Abstract
Background
Children exposed to early-life psychosocial deprivation associated with institutional rearing are at markedly elevated risk of developing ADHD. Neurodevelopmental mechanisms that explain the high prevalence of ADHD in children exposed to institutionalization are unknown. We examined whether abnormalities in cortical thickness and sub-cortical volume were mechanisms explaining elevations in ADHD among children raised in institutional settings.
Methods
Data were drawn from the Bucharest Early Intervention Project, a cohort of children raised from early infancy in institutions in Romania (n=58) and age-matched community controls (n=22). Magnetic resonance imaging data were acquired when children were aged 8–10 years, and ADHD symptoms were assessed using the Health and Behavior Questionnaire (HBQ).
Results
Children reared in institutions exhibited widespread reductions in cortical thickness across prefrontal, parietal, and temporal regions relative to community controls. No group differences were found in the volume of sub-cortical structures. Reduced thickness across numerous cortical areas was associated with higher levels of ADHD symptoms. Cortical thickness in lateral orbitofrontal cortex, insula, inferior parietal cortex, precuneus, superior temporal cortex, and lingual gyrus mediated the association of institutionalization with inattention and impulsivity; additionally, supramarginal gyrus thickness mediated the association with inattention and fusiform gyrus thickness mediated the association with impulsivity.
Conclusion
Severe early-life deprivation disrupts cortical development resulting in reduced thickness in regions with atypical function during attention tasks in children with ADHD, including the inferior parietal cortex, precuneus, and superior temporal cortex. These reductions in thickness are a neurodevelopmental mechanism explaining elevated ADHD symptoms in children exposed to institutional rearing.
Keywords: cortical development, institutionalization, deprivation, childhood adversity, attention-deficit/hyperactivity disorder (ADHD), brain development
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder estimated to affect approximately 5% of children worldwide. (1–3) Children with ADHD exhibit deficits in numerous aspects of executive functioning including working memory, response inhibition, attentional and motor control, and planning. (4–9) Meta-analyses of fMRI studies have identified abnormalities in neural function among children with ADHD including blunted activation in right hemisphere dorsolateral prefrontal cortex (PFC), striatum, and thalamus during inhibition and attention tasks, reduced inferior parietal cortex, precuneus, and superior temporal cortex activation during attention tasks, and hypo-activation in left hemisphere frontal-parietal-cerebellar circuits during timing tasks. (10, 11).
ADHD is also associated with atypical neural structure, including smaller volume of the PFC and basal ganglia (12–14) and reductions in cortical thickness across the prefrontal, parietal, and temporal cortex. (15, 16) Children with ADHD experience 2–5 year delays in reaching peak cortical thickness in these regions, (17) and cortical thickness in children with ADHD does not “catch up” to levels seen in typically developing children in most areas. (16, 18). Children with ADHD whose developmental trajectory of cortical thickness is more similar to that of typically developing children have better functional outcomes than children with persistent thickness reductions, (16) suggesting that this pattern of cortical development may be central to the pathophysiology of ADHD.
What factors lead to these neurodevelopmental deficits in children with ADHD? The high heritability of the disorder and early age-of-onset suggest strong genetic underpinnings. (19, 20) However, early-life psychosocial deprivation is also associated with ADHD, (21–23) indicating that adverse early experiences may contribute to atypical patterns of brain development. The prevalence of ADHD among children raised in institutional settings is 4–5 times higher than in the general population, raising questions about neurodevelopmental mechanisms involved in ADHD following psychosocial deprivation. (21–23) Institutional rearing is associated with atypical structural development that might contribute to ADHD risk in previously-institutionalized children. Reduced cerebral and cortical white and grey matter volumes have been observed in institutionally-reared children (24, 25), as well as white matter microstructure abnormalities in tracts linking the PFC to temporal and parietal regions. (26–28) Larger right amygdala volume was reported in one study of institutionally-reared children, (24) and another found larger amygdala volume among late-adopted children compared to early-adopted and control children. (29) Reduced cerebellar volume has also been observed in previously-institutionalized children. (30)
We investigated whether atypical neural structure is responsible for elevations in ADHD among children raised in institutional settings. We anticipated that institutional rearing would be associated with reduced cortical thickness and sub-cortical volume in regions implicated in ADHD pathology, including the dorsolateral PFC, inferior parietal cortex, superior temporal cortex, and striatum. In addition, we hypothesized that reduced cortical thickness and sub-cortical volume in these regions would be associated with ADHD pathology. Finally, we investigated whether disrupted cortical and sub-cortical development is a mechanism explaining the association between early psychosocial deprivation and ADHD.
Methods
Sample
The Bucharest Early Intervention Project (BEIP) is a longitudinal study of early institutionalization of young children in Bucharest, Romania. (31) A sample of 136 children (age range 6–30 months, M = 23 months) was recruited from each of the six institutions for young children in Bucharest, excluding participants with genetic syndromes (e.g., Down syndrome), fetal alcohol syndrome, and microcephaly. (31) An age-matched sample of 72 community-reared children was recruited from pediatric clinics in Bucharest and comprised the never-institutionalized group (NIG). Half of children in the institutionalized group were randomized to a foster care intervention, resulting in two groups: the foster care group (FCG) and the group who received care as usual (prolonged institutional care [CAUG]). The study design and methods have been described in detail previously. (31)
Structural magnetic resonance imaging (MRI) was acquired when children were between 8 and 10 years of age for all children whose guardians provided consent for imaging. Of the 86 children who completed MRI assessments, 80 were included in analysis: 31 CAUG children (15 female), 27 FCG children (13 female), and 22 NIG children (12 female). Four participants were excluded from analysis because of poor scan quality (2 CAUG, 1 FCG, and 1 NIG) and two children were excluded due to frank neurological abnormality (1 FCG, 1 NIG). Four participants were taking stimulant medication for ADHD at the time of the scan (3 CAUG, 1 FCG).
