Abstract
Selective inhibition of vascular endothelial growth factor (VEGF) increases the efficacy of chemotherapy and has beneficial effects on multiple advanced cancers, but response is often limited and the disease eventually progresses. Changes in the tumour microenvironment — hypoxia among them — that result from vascular pruning, suppressed angiogenesis and other consequences of VEGF inhibition can promote escape and tumour progression. New therapeutic approaches that target pathways that are involved in the escape mechanisms add the benefits of blocking tumour progression to those of slowing tumour growth by inhibiting angiogenesis.
Most of the angiogenesis inhibitors that are currently used for the treatment of cancer achieve their effects by blocking vascular endothelial growth factor (VEGF), a cytokine that promotes blood vessel growth and survival1-3. Inhibitors of VEGF not only stop angiogenesis and destroy part of the tumour vasculature, but they also normalize some tumour vessels3-5.
Through rapid and robust effects on the tumour vasculature, angiogenesis inhibitors slow the growth of many primary tumours and metastases, and selective VEGF blockade increases the efficacy of certain types increases the efficacy of certain types of chemotherapy6,7. Clinical benefit is reflected by lengthening of progression-free survival in advanced colorectal, lung, renal, pancreatic neuroendocrine and ovarian cancer, and by longer overall survival in metastatic colorectal and renal cancer. Although the clinical benefit is not usually sustained, and is small or absent in some types of cancer, these limitations are not unique to angiogenesis inhibitors. Many cancer therapies have modest effects on overall survival. Improved overall survival was found in only 12% of 73 randomized Phase III trials of bevacizumab, trastuzumab and other targeted therapies, as well as a range of chemotherapeutic agents for metastatic breast cancer over the past 30 years8.
Experience shows that most advanced cancers can escape from therapy. When a VEGF inhibitor is combined with chemotherapy or radiation, escape can be from one or both. Preclinical studies raise the additional possibility that VEGF signalling inhibitors that slow tumour growth can also promote tumour escape and progression9.
Multiple strategies for preventing escape are being developed and evaluated in the laboratory and in clinical trials. This Opinion article explores the rationale, evidence and potential strategies for treating advanced cancers by targeting angiogenesis concurrently with mechanisms of tumour progression.
Benefits and limitations
Hundreds of thousands of patients worldwide are being treated with angiogenesis inhibitors for cancer. Angiogenesis inhibitors have been approved for a wide range of cancer types, including hepatocellular carcinoma and renal cell carcinoma that respond poorly to other agents. Bevacizumab, a function-blocking antibody to VEGF, is approved for use with chemotherapy to treat metastatic colorectal cancer and non-small-cell lung cancer; with interferon-α to treat metastatic renal cell cancer; and as a single agent for recurrent glioblastoma (see the Genentech website; see Further information) (TABLE 1). Bevacizumab with chemotherapy significantly prolongs overall survival as first-line treatment for metastatic colorectal cancer10. Ziv-aflibercept, a recombinant fusion protein that as a decoy VEGF receptor (VEGFR) binds VEGFA, VEGFB and placental growth factor (PLGF; also known as PGF), is approved for use with chemotherapy to treat metastatic colorectal cancer (see the Regeneron website; see Further information) (TABLE 1).
Table 1.
Angiogenesis inhibitors currently approved for use in cancer patients
| Angiogenesis inhibitor |
Developer | Type of inhibitor | Targets | Use | Indication (approval region*) |
|---|---|---|---|---|---|
| Bevacizumab (Avastin) |
Genentech/ Roche |
Monoclonal antibody | VEGFA | First or second line |
Metastatic colorectal cancer (United States and Europe) |
| First line | Metastatic non-small-cell lung cancer (United States and Europe) |
||||
| First line | Metastatic renal cell cancer (United States and Europe) |
||||
| First line | Recurrent glioblastoma (United States) | ||||
| First line | Metastatic breast cancer (Europe) | ||||
| First line | Advanced ovarian cancer (Europe) | ||||
| Ziv-aflibercept (Zaltrap, VEGF Trap) |
Regeneron/ Sanofi |
Recombinant fusion protein acting as a soluble decoy receptor |
VEGFA, VEGFB and PLGF |
Second line | Metastatic colorectal cancer (United States) |
| Sorafenib (Nexavar, Bay 43–9006) |
Bayer | Tyrosine kinase inhibitor | VEGFR2, VEGFR3, PDGFRβ, FGFR1, KIT and RAF |
First line | Advanced renal cell carcinoma (United States and Europe) |
| First line | Unresectable hepatocellular carcinoma (United States and Europe) |
||||
| Sunitinib (Sutent, SU11248) |
Pfizer | Tyrosine kinase inhibitor | VEGFRs, PDGFRs, FLT3, KIT and RET |
First line | Advanced renal cell carcinoma (United States and Europe) |
| Second line | Gastrointestinal stromal tumour (United States and Europe) |
||||
| First line | Unresectable pancreatic neuroendocrine tumours with locally advanced or metastatic disease (United States and Europe) |
||||
| Axitinib (Inlyta, AG-013736) |
Pfizer | Tyrosine kinase inhibitor | VEGFRs | Second line | Advanced renal cell carcinoma (United States and Europe‡) |
| Pazopanib (Votrient, GW786034B) |
GlaxoSmithKline | Tyrosine kinase inhibitor | VEGFRs, PDGFRs and KIT |
First line | Renal cell carcinoma (United States and Europe) |
| Second line | Advanced soft tissue sarcoma (United States and Europe‡) |
||||
| Vandetanib (Caprelsa, ZD6474) |
AstraZeneca | Tyrosine kinase inhibitor | VEGFRs, EGFR and RET |
First line | Late-stage medullary thyroid cancer (United States and Europe) |
| Everolimus (Afinitor, RAD001) |
Novartis | Rapamycin derivative inhibitor of mTORC1 |
mTOR | First line | Advanced pancreatic neuroendocrine tumours (United States and Europe) |
| First line | Subependymal giant cell astrocytoma with tuberous sclerosis (United States and Europe) |
||||
| First line | Renal angiomyolipoma associated with tuberous sclerosis complex (United States) |
||||
| Second line | Advanced HER2-negative breast cancer (United States and Europe) |
||||
| Second line | Advanced renal cell carcinoma (United States and Europe) |
EGFR, epidermal growth factor receptor; FGFR1, fibroblast growth factor receptor 1; FLT3, fms-related tyrosine kinase 3; mTORCl, mTOR complex 1; PDGFR, platelet-derived growth factor receptor; PLGF, placental growth factor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Europe refers to approval by the European Medicines Agency (EMA); United States refers to approval by the US Food and Drug Administration (FDA).
A positive opinion was received in May 2012 for the use in this indication from the Committee for Medicinal Products for Human Use (CHMP) of the EMA but final approval has not yet been granted.
Multiple other angiogenesis inhibitors that are approved for cancer therapy (such as sorafenib, sunitinib, axitinib, pazopanib and vandetanib) inhibit VEGF signalling by targeting receptor tyrosine kinases11,12 or reduce VEGF production by blocking the mTOR pathway (for example, everolimus and temsirolimus)13-15 (TABLE 1). Rapamycin analogues that block both mTOR complex 1 (mTORC1) and mTORC2 — OSI-027 being an example16 — have a more potent antiangiogenic action in preclinical models than agents that block only mTORC1 (REFS 15,17).
