The only documented racial predisposition is that oral mucosa melanomas occur more frequently in blacks.10 Patients with a sinonasal mucosal melanoma usually present with nasal obstruction and/or epistaxis.10
Of the 5 previously reported cases of a primary mucosal melanoma arising from the eustachian tube (table),3-6 2 represented incidental findings. Of the other 3 cases, 2 patients presented with epistaxis, and 1 presented with aural fullness and tinnitus. It interesting that 4 of the 5 patients were Asians; this finding is especially striking because studies of mucosal melanomas of the head and neck have not demonstrated any racial predilection previously. In view of the rarity of mucosal melanoma of the eustachian tube, it is difficult to conclude whether or not there is a true correlation between mucosal melanoma at this particular site and Asian heritage. Our patient was white.
Mucosal melanomas may be macular, ulcerated, or nodular, and their coloring varies greatly; white, dark-brown, violaceous, and pink lesions have been seen. The cellular morphology is also variable, ranging from epithelioid to plasmacytoid to spindly. Immunohistochemistry with S-100 protein, HMB-45, Melan-A, PNL-2, and MITF can aid in making a definitive diagnosis of mucosal melanoma.
The etiology of mucosal melanoma is still unclear. Several risk factors are thought to be related to malignant melanoma, including exposure to sunlight, human papillomavirus, chronic irritants, and carcinogenic compounds.2 Some recent studies have found that the pathogenesis of malignant melanomas may be related to an altered immune system. Several genes have been studied in cutaneous melanoma, including CTLA-4, IL-17, Foxp3, and RORγt. These genes are of interest for immunomodulator development.7 Most immunomodulators, including high-dose INF-α2b, are still in clinical trials, mostly in phase I/II. But because of the rarity of mucosal melanomas, such studies have not been examined.
The Wnt/β-catenin pathway is implicated in various cellular activities, including determination, proliferation, migration, and differentiation.11 CTLA-4 has been observed in human epidermal melanocytes and in patient-derived primary melanoma,12 as well as in activated T cells and a subset of regulatory T cells.13,14 Our group's studies have yielded the interesting finding that the tumorigenesis of melanoma has been linked to upregulation of the Wnt/β-catenin pathway. 11 The most highly induced gene of this pathway is CTLA-4. Therefore, blocking CTLA-4 in patients with mucosal melanoma may represent an alternate form of treatment for unresectable mucosal melanomas. Recent research has shown that CTLA-4 blockade increases the number of Th17 cells in patients with metastatic melanoma.15
Th17 is a subset of CD4 cells, which produce IL-17. It has been implicated in an array of autoimmune disorders, including rheumatoid arthritis and psoriasis. But the nature and regulation of Th17 in the context of tumor immunity remains unclear. At least six members of the IL-17 family have been identified. IL-17A and IL-17C have been shown to have the ability to induce mRNA expression of inflammatory cytokines, including IL-1β, IL-6, and IL-23.16 This finding is promising because IL-23 has recently been found to have a possible role as a cancer vaccine adjuvant for melanoma. Its antitumor effect has been well established in murine melanoma.17 IL-17E is a potent inflammatory cytokine that has the ability to activate NF-κβ, which can induce several signaling pathways. The balance of regulatory T cells and Th17 cells in the tumor microenvironment may influence antitumor immunity. It has been speculated that regulatory T cells may inhibit the function of tumor-infiltrating effector T cells. Therefore, a medication that can suppress regulatory T cells at the optimal level may hold promise as a therapeutic option.
Our patient exhibited increased CTLA-4, IL-17A, IL-17C, and IL-17E expression in his mucosal melanoma. Based upon CTLA-4 upregulation, it may be suggested that our patient might respond well to CTLA-4 blockade. On the other hand, upregulated IL-17A and IL-17E might reflect similar results seen in patients treated with IL-2 or INF-α. This could be interpreted as an increase in gene expression of Th17, a good marker of antitumor immunity. Despite CTLA-4 upregulation, our patient might have benefitted from an immunomodulator that can potentiate the effect of Th17. These findings should encourage more exploration. These genes have great potential to be used as biomarkers for predicting prognosis and optimizing treatment in the future.