No differences in ADHD symptoms of inattention, t(51) = 0.46, p = .646, or impulsivity, t(51) = 0.69, p = .497, or in cortical thickness or sub-cortical volume were observed at age 8–10 years based on foster care placement. As such, children in the FCG and CAUG were collapsed into one ever-institutionalized group (EIG) for all analysis. No differences in gender distribution or age were observed for EIG and NIG children, although differences in IQ, birth weight, and cerebral gray and white matter were present across groups (Table 1).
Table 1.
Socio-demographic and developmental characteristics among children reared in institutions and community controls in the Bucharest Early Intervention Project (n=80)
| Ever Institutionalized Group (n=58) |
Never Institutionalized Group (n=22) |
Group Difference | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p-value | |
| Female, No. (%) | 48.3% | 54.5% | χ21 = 0.25 | .617 | ||
| Age, (months) | ||||||
| Age at Study Entry | 17.7 | (7.8) | 20.0 | (7.2) | 1.21 | .276 |
| Age at MRI Scan | 116.3 | (9.0) | 117.9 | (10.6) | 0.1 | .816 |
| Age at HBQ Assessment | 103.2 | (4.6) | 101.4 | (4.0) | 2.49 | .149 |
| Birth Weight, (grams) | 2780.0 | (623.3) | 3150.0 | (411.8) | 4.41* | .040 |
| Head Circumference at birth (cm.) | 46.07 | (2.61) | 46.5 | (2.08) | 0.04 | .843 |
| Full-scale IQ | 72.0 | (15.8) | 107.9 | (14.67) | 96.49* | .001 |
| Intracranial Volume1 | 1,456,490 | (132,948) | 1,499,091 | (109,367) | 1.79 | .184 |
| Cerebral Grey Matter1 | 790,429 | (71,160) | 833,849 | (62,887) | 6.31* | .014 |
| Cortical Grey Matter | 577,432 | (57,120) | 613,899 | (48,391) | 7.04* | .010 |
Significant at the p<.05 level, 2-sided test
Image acquisition
Structural magnetic resonance images were acquired at Regina Maria Health Center on a Siemens Magnetom Avanto 1.5 Tesla syngo system. Images were obtained using a transverse magnetization-prepared rapid gradient echo three-dimensional sequence (TE=2.98ms, TI=1000ms, flip angle= 8°, 176 slices with 1×1×1 mm isometric voxels) with a 16-channel head coil. The TR for this sequence was 1710 ms for most participants (n=59) and varied between 1650–1910 ms for remaining participants. Four subjects were acquired in the sagittal plane; one was acquired in the coronal plane. Acquisition parameters did not differ by group membership nor were they associated with scan quality; all scans were therefore considered together and a covariate for TR length was included in all analysis.
Image Processing
Cortical reconstruction and volumetric segmentation were performed with FreeSurfer (Version 5.0, http://surfer.nmr.mgh.harvard.edu). Technical details of these procedures have been described previously. (32–36) Gray/white matter and gray matter/CSF boundaries are constructed using spatial intensity gradients across tissue classes. A segmentation process is used to identify sub-cortical grey matter structures. Following reconstruction, the cerebral cortex is parcellated into regions based on the structure of gyri and sulci. (34, 37) Intensity and continuity information is used to generate measurements of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface. (33) The resulting surface maps are not restricted to the voxel resolution of the original data and are capable of detecting sub-millimeter differences between groups.
FreeSurfer morphometric procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths, (38, 39) and methods for measuring cortical thickness have been validated against manual measurement (40, 41) and histological analysis, (42) and have been used in studies of children aged 8–10 years. (25,29–43) The results of the automated segmentation and parcellation process were manually inspected for all participants. Where necessary, manual edits were performed as recommended to optimize accurate placement of gray/white and gray/CSF borders based on shifts in the image intensity gradient. (32, 33) No differences were present in the degree to which manual edits were required across groups.
ADHD
The MacArthur Health and Behavior Questionnaire (HBQ) (44) was completed by the primary teacher of each child when they were between 8 and 10 years old (M = 8.5 years, SD = 0.4 years). The HBQ assesses emotional and behavior problems and has been widely used in studies of children ranging from preschool age to adolescence, including previously-institutionalized children. (45) The ADHD sub-scale assesses inattention and impulsivity. Teacher reports of ADHD behaviors on the HBQ have demonstrated excellent test-retest reliability in community and clinical samples, acceptable concordance with parent reports, and high discriminant validity. (44, 46)
Statistical Analysis
We investigated whether elevations in ADHD symptoms among institutionalized children relative to controls were accounted for by differences in brain structure using standard tests of statistical mediation. To provide evidence for mediation, four criteria must be met. (47, 48) First, an association between the exposure and outcome must be established. Here, we examined differences in ADHD symptoms between children reared in institutions versus the community using univariate ANOVAs with group (EIG, NIG) as a between-subjects factor.
Second, the exposure must be associated with the mediator. We examined group differences in brain structure using the Qdec surface-based group analysis tool (Version 1.4) in FreeSurfer. Following spatial normalization to an averaged spherical surface and smoothing with a 10mm full-width half-maximum Gaussian kernel, Qdec applies a general linear model (GLM) to cortical thickness at each vertex, separately by hemisphere. A discrete variable for group was included in the GLM, along with covariates for age, gender, total brain volume, and TR. No interactions were found between group and any of these covariates. To reduce Type I error associated with multiple comparisons, we applied a false discovery rate (FDR) correction. (49) Group differences in sub-cortical volume was examined using univariate ANOVAs with group as a between-subjects factor and the covariates outlined above for the caudate, putamen, globus pallidus, nucleus accumbens, amygdala, hippocampus, thalamus, and cerebellum. FDR correction was applied to correct for multiple comparisons.