The responses to VEGF inhibition that are found in metastatic colorectal or lung cancer are not matched in metastatic breast cancer, for which the addition of bevacizumab to chemotherapy extends progression-free survival by a few months but does not increase overall survival18-22. However, although the patient population as a whole has a limited benefit from this treatment, some patients have striking responses and live significantly longer. These exceptions highlight the importance of finding biomarkers that predict response23-29.
The search for biomarkers (BOX 1) is providing insight not only into approaches for identifying patients who will respond favourably but also into mechanisms of escape in patients who respond initially but then progress during treatment27,30. As an example, a single nucleotide polymorphism (SNP) in the tyrosine kinase domain of the VEGFR1 gene in genomic DNA was found to be significantly correlated with progression-free survival and overall survival in bevacizumab-treated patients with metastatic pancreatic cancer in the Avastin and Tarceva (AViTA) trial29. Bevacizumab-treated patients with the AA genotype — but not placebo-treated patients with this genotype — lived longer than those with AC or CC genotypes, and also longer than the entire cohort of bevacizumab-treated patients undivided by genotype.
Box 1. Biomarkers to predict response to angiogenesis inhibitors.
Functional imaging
Although no biomarker currently available is uniformly predictive of clinical response to inhibition of vascular endothelial growth factor (VEGF) signalling, changes in tumour vascular perfusion and leakage, as surrogate indices of bevacizumab efficacy, can be monitored by dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI)174, and changes in proliferation, metabolism and hypoxia can be assessed by positron emission tomography (PET)175-177.
Hypertension
Treatment-associated hypertension, which results from the suppression of VEGF-mediated vasodilatation, is a surrogate marker of VEGF signalling inhibition that is predictive of survival benefit in some trials but not in others27,178-180.
Circulating proteins
Baseline plasma VEGF concentration is higher in many patients who respond to bevacizumab28. Plasma levels of short VEGF isoforms, VEGF110 and VEGF121, can be particularly informative28. Baseline plasma levels or treatment-induced changes in placental growth factor (PLGF), soluble VEGF receptor 2 (VEGFR2) and multiple other factors can predict response or signal progression of some tumours73,74,181-185.
Circulating cells
The relationship of circulating endothelial cells or tumour cells to therapeutic response has not been consistent in clinical trials74,186-188. One challenge is identifying small numbers of cells in the blood, but more sensitive methods are being developed, and mechanistic insights are coming from preclinical studies171.
Polymorphisms
Single nucleotide polymorphisms (SNPs) in genes that are relevant to VEGF signalling can be predictive of response to bevacizumab in some cancers29.
Tumour biomarkers
Tumour vascularity, VEGF pathway components, and markers of tumour cells, endothelial cells and inflammatory cells can help in assessing the response to inhibitors of VEGF signalling. One dose of bevacizumab reduces CD31-positive tumour vessels and DCE-MRI indices of tumour vascularity and leakiness, and increases tumour cell apoptosis in inflammatory breast cancer189,190. Partial responses to bevacizumab plus chemotherapy correlate with the abundance of CD31-positive tumour vessels at baseline190, and overall survival correlates with the baseline presence of tumour cells that are p53-negative and that have low apoptosis rates191. Tumour neuropilin 1 immunoreactivity has prognostic value in gastric cancer28, and plasma levels of intercellular adhesion molecule 1 (ICAM1) correlate with clinical outcome in non-small-cell lung cancer23.
Angiogenesis inhibitors are approved for cancer therapy in many countries. The approved indications are generally the same but can differ in some cases (TABLE 1). Bevacizumab has received approval for use in the treatment of metastatic breast cancer and metastatic ovarian cancer by the European Medicines Agency (EMA) but not by the US Food and Drug Administration (FDA), and for recurrent glioblastoma by the FDA but not by the EMA. Regulatory agencies can have different views of whether the beneficial effects of angiogenesis inhibitors are sufficient in some types of cancer to balance the risk and expense. For example, the EMA did not follow the recent FDA decision to revoke the approval of bevacizumab plus paclitaxel for metastatic breast cancer.
Overcoming resistance
Disease progression, as reflected by tumour growth and metastasis during treatment with inhibitors of VEGF signalling, is attributed to multiple interacting mechanisms (BOX 2). Among them are compensatory actions of angiogenic growth factors that are not blocked by inhibitors of VEGF signalling, blood flow alterations owing to tumour-vessel pruning and normalization, co-option of normal peritumoural blood vessels, exaggeration of intratumoural hypoxia, activation of pathways that favour epithelial–mesenchymal transition (EMT), promotion of tumour invasiveness, suppression of immune surveillance, induction of tolerance and activation of cancer stem cells9,25,30,31.
Box 2. Putative mechanisms of resistance to angiogenesis inhibitors.
Actions of angiogenic growth factors other than vascular endothelial growth factor (VEGF)
Blood flow alterations owing to vessel pruning and normalization
Co-option of normal peritumoural blood vessels
Exaggeration of intratumoural hypoxia
Activation of pathways that favour epithelial-mesenchymal transition (EMT)
Promotion of tumour invasiveness
Suppression of immune surveillance and induction of tolerance
Activation of cancer stem cells
Changes in dominant VEGF isoform VEGF121, VEGF165 or VEGF189
Changes in neuropilin 1 as a co-receptor of VEGF receptor (VEGFR)
Loss of endothelial cell VEGF dependence resulting from VEGFR2 downregulation
Under-dosing owing to more rapid clearance of small-molecule tyrosine kinase inhibitors
Tyrosine kinase inhibitor inactivation by uptake and lysosomal sequestration in tumour cells
Other proposed mechanisms that contribute to resistance include changes in the dominant VEGF isoform (VEGF121, VEGF165 or VEGF189) or in neuropilin 1 as a VEGFR co-receptor28, and loss of endothelial cell VEGF dependence from downregulation of VEGFR2 (REFS 32,33). For small-molecule receptor tyrosine kinase inhibitors, additional mechanisms include under-dosing owing to increasingly rapid clearance of the inhibitor34, and inhibitor inactivation by uptake and lysosomal sequestration in tumour cells35.
Disease progression during treatment with bevacizumab or ziv-aflibercept paired with chemotherapy does not necessarily mean that the inhibitor has lost efficacy. The resistance could be to the chemotherapy36. Evidence of better overall survival in metastatic colorectal cancer, when bevacizumab is continued beyond progression in the presence of diverse types of chemotherapy, reflects the continued involvement of VEGF37,38.
Preventing, decreasing or reversing resistance are major unmet needs in cancer therapy and are necessary steps towards reducing morbidity and mortality39. This is a serious challenge because of the complex underlying biology and patient diversity. Fortunately, advances in understanding the effects of angiogenesis inhibitors on blood vessels and tumour cells and the mechanisms of tumour invasion and metastasis have led to new treatment strategies. One approach is to target tumour angiogenesis and progression together (FIG. 1).
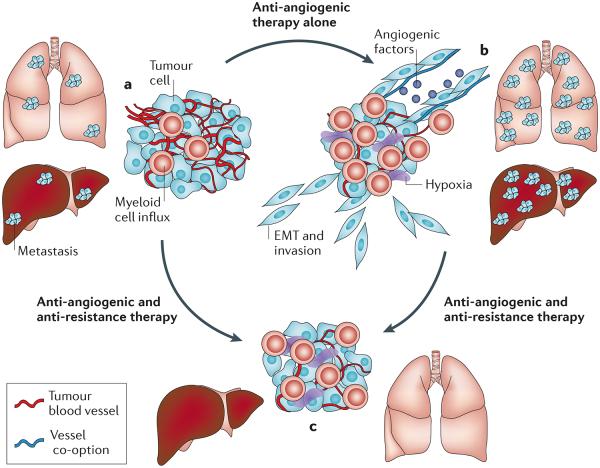
Figure 1. Overcoming resistance to inhibitors of VEGF signalling by blocking angiogenesis and tumour progression.