In the meantime, surgery remains the mainstay of treatment for mucosal melanoma of the head and neck, although wide resection can be difficult in some cases because of the tumor's location. The role of postoperative radiation therapy remains controversial. A recent analysis of mucosal melanoma of the head and neck found that 3-year local control was significantly better with postoperative radiotherapy than with surgery alone.2
Immunotherapy has been one of the major treatment options for patients with advanced melanoma. IL-2 is one of a few drugs that has been approved by the Food and Drug Administration as an adjuvant treatment for melanoma. Several other immunomodulators, including CTLA-4 blockers and IL-21, are in phase I to III clinical trials. Essentially, these agents stimulate the immune system to attack the neoplastic cells.
In summary, we report a case of a primary malignant melanoma arising from the eustachian tube with CTLA-4, IL-17A, IL-17C, and IL-17E upregulation. This gene expression may lead us to a better way of tailoring treatment and help patients with advanced melanoma receive suitable immunotherapy. Additionally, this gene expression may allow us to better predict prognosis. Long-term follow-up and a larger number of cases are required for better understanding of the functions of these genes.
Acknowledgments
This research was supported in part by the grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health.
References
- 1.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M, Morris CG, Werning JW, Mendenhall WM. Mucosal melanoma of the head and neck. Am J Clin Oncol. 2008;31(1):43–8. doi: 10.1097/COC.0b013e318134ee88. [DOI] [PubMed] [Google Scholar]
- 3.Lai CC, Tsay SH, Ho CY. Malignant melanoma of the eustachian tube. J Laryngol Otol. 2001;115(7):567–9. doi: 10.1258/0022215011908243. [DOI] [PubMed] [Google Scholar]
- 4.Racic G, Kurtovic D, Roje Z, et al. Primary mucosal melanoma of the eustachian tube. Eur Arch Otorhinolaryngol. 2004;261(3):139–42. doi: 10.1007/s00405-003-0633-8. [DOI] [PubMed] [Google Scholar]
- 5.Baek SJ, Song MH, Lim BJ, Lee WS. Mucosal melanoma arising in the eustachian tube. J Laryngol Otol. 2006;120(3):E17. doi: 10.1017/s0022215106004762. [DOI] [PubMed] [Google Scholar]
- 6.Yang BT, Wang ZC, Xian JF, Chen QH. MR imaging features of primary mucosal melanoma of the eustachian tube: Report of 2 cases. AJNR Am J Neuroradiol. 2009;30(2):431–3. doi: 10.3174/ajnr.A1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Edington HD, Rao UN, et al. Effects of high-dose IFNα2b on regional lymph node metastases of human melanoma: Modulation of STAT5, FOXP3, and IL-17. Clin Cancer Res. 2008;14(24):8314–20. doi: 10.1158/1078-0432.CCR-08-0705. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytologic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–17. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore ES, Martin H. Melanoma of the upper respiratory tract and oral cavity. Cancer. 1955;8(6):1167–76. doi: 10.1002/1097-0142(1955)8:6<1167::aid-cncr2820080613>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Tomicic J, Wanebo HJ. Mucosal melanomas. Surg Clin North Am. 2003;83(2):237–52. doi: 10.1016/S0039-6109(02)00100-7. [DOI] [PubMed] [Google Scholar]
- 11.Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–42. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 12.Shah KV, Chien AJ, Yee C, Moon RT. CTLA-4 is a direct target of Wnt/β-catenin signaling and is expressed in human melanoma tumors. J Invest Dermatol. 2008;128(12):2870–9. doi: 10.1038/jid.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wing K, Onshi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh B, O'Brien S, Lee D, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Euw E, Chodon T, Attar N, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi Y, Fujio K, Shoda H, et al. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179(10):7128–36. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 17.Overwijk WW, de Visser KE, Tirion FH, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176(9):5213–22. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]