Third, the mediator must be associated with the outcome. Here, we examined the associations of cortical thickness and sub-cortical volume with ADHD symptoms using linear regression. We examined the association of cortical thickness in each cluster that differed between children raised in institutions versus the community after FDR correction with ADHD symptoms. To do so, we created a region of interest (ROI) for each FDR-corrected cluster that was significantly different between groups. This normalized ROI was mapped back to each participant (using deformation tools in FreeSurfer) to generate a mean thickness value for that ROI for each participant. Gender, age, total brain volume, and TR were included as covariates.
Finally, we tested the significance of the indirect effect using a bootstrapping approach that provides bias-corrected confidence intervals and is appropriate for use in small samples. (50) Confidence intervals that do not include zero indicate significant mediation. We required that a brain region differed in thickness or volume as a function of institutionalization and be associated with ADHD symptoms at the FDR-corrected threshold to be included in the mediation analysis.
Results
Institutionalization and ADHD
ADHD symptoms varied as a function of institutionalization for inattention, F(1,70) = 29.48, p < .001, and impulsivity, F(1,69) = 17.94, p < .001. Children with histories of institutional rearing (EIG) exhibited higher levels of inattention (M = 6.46, SD = 2.86) and impulsivity (M = 8.73, SD = 5.53) than community-reared children (inattention M = 1.90, SD = 2.86; impulsivity M = 3.14, SD = 4.33).
Institutionalization and Cortical Thickness
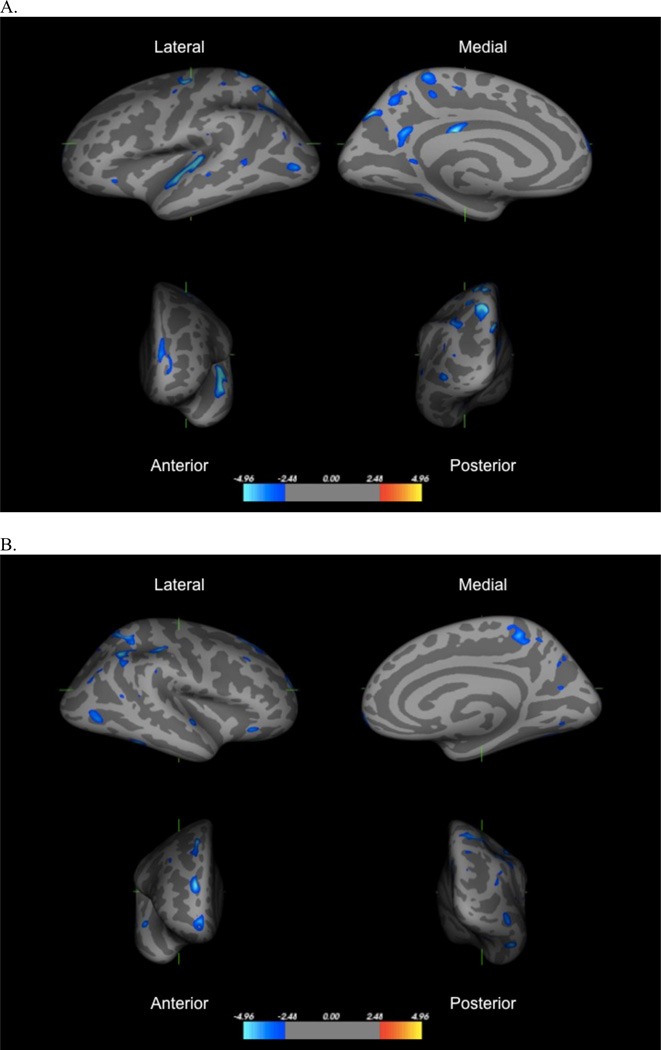
Results from the left hemisphere GLM revealed 34 clusters that differed significantly in thickness as a function of institutionalization. Institutionally-reared children had reduced thickness compared to never-institutionalized children in all 34 clusters. Table 2 provides the Montreal Neurologic Institute (MNI) coordinates and peak of each cluster, and Figure 1 displays results. Significant differences in cortical thickness were observed in multiple clusters and were most pronounced in the superior and inferior parietal cortex (5 and 4 clusters, respectively), precuneus (4 clusters), superior temporal gyrus and sulcus (3 clusters), precentral gyrus (2 clusters), and posterior cingulate (2 clusters). Significant differences were also present in the superior frontal gyrus, middle frontal gyrus (MFG), fusiform gyrus, supramarginal gyrus, lateral orbitofrontal cortex (OFC), lateral occipital cortex, and insula.
Table 2.