A schematic representation of the development and resolution of resistance to angiogenesis inhibitors, based on evidence from preclinical studies, is shown. a | Before treatment with an angiogenesis inhibitor the tumour is highly vascular, rapidly growing and accompanied by metastases. b | After treatment with an inhibitor of vascular endothelial growth factor (VEGF) signalling, the main tumour is smaller and less vascular but is more hypoxic, has more myeloid cells and is more invasive. Tumour growth continues without angiogenesis by co-option of normal blood vessels. Tumour cells undergo epithelial–mesenchymal transition (EMT), become more mesenchymal, invade surrounding tissues and metastasize. c | After inhibition of the pathways involved in tumour progression together with VEGF signalling, the tumour is smaller, has a ball-like shape, is less invasive and has no metastases.
Contribution of chemotherapy
Chemotherapy used together with angiogenesis inhibitors can contribute to resistance and can potentially be used to overcome resistance36. Chemotherapy and anti-VEGF therapy have complex — and not simply additive — actions when combined. The value of using bevacizumab with chemotherapy is well documented40, but its benefit depends on the type, dose and the treatment schedule of the chemotherapeutic agent or drug combination, as well as on the tumour type40,41. Ziv-aflibercept is also more effective when combined with chemotherapy42.
Outcome is better when bevacizumab is paired with uninterrupted, low-dose (metronomic) chemotherapy, both in preclinical models and in some clinical settings40,43-45. The underlying mechanism of the added benefit is currently under debate, but metronomic administration of a topoisomerase I (TOP1) inhibitor or anthracycline chemotherapy as a single agent inhibits hypoxia-inducible factor 1α (HIF1α)46,47. HIF1α blockade could offset the hypoxic effects of vascular pruning when paired with an angiogenesis inhibitor48.
Unlike bevacizumab and ziv-aflibercept, small-molecule tyrosine kinase inhibitors of angiogenesis are not so effectively paired with chemotherapy. Most clinical trials of sunitinib, axitinib or sorafenib have found dose-limiting toxicities or no further benefit when the agent was combined with chemotherapy49-54.
Inhibition of multiple angiogenic factors
Compensatory actions of multiple growth factors can contribute to escape by promoting angiogenesis in the presence of inhibitors that block VEGF signalling. Angiopoietins, angiopoietin 1 (ANG1) and ANG2, PLGF, fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) family members are among those linked to escape from the effects of inhibition of VEGF signalling55-60. VEGFC and VEGFD, which activate signalling of VEGFR3 and VEGFR2, have been implicated in resistance, and the inhibition of both pathways together has been proposed as a solution61-63. In addition to factors that drive angiogenesis directly64-66, some released substances function by recruiting myeloid cells and macrophages that release angiogenic and other factors67. Inhibition of FGF receptor signalling or ANG2 can increase the efficacy of VEGF signalling inhibitors in preclinical tumour models55,68.
Function-blocking antibodies to PLGF reduce tumour angiogenesis, growth and metastasis, and can render some preclinical tumour models more responsive to inhibitors of VEGF signalling69,70. Other evidence raises questions about the anti-angiogenic potency of PLGF-specific antibodies in tumours but supports the anti-metastatic effects of PLGF blockade71. Inhibition of PLGF does not seem to exaggerate intratumoural hypoxia.
Plasma PLGF levels increase in patients with colorectal cancer, renal cell cancer or glioblastoma after treatment with an inhibitor of VEGF signalling72-74. The fusion protein sFLT01, which sequesters both VEGF and PLGF, slows tumour growth and prolongs survival in mice, but circulating PLGF levels remain elevated, raising the question of whether tumour progression could eventually be promoted75. The antibody RO5323441 against human PLGF in combination with bevacizumab for recurrent glioblastoma or hepatocellular carcinoma had an acceptable tolerability in an early clinical trial, but it is unclear whether PLGF blockade adds to the benefit of blocking VEGF signalling (TABLE 2). Whether an anti-PLGF strategy is an effective treatment option for cancers that are refractory to VEGF inhibition is still to be determined.
Table 2.
Clinical trials of agents targeting VEGF signalling in combination with other targeted agents
| ClinicalTrials.gov identifier | Combination strategy | Stage | Cancer type |
|---|---|---|---|
| NCT01496742 | Bevacizumab plus MET-specific antibody onartuzumab (MetMAb) and standard therapies |
Phase II | Non-small-cell lung cancer |
| NCT01186991 | Bevacizumab plus MET-specific antibody onartuzumab (MetMAb) and paclitaxel |
Phase II | Metastatic triple-negative breast cancer |
| NCT01418222 | Bevacizumab plus MET-specific antibody onartuzumab (MetMAb) and standard therapies |
Phase II | Metastatic colorectal cancer |
| NCT01339039 | Bevacizumab plus CXCR4 inhibitor plerixafor (mozobil and AMD3100) |
Phase I | Recurrent high-grade glioma |
| NCT01251926 | Bevacizumab plus topoisomerase I inhibitor EZN-2208 (pegylated SN-38) |
Phase I | Refractory solid malignancies |
| NCT00811993 | Bevacizumab plus IGF1R inhibitor R1507 and standard therapies | Phase I | Advanced solid malignancies |
| NCT01308684 | Bevacizumab plus PLGF-specific antibody RO5323441 (TB-403) | Phase I | Recurrent high-grade glioma |
| NCT01605227 | Cabozantinib (XL184, inhibitor of VEGFR, MET, AXL and other kinases) versus prednisone (COMET-1 trial) |
Phase III | Castration-resistant prostate cancer metastatic to bone |
| NCT01522443 | Cabozantinib (XL184, inhibitor of VEGFR, MET, AXL and other kinases) versus mitoxantrone and prednisone (COMET-2 trial) |
Phase III | Castration-resistant prostate cancer metastatic to bone |
| NCT00726323 | Foretinib (GSK1363089, XL880, inhibitor of VEGFR, MET and other kinases) |
Phase II | Papillary renal cell carcinoma |
| NCT01468922 | Pazopanib (inhibitor of VEGFRs, PDGFRs and other kinases) and tivantinib (ARQ 197, inhibitor of MET) |
Phase IB | Refractory advanced solid tumours |
CXCR4, chemokine (C-X-C motif) receptor 4; IGF1R, insulin-like growth factor 1 receptor; PDGFR, platelet-derived growth factor receptor; PLGF, placental growth factor; VEGFR, vascular endothelial growth factor receptor.
Inhibition of PLGF, ANG2 or FGF along with VEGF could overcome some aspects of resistance to VEGF blockade, but the diversity of other growth factors that can drive angiogenesis and promote escape raises questions about the durability of this approach.
Destabilization of resistant tumour blood vessels
Tumour vessels that do not regress after inhibition of VEGF signalling tend to have more normal endothelial cells, more complete and tighter pericyte coverage, and less leakiness4,76. This ‘normalization’ is considered by some to be therapeutically beneficial because more efficient vessels and lower tumour interstitial fluid pressure owing to less leakage could improve drug delivery77-82. Vessels with normalized endothelial cells could also be less vulnerable to tumour cell intravasation30.