Left hemisphere regions with significant differences in cortical thickness (mm) among children reared in institutions relative to community controls in the Bucharest Early Intervention Project (n=80)1,2
| Cluster size | Peak within Cluster |
Approximate coordinates in MNI space (x, y, z) |
|||
|---|---|---|---|---|---|
| Brain Area | (mm2) | t | x | y | z |
| 1. Superior Parietal Cortex | 307.0 | −6.65 | −15.2 | −66.1 | 50.1 |
| 2. Superior Temporal Gyrus | 567.7 | −6.65 | −52.8 | −10.3 | −0.7 |
| 3. Precentral Gyrus | 179.4 | −6.02 | −35.2 | −14.6 | 64.7 |
| 4. Superior Parietal Cortex | 337.4 | −5.91 | −16.8 | −52.4 | 60.5 |
| 5. Superior Parietal Cortex | 168.0 | −5.24 | −16.7 | −74.2 | 37.6 |
| 6. Posterior Cingulate | 67.7 | −5.10 | −3.9 | −26.7 | 26.6 |
| 7. Inferior Parietal Cortex | 251.9 | −4.22 | −36.2 | −63.8 | 40.9 |
| 8. Precuneus | 158.6 | −4.16 | −8.2 | −57.5 | 22.4 |
| 9. Paracentral | 130.8 | −4.04 | −15.4 | −41.5 | 62.8 |
| 10. Lateral Occipital | 121.6 | −4.00 | −43.9 | −81.0 | 1.7 |
| 11. Superior Parietal Cortex | 53.9 | −3.96 | −28.6 | −56.2 | 56.7 |
| 12. Superior Frontal Gyrus | 179.4 | −3.92 | −9.1 | 62.9 | 17.4 |
| 13. Precuneus | 121.1 | −3.74 | −9.9 | −59.9 | 45.2 |
| 14. Fusiform Gyrus | 55.6 | −3.49 | −33.9 | −47.4 | −11.9 |
| 15. Paracentral | 15.5 | −3.48 | −8.5 | −25.9 | 51.1 |
| 16. Precuneus | 30.3 | −3.35 | −18.3 | −41.2 | 45.5 |
| 17. Posterior Cingulate | 25.7 | −3.29 | −10.4 | −48.3 | 3.5 |
| 18. Middle Frontal Gyrus | 79.5 | −3.20 | −19.7 | 61.2 | 4.0 |
| 19. Lateral Orbitofrontal Cortex | 32.8 | −3.18 | −35.8 | 28.4 | −7.2 |
| 20. Superior Temporal Gyrus | 20.3 | −3.15 | −49.9 | −29.0 | −2.9 |
| 21. Precentral Gyrus | 22.3 | −3.03 | −41.6 | −7.3 | 53.3 |
| 22. Inferior Parietal Cortex | 12.3 | −2.99 | −40.6 | −60.9 | 42.9 |
| 23. Postcentral Gyrus | 22.8 | −2.93 | −32.7 | −35.9 | 55.6 |
| 24. Inferior Parietal Cortex | 21.0 | −2.91 | −44.2 | −72.6 | 25.9 |
| 25. Supramarginal Gyrus | 17.8 | −2.89 | −47.5 | −48.2 | 42.7 |
| 26. Insula | 11.1 | −2.89 | −31.4 | −29.7 | 13.4 |
| 27. Superior Temporal Sulcus | 39.6 | −2.87 | −53.1 | −51.5 | 3.4 |
| 28. Inferior Parietal Cortex | 10.5 | −2.85 | −38.2 | −84.3 | 14.5 |
| 29. Precuneus | 6.5 | −2.79 | −13.0 | −63.4 | 28.4 |
| 30. Supramarginal Gyrus | 5.8 | −2.76 | −56.6 | −29.4 | 18.9 |
| 31. Supramarginal Gyrus | 0.4 | −2.72 | −44.5 | −50.2 | 40.4 |
| 32. Superior Parietal Cortex | 1.7 | −2.72 | −36.6 | −48.6 | 51.5 |
| 33. Postcentral Gyrus | 0.3 | −2.71 | −29.8 | −32.3 | 68.2 |
| 34. Superior Frontal Gyrus | 0.7 | −2.71 | −15.0 | 58.7 | 13.9 |
Analyses control for age, gender, total brain volume, and TR.
Significant group differences are shown at the p<.05 level, corrected for the false discovery rate (FDR). All significant group differences represent reduced cortical thickness in children reared in institutions relative to controls.
Figure 1.
Regions in left hemisphere (panel a) and right hemisphere (panel b) with significant reductions in thickness among children exposed to institutional rearing relative to controls, following FDR correction. Images represent (clockwise from top left), lateral, medial, posterior, and anterior views of the group average brain.
The right hemisphere GLM revealed 27 clusters that differed significantly between groups, with institutionally-reared children exhibiting reduced cortical thickness than controls in all clusters (Table 3, Figure 2). Findings mirrored those from the left hemisphere, with the exception of greater differences in the MFG. Areas with multiple significant clusters and the largest group differences were the MFG (2 clusters), superior and inferior parietal cortex (3 and 4 clusters, respectively), precuneus (4 clusters), supramarginal gyrus (2 clusters), and superior temporal gyrus and sulcus (2 clusters). Additional regions differing in thickness included the superior frontal gyrus, inferior temporal gyrus, frontal pole, lateral OFC, lateral occipital cortex, fusiform gyrus, lingual gyrus, and insula.
Table 3.