Some clinical studies have been interpreted as indicating that vessel normalization after treatment with bevacizumab can improve the efficacy of radiation therapy83, perhaps by improving tumour oxygenation and radiosensitivity. An alternative mechanism, whereby radiotherapy sensitizes endothelial cells to bevacizumab, has been suggested by preclinical studies showing that bevacizumab causes greater slowing of tumour growth when preceded by radiation than when followed by radiation84. Neoadjuvant (pre-surgery) bevacizumab sequenced with chemotherapy plus radiation followed by adjuvant (post-surgery) chemotherapy has shown favourable results in a trial of locally invasive rectal cancer85. These promising findings await confirmation by randomized prospective trials that determine long-term outcomes.
Vessel normalization also has potential downsides. Stabilized tumour vessels with tight pericyte coverage can resist regression and support tumour growth with little or no angiogenesis86,87. Reduced tumour vascularity and tighter endothelial barrier function after inhibition of VEGF signalling impairs the delivery of some agents88-91. The measurement of radiolabelled water and docetaxel in ten patients with non-small-cell lung cancer by positron emission tomography (PET) revealed significant reductions in both tumour perfusion and tumour drug delivery at 5 hours after bevacizumab infusion90. Systemic exposure to docetaxel increased owing to slower plasma clearance despite the decrease in tumour accumulation that persisted for at least 4 days.
Co-option of peritumoural blood vessels by invading tumour cells, which is a common feature of glioblastoma92,93 and lung cancer94,95, enables tumour growth without angiogenesis by exploiting existing normal vasculature. Although this process has not been studied in detail, co-opted blood vessels seem to be less sensitive than tumour vessels to inhibition of VEGF signalling96.
Therapeutic destabilization (‘abnormalization’) of tumour vessels is a potential approach for overcoming resistance to VEGF inhibition. The inhibition of Notch signalling by blocking delta-like protein 4 (DLL4) stimulates the growth of abundant vascular structures but slows tumour growth in preclinical models because the vessels that are derived from endothelial hypersprouting are poorly functional and intratumoural hypoxia is increased97-99. The abnormal vasculature is more responsive to inhibition of VEGF signalling, and tumour growth is slowed even in some models that are usually unresponsive to VEGF inhibition98. Broad inhibition of Notch signalling by the γ-secretase inhibitor dibenzazepine (DBZ) also increases the efficacy of bevacizumab in preclinical models100.
Clinical studies of DLL4 inhibitors such as REGN421 should determine whether this strategy can overcome resistance to inhibitors of VEGF signalling. However, a report of vascular neoplasms and other pathologies in mice, rats and monkeys after administration of a function-blocking DLL4 antibody, soluble DLL4 fusion protein or DBZ101 has slowed the development of DLL4 inhibitors.
High levels of DLL4 are correlated with resistance to inhibitors of VEGF signalling in some tumour types. Patients with breast cancer who are treated with capecitabine plus bevacizumab and who have tumours with little or no expression of DLL4 have significantly longer progression-free survival than those treated with capecitabine alone102. This benefit of bevacizumab is not found in similarly treated patients when the tumours have high DLL4 expression102.
Inhibition of immune cell recruitment.
The recruitment of bone marrow-derived myeloid cells and other immune cells that produce angiogenic factors and that contribute to the suppression of immune surveillance can accompany escape from inhibition of VEGF signalling in preclinical tumour models31,103-107. Tumour-infiltrating immune cells are an important source of matrix metalloproteinases that degrade the extracellular matrix, disrupt cell–matrix contacts and promote tumour cell invasion108,109. These proteases also release growth factors from the matrix and activate growth factors secreted as propeptides.
Some tumours resistant to inhibitors of VEGF signalling secrete cytokines that recruit myeloid cells and other cells that promote angiogenesis and immune tolerance110. Among these are stromal cell-derived factor 1 (SDF1; also known as CXCL12), colony-stimulating factors (CSFs) M-CSF, G-CSF and GM-CSF, BV8 (also known as prokineticin 2), interleukin-8 (IL-8) and C-C motif chemokine 28 (CCL28) (REFS 111-114). Cancer-associated fibroblasts (CAFs) are another source of SDF1 and angiogenic factors109,115-117.
Plasma levels of SDF1 correlate with metastasis in bevacizumab-treated patients with advanced rectal cancer118. Plasma SDF1 levels are also increased in patients with glioblastoma who have radiographic evidence of progression on treatment with cediranib74. SDF1, a ligand for CXC motif receptor 4 (CXCR4), promotes myeloid cell recruitment to the hypoxic regions of tumours. After radiation therapy, glioblastoma xenografts become revascularized and regrow through a HIF1α-mediated process that leads to CXCR4-driven recruitment of myeloid cells119,120. Tumour regrowth does not occur when myeloid cell influx is blocked by the inhibition of CXCR4 using plerixafor (AMD3100)119,120. Plerixafor, which is approved for use in non-Hodgkin’s lymphoma and multiple myeloma, is currently being used in combination with bevacizumab in early clinical trials of glioblastoma (TABLE 2). These trials will test whether escape from inhibition of VEGF signalling can be prevented by blocking SDF1-driven recruitment of myeloid cells.
Myeloid cell recruitment can also be suppressed by inhibition of M-CSF-mediated signalling through its receptor CSF1R (also known as c-FMS). Lung cancer growth in preclinical studies is reduced to a greater extent by blocking VEGF signalling (using the VEGFR2-specific antibody DC101) together with CSF1R signalling (using GW2580) than by blocking VEGF signalling alone121.
Inhibition of processes that favour tumour progression
Some effects of VEGF signalling inhibitors that slow tumour growth can also promote invasion and metastasis in some preclinical models122-126. The propensity for these effects seems to be dependent on multiple factors, including the tumour type and microenvironment, as well as drug-specific properties, such as dose and schedule, as these effects have been found in some studies122-132 but not in others7,133,134.
Among the mechanisms that could causally link angiogenesis inhibitors to tumour progression are vascular pruning, intratumoural hypoxia and other changes in the tumour microenvironment. Additional factors include conditions that favour crosstalk between VEGF receptors and other receptor tyrosine kinases (for example, MET, the receptor for hepatocyte growth factor) through heterodimerization, receptor complex formation, transphosphorylation, shared endocytosis and recycling or other interactions126,135-138. Changes in VEGF signalling can influence other receptors and their downstream pathways through these processes.
Inhibitors of VEGF signalling slow tumour growth by stopping angiogenesis and causing the rapid pruning of tumour vessels. Vascular pruning can exaggerate intratumoural hypoxia and can stabilize HIF1α47,139. Intratumoural hypoxia selects for tumour cells that survive in a low oxygen environment, undergo EMT, are more motile and invasive, and have gene expression changes that are driven by HIF1α activation140. Downstream effects of HIF1α are influenced by the tumour microenvironment. Deletion of HIF1α in astrocytoma cells leads to rapidly growing, invasive tumours in the brain but results in poorly vascularized, slowly growing, necrotic tumours when grown subcutaneously141.
The association of intratumoural hypoxia with evasive resistance to inhibitors of VEGF signalling makes the hypoxic microenvironment an attractive therapeutic target. Targeting HIF1α and angiogenesis together is a potential strategy for preventing escape from inhibitors of VEGF signalling142,143. Preclinical and clinical trials are currently underway to test whether HIF1α block-ade increases the therapeutic benefit of inhibitors of VEGF signalling. The TOP1 inhibitor topotecan blocks the accumulation of α-subunits of HIF1 through a TOP1-dependent effect on RNA transcription that is independent of DNA replication and proteasome degradation of HIF1α144. Daily low-dose topotecan in combination with bevacizumab reduces HIF1α accumulation, decreases tumour cell proliferation, increases apoptosis and promotes tumour regression in preclinical models47. EZN-2208, a pegylated TOP1 inhibitor that results in sustained downregulation of HIF1α145, is being tested in combination with bevacizumab in patients with refractory solid tumours (TABLE 2).
Like hypoxia, insulin and insulin-like growth factor I (IGF1) increase HIF1α activity and expression of VEGF146,147. IGF1–IGF1 receptor (IGF1R) signalling also increases tumour cell proliferation, decreases apoptosis and promotes progression to a more invasive and metastatic phenotype148. Downregulation of IGF1R suppresses the growth of some tumours through a mechanism that is complementary to VEGF blockade147. The suppression of tumour growth can also require inhibition of IGF2 or insulin receptors149. A function-blocking antibody to IGF1R in combination with bevacizumab is being tested on advanced solid tumours in early clinical trials (TABLE 2).
Through HIF1α, hypoxia increases the expression of various proteins that are involved in glycolytic metabolism, oxygen consumption, resistance to apoptosis, immune evasion, angiogenesis, invasion and metastasis140; these proteins include SDF1, CXCR4 and MET150-152.
Tumours with high MET expression or activating mutations of MET are generally more aggressive and have a less favourable prognosis153. The activation of MET can promote EMT and tumour invasiveness151,154, partly by increasing the activity of transcriptional repressors, such as snail homolog 1 (SNAI1; also known as SNAIL), ZEB1 and TWIST1, that reduce E-cadherin expression, increase N-cadherin expression and turn on the expression of other mesenchymal markers155. Inhibition of VEGF signalling can result in decreased expression of epithelial markers and increased expression of SNAI1, TWIST1 and other mesenchymal markers in some preclinical models124-126,129. The expression of the mesenchymal marker fascin increases in some glioblastomas after treatment with bevacizumab156.
Targeting tumour progression and angiogenesis together has recently shown promise as a strategy for preventing escape from inhibitors of VEGF signalling in preclinical models124-126,157,158. One approach is the inhibition of MET and VEGF signalling together, either by concurrent administration of selective inhibitors (PF-04217903 plus a VEGF-specific antibody) or by single agents that block both receptors (cabozantinib (also known as XL184) or foretinib (also known as XL880))125,158. Concurrent inhibition of MET and VEGF signalling can slow tumour growth, decrease invasion and metastasis, and change invasive tumours into a shape with a more ball-like appearance (FIG. 2a,b) in preclinical models125,158. The reduction in tumour progression is attributed to synergistic effects of blocking MET and VEGF receptor signalling and crosstalk125,126,135,158,159. The therapeutic benefit of blocking MET and VEGF together is currently being evaluated in clinical trials of multiple tumour types (TABLE 2).
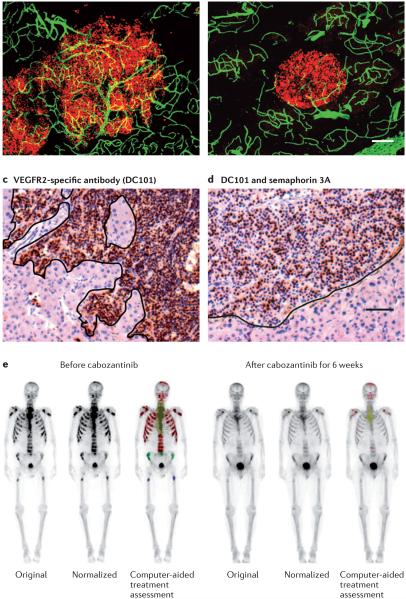
Figure 2. Reversal of tumour progression.
Pancreatic neuroendocrine tumours from RIP1-Tag2 transgenic mice are shown (parts a–d). Tumour cells stained for SV40 T- antigen are indicated (shown in red in parts a,b; shown in brown in parts c,d). Blood vessels stained for CD31 are shown (green in parts a,b). The scale bar shown in part b (80 μm) also applies to part a. The scale bar in part d (50 μm) also applies to part c. The irregular borders of invasive tumours treated with a vascular endothelial growth factor (VEGF)-specific antibody (part a) or a VEGF receptor 2 (VEGFR2)-specific antibody DC101 (part c) contrast with the smooth borders of tumours treated with cabozantinib, which is a small-molecule tyrosine kinase inhibitor targeting VEGF receptors and MET (part b), or with VEGFR2-specific antibody plus semaphorin 3A124 (part d) (parts a and b are similar to data reported in REF. 125). Bone scans of a patient with castration-resistant metastatic prostate cancer before and after treatment with cabozantinib are shown (part e)166. Metastases are highlighted and are coloured (red, green and blue) by computer-aided normalization and enhancement algorithms. Parts c and d reproduced, with permission, from REF. 124 © Am. Soc. Clin. Investigation (2012). Part e reproduced, with permission, from REF. 166 © Lippincott Williams & Wilkins (2012).
Administration of semaphorin 3A (SEMA3A), a secreted ligand for neuropilin 1, plexins and integrins via RHO-family GTPases, is another approach that has been shown to prevent escape from the inhibition of VEGF signalling124. Neuroendocrine tumours of the pancreas and carcinomas of the uterine cervix are more invasive and metastatic in mice treated with sunitinib or with the VEGFR2-specific antibody DC101. However, administration of SEMA3A by adeno-associated virus along with a VEGF signalling inhibitor improves tumour vascular function, reduces intratumoural hypoxia, MET expression and EMT, decreases invasion (FIG. 2c,d) and metastasis, and prolongs survival124.
AXL, a member of the TYRO-AXL-MER (TAM) family of receptor tyrosine kinases, is a further target currently under examination for the prevention of escape from VEGF signalling inhibitors160. AXL and its ligand, growth arrest-specific gene 6 (GAS6), promote tumour cell survival, proliferation and migration161. Expression of AXL increases during EMT, contributes to invasion and metastasis, and is a negative predictor of overall survival in breast cancer160. Knockdown of AXL by short hairpin RNA eliminates the metastatic potential of MDA-MB-231 breast cancer xenograft tumours160. Similarly, a small-molecule inhibitor of AXL (R428) reduces invasion and metastasis in multiple tumour models162, and the AXL-specific antibody YW327.6S2 reduces metastasis and increases the efficacy of inhibitors of VEGF signalling in lung and breast cancer xenograft tumours157.
Cabozantinib, which inhibits MET, AXL and VEGF receptors, as well as multiple other receptor tyrosine kinases, is a potent inhibitor of invasion and metastasis in spontaneous and xenograft tumours in mice125,158,159. Cabozantinib has greater effects on tumour angiogenesis and overall survival than those found with combinations of selective inhibitors of MET and VEGF signalling in the same preclinical model, suggesting that AXL or other targets (such as RET, KIT and TIE2) contribute to the efficacy of cabozantinib125,159. Cabozantinib is showing promising results in clinical trials of castration-resistant metastatic prostate cancer (FIG. 2e; TABLE 2), medullary thyroid cancer, breast cancer, non-small-cell lung cancer, melanoma, liver cancer and ovarian cancer163-166.
Inhibition of cancer stem cell activation.