Right hemisphere regions with significant differences in cortical thickness (mm) among children reared in institutions relative to community controls in the Bucharest Early Intervention Project (n=80)1,2
| Cluster size | Peak within Cluster |
Approximate coordinates in MNI space (x, y, z) |
|||
|---|---|---|---|---|---|
| Brain Area | (mm2) | t | x | y | z |
| 1. Middle Frontal Gyrus | 197.7 | −5.26 | 18.7 | 53.7 | 21.4 |
| 2. Inferior Parietal Cortex | 353.6 | −5.23 | 41.7 | −55.9 | 41.1 |
| 3. Superior Frontal Gyrus | 223.1 | −5.10 | 20.6 | 23.4 | 55.1 |
| 4. Inferior Temporal Gyrus | 136.5 | −4.80 | 47.3 | −45.1 | −15.3 |
| 5. Supramarginal Gyrus | 142.3 | −4.76 | 40.7 | −27.2 | 40.3 |
| 6. Superior Parietal Cortex | 330.8 | −4.71 | 26.7 | −55.4 | 59.6 |
| 7. Frontal Pole | 200.6 | −4.47 | 10.0 | 64.0 | −8.0 |
| 8. Precuneus | 173.1 | −4.33 | 16.2 | −42.9 | 55.0 |
| 9. Superior Temporal Gyrus | 73.0 | −4.11 | 57.1 | −4.8 | −2.6 |
| 10. Lateral Orbitofrontal Cortex | 62.9 | −4.03 | 40.1 | 25.2 | −13.7 |
| 11. Lateral Occipital Cortex | 181.7 | −4.02 | 40.6 | −70.3 | −1.9 |
| 12. Fusiform Gyrus | 58.9 | −3.83 | 29.3 | −66.1 | −13.8 |
| 13. Precuneus | 22.0 | −3.59 | 8.3 | −54.7 | 52.1 |
| 14. Middle Frontal Gyrus | 34.9 | −3.51 | 36.9 | 26.8 | 43.4 |
| 15. Inferior Parietal Cortex | 47.1 | −3.49 | 42.2 | −77.4 | 24.9 |
| 16. Insula | 1.2 | −3.29 | 31.0 | 13.6 | −12.4 |
| 17. Inferior Parietal Cortex | 24.1 | −3.26 | 41.9 | −71.0 | 35.0 |
| 18. Precuneus | 27.6 | −3.23 | 22.0 | −67.3 | 15.1 |
| 19. Precuneus | 26.6 | −3.13 | 15.3 | −69.8 | 39.0 |
| 20. Inferior Parietal Cortex | 17.1 | −3.11 | 51.7 | −56.1 | 13.2 |
| 21. Lingual Gyrus | 32.6 | −3.11 | 15.7 | −73.0 | −5.8 |
| 22. Superior Parietal Cortex | 26.0 | −3.09 | 18.4 | −66.3 | 46.8 |
| 23. Supramarginal Gyrus | 8.9 | −3.05 | 55.5 | −34.5 | 35.2 |
| 24. Precuneus | 19.9 | −2.94 | 10.7 | −66.1 | 34.9 |
| 25. Superior Temporal Sulcus | 3.5 | −2.93 | 49.8 | −41.8 | 14.3 |
| 26. Superior Parietal Cortex | 5.9 | −2.92 | 24.1 | −84.3 | 30.7 |
| 27. Insula | 0.3 | −2.92 | 29.6 | 13.4 | −13.7 |
Analyses control for age, gender, total brain volume, and TR.
Significant group differences are shown at the p<.05 level, corrected for the false discovery rate (FDR). All significant group differences represent reduced cortical thickness in children reared in institutions relative to controls.
We also examined the association of duration of institutionalization with cortical thickness in institutionally-reared children. Similar regions in the prefrontal, parietal, and temporal cortex that emerged in the between-groups analysis were associated with duration of institutionalization; however, none of these associations survived FDR correction.
Institutionalization and Sub-Cortical and Cerebellar Volume
Consistent with a previous report, (25) no differences in the volume of the striatum (including the caudate, putamen, globus pallidus, and nucleus accumbens), amygdala, hippocampus, thalamus, or cerebellum were observed as a function of institutionalization (Table 4).
Table 4.
Group differences in the volume of sub-cortical structures and the cerebellum among children reared in institutions and community controls in the Bucharest Early Intervention Project (n=80)1
| Ever Institutionalized Group (n=58) |
Never Institutionalized Group (n=22) |
Group Difference1 | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p-value | |
| Striatum | ||||||
| Caudate | 8338.2 | 865.1 | 8693.7 | 878.1 | 1.03 | .512 |
| Putamen | 11,742.7 | 1052.2 | 11,804.0 | 1058.4 | 0.31 | .763 |
| Globus Pallidus | 4242.6 | 485.5 | 4417.8 | 484.2 | 1.00 | .512 |
| Nucleus Accumbens | 1447.2 | 216.3 | 1389.5 | 187.7 | 3.68S | .472 |
| Amygdala | 3759.2 | 395.8 | 3893.8 | 336.4 | 1.43 | .512 |
| Hippocampus | 9254.7 | 792.5 | 9382.8 | 749.7 | 0.00 | .992 |
| Thalamus | 14,465.7 | 1459.8 | 15.046.2 | 1187.1 | 1.47 | .512 |
| Cerebellum | 130,490.5 | 12,289.3 | 134,281.3 | 13,773.0 | 0.19 | .763 |
Analyses control for age, gender, total brain volume, and TR and p-values corrected for the false discovery rate (FDR).
Cortical Thickness and ADHD
Cortical thickness was significantly associated with inattention in 15 of the 34 left hemisphere regions and 13 of the 27 right hemisphere regions that differed in thickness between children with and without exposure to institutionalization, such that reduced thickness was associated with higher symptoms levels (Table 5). Reduced cortical thickness was associated with greater impulsivity in 10 of the 34 left hemisphere regions and 13 of the 27 right hemisphere regions that differed according to institutionalization. Cortical thickness was significantly associated with both inattention and impulsivity in the superior and inferior parietal cortex, MFG, superior temporal gyrus and sulcus, supramarginal gyrus, and precuneus. Additional regions associated with ADHD symptoms included the lateral OFC, frontal pole, postcentral gyrus, fusiform gyrus, inferior temporal gyrus, insula, and lingual gyrus.
Table 5.