Treatment with angiogenesis inhibitors can increase the population of cancer cells with stem cell-like properties in hypoxic regions of a tumour128,154,167,168. A recent study has shown that breast cancer xenograft tumours in mice treated with sunitinib or bevacizumab have less vascularity, more hypoxia, slower growth and more abundant stem cells — identified by aldehyde dehydrogenase immunofluorescence — in hypoxic regions128. This process is dependent on HIF1α activation of AKT–β-catenin signalling. These findings are consistent with a contribution of breast cancer stem cells to hypoxia-related mechanisms of escape from inhibitors of VEGF signalling. The presence of MET overexpression, EMT, invasion and metastasis and potential strategies for inhibiting this escape mechanism are yet to be examined.
Future directions
The expectations for the use of angiogenesis inhibitors as cancer therapeutics have evolved during the years following their approval for clinical use. Many preclinical experiments and clinical trials have documented their potential, as well as their limitations, as with other anticancer drugs, and have shown that VEGF blockade by bevacizumab or zivaflibercept is usually more effective when used in combination with chemotherapy.
As the actions of angiogenesis inhibitors are becoming better understood, targeted therapies are being developed to block key steps in tumour growth, invasion and metastasis, and the range of drugs is expanding as mechanisms of tumour progression are elucidated. Preclinical studies have already provided proof of concept for inhibiting mechanisms of escape together with VEGF-driven angiogenesis, with the goal of slowing both tumour growth and progression. Although initial results are promising, important questions remain about how well the preclinical findings will translate to human cancer and whether the benefits will be durable, apply to multiple tumour types and not be limited by other escape mechanisms. More work is needed to identify inhibitors of escape mechanisms that can be used safely and effectively in combination with inhibitors of VEGF signalling over extended periods.
Among other issues to be resolved is why chemotherapy is required for meaningful clinical benefit of bevacizumab and zivaflibercept in multiple tumour types. Do selective inhibitors of VEGF improve the delivery of other agents and sensitivity to radiotherapy by normalizing tumour blood vessels? Or does chemotherapy or radiation augment the effects of VEGF inhibitors by sensitizing endothelial cells to their actions? Does chemotherapy suppress hypoxia-driven tumour progression that would otherwise develop with a VEGF inhibitor used as monotherapy? How does VEGF blockade influence myeloid cell recruitment and immune surveillance? Why do most small-molecule tyrosine kinase inhibitors not pair well with chemotherapy? Is this due to drug–drug interactions, their multi-targeted nature or due to additive toxicities? As the number of druggable targets increases, the relative cost, benefit and safety of combinations of expensive targeted therapies will need to be balanced against the efficacy and toxicity profile of single agents that block multiple targets.
The 90% of cancer patients who die as a consequence of tumour invasion and metastasis39,169 are telling reminders of the importance of these processes in outcome. In colorectal cancer, in which the primary tumour is generally removed by surgery, metastases can occur despite adjuvant bevacizumab treatment plus chemotherapy170, presumably because tumour cell dissemination occurs before surgery171 or because bevacizumab promotes evasive resistance via heightened invasion and metastasis9.
If the growth of metastases in humans is angiogenesis dependent, and selective VEGF blockade can stop angiogenesis in humans as in preclinical models6,7,133,134, why does bevacizumab not prolong disease-free survival in the adjuvant setting? In the 3-year Phase III C-08 trial of adjuvant therapy after surgery for colorectal cancer, the addition of bevacizumab significantly improved disease-free survival during the initial year of treatment but not during the remaining period after treatment ended170. This finding illustrates an effect of treatment duration on outcome and supports the continued administration of bevacizumab beyond progression. Withdrawal of VEGF signalling inhibition is followed by tumour revascularization and regrowth as the actions of VEGF resume7,172,173. In the Bevacizumab Regimens: Investigation of Treatment Effects and Safety (BRiTE) trial in metastatic colorectal cancer, 74% of 1,953 patients who experienced disease progression while on bevacizumab and chemotherapy first-line had significantly better survival when bevacizumab was continued beyond progression37. Bevacizumab treatment beyond progression also resulted in prolonged overall survival in a retrospective study of metastatic colorectal cancer38.
More needs to be learned about the effects of sustained inhibition of VEGF signalling on the growth and further spreading of metastases. Knowing whether sustained anti-VEGF therapy in the adjuvant setting can slow the appearance of metastases and whether treatment beyond progression can slow further growth and dissemination of metastases would help to resolve these issues.
Blocking both angiogenesis and escape pathways that drive tumour progression is now an attainable step in the evolution of the use of agents that inhibit VEGF signalling together with other targets. This approach takes advantage of the current knowledge of tumour vascular biology and mechanisms of tumour growth, invasion and metastasis. Key steps that still need to be taken include learning more about escape mechanisms and how to control them, identifying additional targeted drugs that act synergistically with angiogenesis inhibitors and finding predictive biomarkers to identify patients who will have sustained benefit.
Acknowledgements
This work was supported in part by US National Institutes of Health (NIH) grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute, and funding from AngelWorks Foundation (to D.McD.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
ClinicalTrials.gov: http://clinicaltrials.gov/
NCT00726323 | NCT00811993 | NCT01186991 | NCT01251926 | NCT01308684 | NCT01339039 | NCT01418222 | NCT01468922 | NCT01496742 | NCT01522443 | NCT01605227
FURTHER INFORMATION
Donald M. McDonald’s homepage: http://mcdonald.ucsf.edu/
Genentech: http://www.gene.com
Regeneron: http://www.regeneron.com
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Reference
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu. Rev. Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 3.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 4.Inai T, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 7.Bagri A, et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin. Cancer Res. 2010;16:3887–3900. doi: 10.1158/1078-0432.CCR-09-3100. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, et al. In the end what matters most? A review of clinical endpoints in advanced breast cancer. Oncologist. 2011;16:25–35. doi: 10.1634/theoncologist.2010-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nature Rev. Clin. Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strickler JH, Hurwitz HI. Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer. Oncologist. 2012;17:513–524. doi: 10.1634/theoncologist.2012-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong H, Bowen JP. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr. Top. Med. Chem. 2011;11:1571–1590. doi: 10.2174/156802611795860924. [DOI] [PubMed] [Google Scholar]
- 12.Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr. Oncol. Rep. 2011;13:103–111. doi: 10.1007/s11912-011-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature Rev. Drug Discov. 2012;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 14.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Falcon BL, et al. Reduced VEGF production, angiogenesis, and vascular regrowth contribute to the antitumor properties of dual mTORC1/mTORC2 inhibitors. Cancer Res. 2011;71:1573–1583. doi: 10.1158/0008-5472.CAN-10-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagwat SV, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol. Cancer Ther. 2011;10:1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CW, Nozawa H, Hanahan D. Survival benefit with proapoptotic molecular and pathologic responses from dual targeting of mammalian target of rapamycin and epidermal growth factor receptor in a preclinical model of pancreatic neuroendocrine carcinogenesis. J. Clin. Oncol. 2010;28:4425–4433. doi: 10.1200/JCO.2010.28.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J. Clin. Oncol. 2009;27:4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert NJ, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 21.Brufsky AM, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2011;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 22.Lohmann AE, Chia S. Patients with metastatic breast cancer using bevacizumab as a treatment: is there still a role for it? Curr. Treat. Options Oncol. 2012;13:249–262. doi: 10.1007/s11864-012-0181-9. [DOI] [PubMed] [Google Scholar]
- 23.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab-an Eastern Cooperative Oncology Group Study. Clin. Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nature Rev. Clin. Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12–25. doi: 10.1177/1947601909356574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duda DG, Ancukiewicz M, Jain RK. Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers? J. Clin. Oncol. 2010;28:183–185. doi: 10.1200/JCO.2009.24.8021. [DOI] [PubMed] [Google Scholar]
- 27.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012;30:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts D, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13:724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- 30.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nature Rev. Clin. Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 31.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arao T, et al. Acquired drug resistance to vascular endothelial growth factor receptor 2 tyrosine kinase inhibitor in human vascular endothelial cells. Anticancer. Res. 2011;31:2787–2796. [PubMed] [Google Scholar]
- 33.Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res. 2011;71:7021–7028. doi: 10.1158/0008-5472.CAN-11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arrondeau J, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest. New Drugs. 2012;30:2046–2049. doi: 10.1007/s10637-011-9764-8. [DOI] [PubMed] [Google Scholar]
- 35.Gotink KJ, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin. Cancer Res. 2011;17:7337–7346. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocci G, Loupakis F. The possible role of chemotherapy in antiangiogenic drug resistance. Med. Hypotheses. 2012;78:646–648. doi: 10.1016/j.mehy.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Grothey A, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J. Clin. Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 38.Cartwright TH, et al. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. 2012 Jun 1; doi: 10.1016/j.clcc.2012.05.005. (doi: 10.1016/j.clcc.2012.05.005) [DOI] [PubMed] [Google Scholar]
- 39.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 40.Kerbel RS. Reappraising antiangiogenic therapy for breast cancer. Breast. 2011;20:S56–S60. doi: 10.1016/S0960-9776(11)70295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat. Rev. 2012;38:484–493. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerbel RS. Improving conventional or low dose metronomic chemotherapy with targeted antiangiogenic drugs. Cancer Res. Treat. 2007;39:150–159. doi: 10.4143/crt.2007.39.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montagna E, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin. Breast Cancer. 2012;12:207–214. doi: 10.1016/j.clbc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Lee K, et al. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl Acad. Sci. USA. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Rapisarda A, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol. Cancer Ther. 2009;8:1867–1877. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto K, et al. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol. Cancer Ther. 2010;9:996–1006. doi: 10.1158/1535-7163.MCT-09-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitt JM, et al. Sunitinib plus paclitaxel in patients with advanced esophageal cancer: a phase II study from the Hoosier Oncology Group. J. Thorac Oncol. 2012;7:760–763. doi: 10.1097/JTO.0b013e31824abc7c. [DOI] [PubMed] [Google Scholar]
- 50.Heath EI, et al. Sunitinib in combination with paclitaxel plus carboplatin in patients with advanced solid tumors: phase I study results. Cancer Chemother. Pharmacol. 2011;68:703–712. doi: 10.1007/s00280-010-1536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kindler HL, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest. New Drugs. 2012;30:382–386. doi: 10.1007/s10637-010-9526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goncalves A, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann. Oncol. 2012 Jul 5; doi: 10.1093/annonc/mds135. (doi:10.1093/annonc/mds135) [DOI] [PubMed] [Google Scholar]
- 53.Bergh J, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J. Clin. Oncol. 2012;30:921–929. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 54.Rugo HS, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J. Clin. Oncol. 2011;29:2459–2465. doi: 10.1200/JCO.2010.31.2975. [DOI] [PubMed] [Google Scholar]
- 55.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, et al. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int. J. Oncol. 2009;34:79–87. [PMC free article] [PubMed] [Google Scholar]
- 57.Alessi P, et al. Anti-FGF2 approaches as a strategy to compensate resistance to anti-VEGF therapy: long-pentraxin 3 as a novel antiangiogenic FGF2-antagonist. Eur. Cytokine Netw. 2009;20:225–234. doi: 10.1684/ecn.2009.0175. [DOI] [PubMed] [Google Scholar]
- 58.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Saylor PJ, Escudier B, Michaelson MD. Importance of fibroblast growth factor receptor in neovascularization and tumor escape from antiangiogenic therapy. Clin. Genitourin. Cancer. 2012;10:77–83. doi: 10.1016/j.clgc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 62.Sallinen H, et al. Antiangiogenic gene therapy with soluble VEGFR-1, -2, and -3 reduces the growth of solid human ovarian carcinoma in mice. Mol. Ther. 2009;17:278–284. doi: 10.1038/mt.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon MS. Antiangiogenic therapies: is VEGF-A inhibition alone enough? Expert Rev. Anticancer Ther. 2011;11:485–496. doi: 10.1586/era.11.5. [DOI] [PubMed] [Google Scholar]
- 64.Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk. Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist. Updat. 2010;13:16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nature Rev. Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 68.Hashizume H, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 70.Van de Veire S, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 71.Bais C, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 72.Willett CG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J. Clin. Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 73.Rini BI, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 74.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bagley RG, et al. Placental growth factor upregulation is a host response to antiangiogenic therapy. Clin. Cancer Res. 2011;17:976–988. doi: 10.1158/1078-0432.CCR-10-2687. [DOI] [PubMed] [Google Scholar]
- 76.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 77.Dickson PV, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin. Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro. Oncol. 2009;11:301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vangestel C, et al. 99mTc-(CO)3 His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab. J. Nucl. Med. 2011;52:1786–1794. doi: 10.2967/jnumed.111.092650. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q, et al. Time-course imaging of therapeutic functional tumor vascular normalization by antiangiogenic agents. Mol. Cancer Ther. 2011;10:1173–1184. doi: 10.1158/1535-7163.MCT-11-0008. [DOI] [PubMed] [Google Scholar]
- 81.Turley RS, et al. Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma. Clin. Cancer Res. 2012;18:3328–3339. doi: 10.1158/1078-0432.CCR-11-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willett CG, et al. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin. Oncol. 2006;33:S35–S40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoang T, Huang S, Armstrong E, Eickhoff JC, Harari PM. Enhancement of radiation response with bevacizumab. J. Exp. Clin. Cancer Res. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willett CG, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sennino B, et al. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helfrich I, et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM. Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 2006;66:1434–1445. doi: 10.1158/0008-5472.CAN-05-0923. [DOI] [PubMed] [Google Scholar]
- 89.Ribatti D. Vascular normalization: a real benefit? Cancer Chemother. Pharmacol. 2011;68:275–278. doi: 10.1007/s00280-011-1683-z. [DOI] [PubMed] [Google Scholar]
- 90.Van der Veldt AA, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 91.Pastuskovas CV, et al. Effects of anti-VEGF on pharmacokinetics, biodistribution, and tumor penetration of trastuzumab in a preclinical breast cancer model. Mol. Cancer Ther. 2012;11:752–762. doi: 10.1158/1535-7163.MCT-11-0742-T. [DOI] [PubMed] [Google Scholar]
- 92.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 93.Rubenstein JL, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pezzella F, et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neoangiogenesis. Am. J. Pathol. 1997;151:1417–1423. [PMC free article] [PubMed] [Google Scholar]
- 95.Offersen BV, Pfeiffer P, Hamilton-Dutoit S, Overgaard J. Patterns of angiogenesis in nonsmall-cell lung carcinoma. Cancer. 2001;91:1500–1509. [PubMed] [Google Scholar]
- 96.Kim ES, et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc. Natl Acad. Sci. USA. 2002;99:11399–11404. doi: 10.1073/pnas.172398399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noguera-Troise I, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 98.Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 99.Kuhnert F, Kirshner JR, Thurston G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc. Cell. 2011;3:20. doi: 10.1186/2045-824X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li JL, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–6083. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 101.Yan M, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:e6–e7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 102.Jubb AM, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin. Cancer Res. 2011;17:372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist. Updat. 2008;11:219–230. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol. Aspects Med. 2011;32:123–145. doi: 10.1016/j.mam.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 106.Finke J, et al. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin. Exp. Immunol. 2012;167:195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 110.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nature Biotech. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 111.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 112.Huang D, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carbone C, et al. Anti-VEGF treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clin. Cancer Res. 2011;17:5822–5832. doi: 10.1158/1078-0432.CCR-11-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 115.Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol. Sci. 2009;30:624–630. doi: 10.1016/j.tips.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 116.Wang W, Ma JL, Jia WD, Xu GL. Periostin: a putative mediator involved in tumour resistance to anti-angiogenic therapy? Cell Biol. Int. 2011;35:1085–1088. doi: 10.1042/CBI20110171. [DOI] [PubMed] [Google Scholar]