Cortical regions significantly associated with symptoms of inattention and impulsivity in the Bucharest Early Intervention Project (n=74)1
| Inattention | Impulsivity | ||||
|---|---|---|---|---|---|
| β | FDR corrected p-value |
β | FDR corrected p-value |
||
| Brain Area | Region2 | ||||
| Left Hemisphere | |||||
| Superior Parietal Cortex | 1 | −.38 | .013 | −.29 | .027 |
| Superior Temporal Gyrus | 2 | −.45 | .001 | −.36 | .009 |
| Superior Parietal Cortex | 5 | −.27 | .036 | −.33 | .013 |
| Inferior Parietal Cortex | 7 | −.34 | .013 | −.27 | .051 |
| Superior Parietal Cortex | 11 | −.43 | .001 | −.34 | .012 |
| Precuneus | 16 | −.36 | .007 | −.35 | .011 |
| Posterior Cingulate | 17 | −.25 | .040 | −.20 | .098 |
| Middle Frontal Gyrus | 18 | −.34 | .009 | −.25 | .053 |
| Lateral Orbitofrontal | 19 | −.37 | .009 | −.31 | .035 |
| Inferior Parietal Cortex | 22 | −.32 | .011 | −.30 | .018 |
| Postcentral Gyrus | 23 | −.27 | .036 | −.19 | .133 |
| Supramarginal Gyrus | 25 | −.27 | .036 | −.24 | .063 |
| Superior Temporal Sulcus | 27 | −.43 | .001 | −.35 | .009 |
| Inferior Parietal Cortex | 28 | −.25 | .049 | −.15 | .211 |
| Superior Parietal Cortex | 32 | −.30 | .018 | −.12 | .314 |
| Right Hemisphere | |||||
| Middle Frontal Gyrus | 1 | −.33 | .008 | −.31 | .018 |
| Inferior Parietal Cortex | 2 | −.43 | .001 | −.42 | .002 |
| Inferior Temporal Gyrus | 4 | −.33 | .014 | −.35 | .013 |
| Supramarginal Gyrus | 5 | −.36 | .005 | −.34 | .011 |
| Superior Parietal Cortex | 6 | −.43 | .001 | −.38 | .009 |
| Frontal Pole | 7 | −.36 | .007 | −.36 | .012 |
| Precuneus | 8 | −.45 | .001 | −.41 | .002 |
| Superior Temporal Gyrus | 9 | −.40 | .002 | −.30 | .023 |
| Fusiform Gyrus | 12 | −.34 | .008 | −.36 | .009 |
| Insula | 16 | −.43 | .001 | −.37 | .009 |
| Lingual Gyrus | 21 | −.40 | .002 | −.36 | .009 |
| Superior Temporal Sulcus | 25 | −.41 | .001 | −.26 | .045 |
| Insula | 27 | −.40 | .002 | −.27 | .039 |
Significant at the .05 level, 2-sided test after correction for false discovery rate (FDR)
Associations reported for symptoms of inattention and impulsivity are based on linear regression controlling for age, gender, total brain volume, and TR.
Mediation Analysis
A significant indirect effect of institutionalization on inattention through cortical thickness was observed for the OFC (95% CI: 0.07, 1.54), insula (95% CI: 0.20, 1.57), inferior parietal cortex (95% CI: 0.01, 2.44), supramaringal gyrus (95% CI: 0.05, 2.16), precuneus (95% CI: 0.43, 2.18), superior temporal cortex (95% CI: 0.86, 3.18), and lingual gyrus (95% CI: 0.07, 1.56). The total effect of institutionalization on inattention, β = 0.54, p < .001, was no longer significant when these regions were added to the model, β = 0.19, p = .15, and was reduced by 64.8% when cortical thickness in these regions was controlled.
A significant indirect effect of institutionalization on impulsivity through cortical thickness was observed for the OFC (95% CI: 0.11, 2.49), insula (95% CI: 0.13, 2.36), inferior parietal cortex (95% CI: 0.10, 3.61), precuneus (95% CI: 0.68, 3.30), superior temporal cortex (95% CI: 0.27, 4.08), fusiform gyrus (95% CI: 0.13, 2.53), and lingual gyrus (95% CI: 0.13, 2.33). The effect of institutionalization on impulsivity, β = 0.43, p < .001, was no longer significant when these regions are added to the model, β = 0.08, p = .58, and was reduced by 81.7% after accounting for cortical thickness in these regions.
Sensitivity Analysis
We conducted sensitivity analyses to determine whether other differences between the groups explained our findings, including birth weight, IQ, and medication status. We used a GLM to examine the association of cortical thickness with a) birth weight in the 66 participants (82.5%) that had this data available; and b) IQ in every vertex in the brain. After correction for FDR, no brain regions in either hemisphere were associated with birth weight or IQ, indicating that these factors were not plausible confounders of the association between institutionalization and neural structure. We also examined group differences in cortical thickness after excluding: a) the 4 participants on psychiatric medications at the time of scan; and b) the 5 participants acquired in a different orientation. Cortical regions that differed in thickness across groups were unchanged (Supplement: Tables S1–S4).
Discussion
ADHD is a common neurodevelopmental disorder. Institutional rearing is strongly associated with ADHD, which has generated questions about the neurodevelopmental pathways linking early-life psychosocial deprivation to ADHD. (21–23) We investigated this issue in a sample of children raised in deprived institutional settings to determine whether atypical neural structure was a mechanism linking institutional rearing to elevations in ADHD symptoms. Our findings provide novel evidence of widespread reductions in cortical thickness as a neurodevelopmental mechanism linking adverse psychosocial experience to the onset of ADHD. We found no evidence for a sub-cortical pathway linking institutionalization to ADHD.