- 117.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 118.Xu L, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1α, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br. J. Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Priceman SJ, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maione F, et al. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J. Clin. Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sennino B, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu KV, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Casazza A, et al. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol. Med. 2012;4:234–250. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conley SJ, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl Acad. Sci. USA. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cooke VG, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by Met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grepin R, et al. Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: the role of CXCL cytokines. Oncogene. 2012;31:1683–1694. doi: 10.1038/onc.2011.360. [DOI] [PubMed] [Google Scholar]
- 131.He S, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012;227:431–445. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shojaei F, Simmons BH, Lee JH, Lappin PB, Christensen JG. HGF/c-Met pathway is one of the mediators of sunitinib-induced tumor cell type-dependent metastasis. Cancer Lett. 2012;320:48–55. doi: 10.1016/j.canlet.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 133.Singh M, et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumor models. J. Pathol. 2012;227:417–430. doi: 10.1002/path.4053. [DOI] [PubMed] [Google Scholar]
- 134.Chung AS, et al. Differential drug-class specific metastatic effects following treatment with a panel of angiogenesis inhibitors. J. Pathol. 2012;227:404–416. doi: 10.1002/path.4052. [DOI] [PubMed] [Google Scholar]
- 135.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Jones MC, et al. VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic. 2009;10:754–766. doi: 10.1111/j.1600-0854.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 137.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nature Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 138.Muller PA, et al. Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene. 2012 May 14; doi: 10.1038/onc.2012.148. (doi:10.1038/ onc.2012.148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ou G, et al. Usefulness of HIF-1 imaging for determining optimal timing of combining bevacizumab and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:463–467. doi: 10.1016/j.ijrobp.2009.02.083. [DOI] [PubMed] [Google Scholar]
- 140.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blouw B, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 142.Rapisarda A, Melillo G. Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug Resist. Updat. 2009;12:74–80. doi: 10.1016/j.drup.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 144.Rapisarda A, et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 145.Sapra P, et al. Potent and sustained inhibition of HIF-1α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effects. Angiogenesis. 2011;14:245–253. doi: 10.1007/s10456-011-9209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zelzer E, et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li H, et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 148.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 149.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc. Natl Acad. Sci. USA. 2010;107:10791–10798. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schioppa T, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pennacchietti S, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 152.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 153.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nature Rev. Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 154.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 155.Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol. Cancer Res. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 156.Narayana A, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J. Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 157.Ye X, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 158.You WK, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–4768. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yakes FM, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 160.Gjerdrum C, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl Acad. Sci. USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holland SJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 163.Hussain M, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): results from a phase II randomized discontinuation trial. J. Clin. Oncol. Abstr. 2011;29:4516. [Google Scholar]
- 164.Kurzrock R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aftab DT, McDonald DM. MET and VEGF: synergistic targets in castration-resistant prostate cancer. Clin. Transl. Oncol. 2011;13:703–709. doi: 10.1007/s12094-011-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown MS, et al. Computer-aided quantitative bone scan assessment of prostate cancer treatment response. Nucl. Med. Commun. 2012;33:384–394. doi: 10.1097/MNM.0b013e3283503ebf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Rev. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 168.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nature Rev. Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 170.Allegra CJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cacheux W, et al. Reversible tumor growth acceleration following bevacizumab interruption in metastatic colorectal cancer patients scheduled for surgery. Ann. Oncol. 2008;19:1659–1661. doi: 10.1093/annonc/mdn540. [DOI] [PubMed] [Google Scholar]
- 174.O’Connor JP, et al. DCE-MRI biomarkers of tumour heterogeneity predict CRC liver metastasis shrinkage following bevacizumab and FOLFOX-6. Br. J. Cancer. 2011;105:139–145. doi: 10.1038/bjc.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dingemans AM, et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann. Oncol. 2011;22:559–566. doi: 10.1093/annonc/mdq391. [DOI] [PubMed] [Google Scholar]
- 176.Kawai N, et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:441–450. doi: 10.1007/s00259-010-1645-4. [DOI] [PubMed] [Google Scholar]
- 177.Harris RJ, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14:1079–1089. doi: 10.1093/neuonc/nos141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dewdney A, Cunningham D, Barbachano Y, Chau I. Correlation of bevacizumab-induced hypertension and outcome in the BOXER study, a phase II study of capecitabine, oxaliplatin (CAPOX) plus bevacizumab as peri-operative treatment in 45 patients with poor-risk colorectal liver-only metastases unsuitable for upfront resection. Br. J. Cancer. 2011;106:1718–1721. doi: 10.1038/bjc.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mir O, et al. An observational study of bevacizumab-induced hypertension as a clinical biomarker of antitumor activity. Oncologist. 2011;16:1325–1332. doi: 10.1634/theoncologist.2010-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Osterlund P, et al. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br. J. Cancer. 2011;104:599–604. doi: 10.1038/bjc.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.DePrimo SE, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J. Transl. Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burstein HJ, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin. Cancer Res. 2008;14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 183.Pena C, Lathia C, Shan M, Escudier B, Bukowski RM. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: results from sorafenib phase III treatment approaches in renal cancer global evaluation trial. Clin. Cancer Res. 2010;16:4853–4863. doi: 10.1158/1078-0432.CCR-09-3343. [DOI] [PubMed] [Google Scholar]
- 184.Nikolinakos PG, et al. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res. 2010;70:2171–2179. doi: 10.1158/0008-5472.CAN-09-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kopetz S, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J. Clin. Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dellapasqua S, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J. Clin. Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 187.Ronzoni M, et al. Circulating endothelial cells and endothelial progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann. Oncol. 2010;21:2382–2389. doi: 10.1093/annonc/mdq261. [DOI] [PubMed] [Google Scholar]
- 188.Rugo HS, et al. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res. Treat. 2012;134:13–20. doi: 10.1007/s10549-011-1918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wedam SB, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J. Clin. Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 190.Denduluri N, et al. Circulating biomarkers of bevacizumab activity in patients with breast cancer. Cancer Biol. Ther. 2008;7:15–20. doi: 10.4161/cbt.7.1.5337. [DOI] [PubMed] [Google Scholar]
- 191.Yang SX, Steinberg SM, Nguyen D, Swain SM. p53, HER2 and tumor cell apoptosis correlate with clinical outcome after neoadjuvant bevacizumab plus chemotherapy in breast cancer. Int. J. Oncol. 2011;38:1445–1452. doi: 10.3892/ijo.2011.966. [DOI] [PMC free article] [PubMed] [Google Scholar]