This is the first study to document the effects of psychosocial deprivation on patterns of cortical thickness in children. Prior research indicates that a wide range of adverse early environments—including institutional rearing, abuse, and neglect—are associated with reduced cerebral and cortical volume. (24,25, 51–54) However, with one exception, (53) these studies have focused on global markers of cortical development and have not identified specific cortical regions associated with environmental adversity. Our findings indicate that institutional rearing is associated with pronounced reductions in cortical thickness in the PFC, including dorsolateral and OFC regions, throughout lateral and medial parietal cortex, including superior and inferior regions, the supramarginal gyrus, precuneus, and posterior cingulate, and in the superior temporal gyrus and sulcus. This pattern of widespread reductions in cortical thickness is consistent with one previous study examining cortical structure in physically abused children, which found reductions in cortical volume in the OFC and in parietal and temporal regions. (53) These findings are also similar to the pattern of pervasive reductions in cortical thickness observed in children with ADHD. (15–17)
We provide novel evidence indicating that reduced cortical thickness is a neurodevelopmental mechanism linking institutionalization to ADHD symptoms. Reductions in cortical thickness associated with institutionalization might reflect either a developmental delay in reaching peak cortical thickness or accelerated cortical thinning in children exposed to psychosocial deprivation. Additional research is needed to adjudicate between these possibilities. In either case, our results suggest that these perturbations in cortical development are associated with the elevated rates of ADHD observed among children exposed to institutional rearing. Although reduced cortical thickness was present in children exposed to institutionalization across numerous regions, only a few areas significantly mediated the association of institutionalization with ADHD symptoms. Specifically, cortical thickness in lateral OFC, insula, inferior parietal cortex, precuneus, superior temporal gyrus and sulcus, and lingual gyrus mediated the association of institutionalization with inattention and impulsivity; supramarginal gyrus thickness additionally mediated the association with inattention and fusiform gyrus thickness additionally mediated the association with impulsivity. This pattern is largely consistent with findings from meta-analyses of fMRI studies, which document blunted activation in dorsolateral PFC, inferior parietal cortex, precuneus, and superior temporal cortex during attention tasks in ADHD. (11) These regions are integral to cognitive processes disrupted in ADHD including working memory storage, target detection, attentional orienting, and attention allocation. (55–59) Additionally, the precuneus and inferior parietal lobule are central nodes in the default mode network. (60, 61) Fluctuations in default mode network activation have been linked to attention lapses, (62) and some have hypothesized that this network underlies attention to external stimuli; (63) it is possible that atypical cortical structure in regions associated with the default mode network are related to the attentional deficits that underlie ADHD. This possibility warrants examination in future research. Finally, the OFC is involved in emotion regulation, social behavior, and decision making in situations involving reward or other emotionally salient cues. (64, 65) Children with ADHD exhibit impulsivity and problems in decision making, particularly in situations with high reward salience, (66) which may be related, in part, to abnormalities in the structure of the OFC.
In contrast, institutionalization was unrelated to the volume of sub-cortical structures, including the striatum, or to cerebellar volume. These findings are surprising for several reasons. First, institutional rearing has been associated with amygdala and cerebellum volume in previous studies. (24,29–30) Second, meta-analyses have identified the caudate and other divisions of the basal ganglia as regions that differ in structure among those with ADHD relative to controls. (13, 14) Third, previous research suggests important functional differences in the basal ganglia, particularly the caudate, among children with ADHD compared to controls. (67–71) Abnormalities in fronto-striatal function are central to theoretical conceptualizations of cognitive deficits in ADHD, including working memory and response selection and inhibition. (9,72–73) Striatal contributions to ADHD symptomatology may reflect predominantly genetic and prenatal influences whereas cortical mechanisms reflect a combination of both pre- and postnatal influences. Future research is needed to evaluate this possibility empirically. It is important to acknowledge, however, that the lack of differences in striatal volume as a function of institutionalization may have resulted from measurement error given the optimization of FreeSurfer algorithms for cortical analysis.
Children exposed to institutionalization exhibited reductions in cortical thickness in numerous regions of the prefrontal, parietal and temporal cortex, and this atypical pattern of neurodevelopment was a mechanism linking institutionalization to ADHD. These findings have important implications for understanding the role of psychosocial experience in the developmental neurobiology of ADHD. Theoretical conceptualizations argue that ADHD involves fundamental deficits in the ability to generate accurate predictions about the type and timing of environmental events and to engage top-down control processes to alter behavior following experiences that violate predictions. (9) The deprived social environment of institutions may contribute to these deficits by affording children few opportunities to detect and learn environmental contingencies in order to facilitate accurate predictions about future events. Moreover, associative learning that occurs in the highly structured and atypical environment of institutions might impair prediction ability once children leave institutional care. In either case, children are provided limited experience engaging top-down control systems to regulate behavior in novel or unexpected circumstances. These experiences likely result in pervasive underutilization of multiple areas in association cortex, which may ultimately lead to the widespread reductions in cortical thickness observed here.
Identifying the specific aspects of psychosocial experience that predict disruptions in cortical development is an important goal for future research in order to elucidate mechanisms linking other types of adverse environments with ADHD. Executive functioning deficits and ADHD are common among children raised in families with low socio-economic status (74, 75) and those exposed to other types of psychosocial adversity. (76, 77) Determining whether the same cortical pathways are involved in these associations warrants examination in future studies. Conversely, other types of experience that lead to the pattern of cortical maturation observed here may also increase propensity for ADHD (e.g., preterm birth). Finally, the degree to which early intervention can mitigate the effects of adverse environmental experiences on cortical development is unknown.
The lack of intervention effect on ADHD and cortical structure among children randomized to foster care in this sample is surprising, given marked improvements resulting from the intervention in other cognitive and psychosocial domains. (21, 78) A previous study of children adopted out of Romanian institutions observed no elevations in ADHD among children placed before 6 months of age. (22, 23) No children were placed in foster care this early in the BEIP, suggesting that psychosocial experience very early in life might exert a lasting influence on cortical development that influences risk of ADHD and is not ameliorated by later intervention.
Several limitations are worth noting. First, ADHD symptoms were assessed using a teacher-report measure rather than a diagnostic interview. However, ADHD behaviors frequently manifest in the school setting, and in this sample teacher reports provide a more standardized method of reporting ADHD symptoms than caregiver reports, given variation across groups in the length and quality of caregiver relationships. Teachers have a unique perspective in having substantial amounts of time in which to observe children of a given age and to evaluate individual differences. Future research is nevertheless needed to replicate these findings in predicting ADHD diagnosis based on structured interviews. Second, the number of control participants was small relative to the number of children exposed to institutional rearing. Third, several previously-institutionalized children were on medications for ADHD at the time of scan. However, sensitivity analysis indicated no difference in results when these children were excluded. Fourth, group differences in ADHD may be related to factors other than postnatal rearing environments, such as prenatal malnutrition, exposure to alcohol or other toxins, or genetic factors. Though we cannot rule out genetic and prenatal differences between children with and without exposure to institutionalization, results were unchanged when we controlled for birth weight. Additionally, although meaningful IQ and birth weight differences exist across study groups, IQ and birth weight were unassociated with cortical thickness, indicating that they are not a plausible confounders of the observed associations with neural structure. Finally, differences in scan acquisition or motion may have contributed to our findings. However, neither differences in scan parameters nor rejection of scans due to artifact differed across groups, reducing concern about this possibility. Moreover, TR was included a covariate in all analysis and sensitivity analysis indicates that removing the five subjects acquired in a different orientation did not change the pattern of results.
We present novel evidence for a neurodevelopmental mechanism linking institutional rearing to ADHD symptomatology. Children reared in institutions exhibited widespread reductions in cortical thickness. Reductions in thickness in the prefrontal, parietal, and temporal cortex explained, at least in part, inattention and impulsivity observed in these children. Early-life psychosocial deprivation appears to disrupt cortical development, culminating in heightened risk of ADHD. Future research is needed to determine whether interventions targeted very early in the life course ameliorate these aberrant patterns of brain development and their behavioral consequences.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by a grant from the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development (Charles A. Nelson, Network Chair) and the National Institutes of Health (R01-MH091363 to Nelson, K01-MH092526 to McLaughlin, and K01-MH092555 to Sheridan). These funders provided support for all data collection and analysis. We thank Sebastian Koga for overseeing the project in Romania; Hermi Woodward and members of the MacArthur Foundation Research Network on Early Experience and Brain Development for input regarding the conceptualization, design, and implementation of the project; the caregivers and children who participated in this project; and the Bucharest Early Intervention Project staff for their tireless work on our behalf.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors, including Drs. McLaughlin, Sheridan, Fox, Zeanah, and Nelson and Mr. Winter, declare that they have no biomedical financial interests or potential conflicts of interest.
References
- 1.Polanczyk G, Silva de Lima M, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Scahill L, Schwab-Stone ME. Epidemiology of ADHD in school-age children. Child and Adolescent Psychiatric Clinics of North America. 2000;9:541–555. [PubMed] [Google Scholar]
- 3.Faraone SV, Sergeant JA, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 4.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 5.Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 7.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 10.Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. Archives of General Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 12.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufman WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 14.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 15.Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, et al. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw P, Lerch JP, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gogtay N, Giedd J, Rapoport JL. Brain development in healthy, hyperactive, and psychotic children. Archives of Neurology. 2002;59:1244–1248. doi: 10.1001/archneur.59.8.1244. [DOI] [PubMed] [Google Scholar]
- 19.Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biological Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- 20.Faraone SV, Doyle AE. The nature and heritability of attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2001;10:299–316. [PubMed] [Google Scholar]
- 21.Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 22.Stevens SE, Sonuga-Barke EJS, Kreppner JM, Beckett C, Castle J, Colvert E, et al. Inattention/overactivity following early severe institutional deprivation: Presentation and associations in early adolescence. Journal of Abnormal Child Psychology. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- 23.Kreppner JM, O'Connor TG, Rutter M, Beckett C, Castle J, Croft C. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- 24.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences. 2012;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Behen ME, Singsoonsud P, Veenstra AL, Wolfe-Christensen C, Helder E, et al. Microstructural abnormalities in language and limbic pathway in orphanage-reared children: A diffusion tensor imaging study. Journal of Child Neurology. 2013 doi: 10.1177/0883073812474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 28.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cerebral Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tottenham N, Hare T, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeanah CH, Nelson CB, Fox NA, Smyke AT, Marshall PJ, Parker SW, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 32.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 37.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 39.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 41.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 42.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 43.Ostby Y. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, et al. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. Journal of Child Psychology and Psychiatry. 2011;52:56–63. doi: 10.1111/j.1469-7610.2010.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemery Chalfant K, Schreiber JE, Schmidt NL, Van Hulle CA, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing, and attention problems in young children: Validation of the MacArthur HBQ. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1315–1323. doi: 10.1097/chi.0b013e3180f616c6. [DOI] [PubMed] [Google Scholar]
- 47.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 48.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 50.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 51.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology Part II: Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 52.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 53.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson JRT, et al. Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in maltreatment-related posttraumatic stress disroder: A sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 55.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 56.Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- 57.Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. An event-related fMRI study of visual and auditory oddball tasks. Journal of Psychophysiology. 2001;15:221–240. [PubMed] [Google Scholar]
- 58.Stevens MC, Pearlson GD, Kiehl KA. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164:1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- 59.Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: Event-related fMRI evidence. American Journal of Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- 60.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 61.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8 doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 62.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on "Wandering minds: the default network and stimulus-independent thought". Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 64.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;20:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 65.O'Doherty JP, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 66.Garon N, Moore C, Waschbusch DA. Decision making in children with ADHD only, AHDH-Anxious/Depressed, and control children using a child version of the Iowa Gambling Task. Journal of Attention Disorders. 2006;9:607–619. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- 67.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention deficit/hyperactivity disorder. JAMA: Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 68.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 69.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 70.Durston S, Tottenham N, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 71.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Toney ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 72.Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfuntion. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 74.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 75.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 76.Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, et al. Family-environment risk factors for attention-deficit hyperactivity disorder: A test of Rutter's indicators of adversity. Archives of General Psychiatry. 1995;52:464–470. doi: 10.1001/archpsyc.1995.03950180050007. [DOI] [PubMed] [Google Scholar]
- 77.Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT. Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29:526–533. doi: 10.1097/00004583-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.