Abstract
The tibial nerve transection model is a well-tolerated, validated, and reproducible model of denervation-induced skeletal muscle atrophy in rodents. Although originally developed and used extensively in the rat due to its larger size, the tibial nerve in mice is big enough that it can be easily manipulated with either crush or transection, leaving the peroneal and sural nerve branches of the sciatic nerve intact and thereby preserving their target muscles. Thus, this model offers the advantages of inducing less morbidity and impediment of ambulation than the sciatic nerve transection model and also allows investigators to study the physiologic, cellular and molecular biologic mechanisms regulating the process of muscle atrophy in genetically engineered mice. The tibial nerve supplies the gastrocnemius, soleus and plantaris muscles, so its transection permits the study of denervated skeletal muscle composed of fast twitch type II fibers and/or slow twitch type I fibers. Here we demonstrate the tibial nerve transection model in the C57Black6 mouse. We assess the atrophy of the gastrocnemius muscle, as a representative muscle, at 1, 2, and 4 weeks post-denervation by measuring muscle weights and fiber type specific cross-sectional area on paraffin-embedded histologic sections immunostained for fast twitch myosin.
Keywords: Medicine, Issue 81, mouse, tibial nerve, gastronemius, soleus, atrophy, denervation, reinnervation, myofiber, transection
Introduction
Skeletal muscle denervation, due to traumatic peripheral nerve injury, disease or pharmacologic intervention, results in the immediate loss of muscle voluntary contractile function. Muscle concomitantly begins to atrophy and this atrophy is reversible if timely, good-quality reinnervation occurs1,2. In the absence of reinnervation, myofiber atrophy progresses, and irreversible biologic changes in the muscle occur with muscle fibrosis and myofiber death. Here we demonstrate the tibial nerve transection model, a model of denervation-induced skeletal muscle atrophy and fibrosis, in mice. This model enables scientists to study the physiologic, cellular and molecular biologic mechanisms that underlie muscle atrophy in vivo in the gastrocnemius and soleus muscles. While historically used predominantly in rats, more recent application of this model to knockout and transgenic mouse lines specifically, allows investigators to assess the role of their particular protein(s) of interest in the induction, development and maintenance, or alternatively the resolution of, muscle atrophy and fibrosis in vivo.
The tibial nerve is a mixed motor-sensory peripheral nerve in the rodent hindlimb, and is one of the three terminal branches of the sciatic nerve. Transection of the tibial nerve denervates the gastrocnemius, soleus and plantaris muscles (and the three small deep flexor muscles of the foot including tibialis posterior, flexor digitorum longus and flexor hallicus longus), and is a well standardized and validated model in rats3,4. The gastrocnemius and soleus muscles can be easily dissected at serial time points post tibial nerve transection, fixed and processed for assessment of muscle histology and muscle fiber morphometrics, or flash frozen for extraction of muscle RNA and protein for the purpose of studying, for example, the cellular signaling networks regulating muscle atrophy. The gastrocnemius muscle is a mixed fiber type muscle (type I and type II, although predominantly type II) and the soleus muscle is composed of a large proportion of type I fibers, thereby providing both fast and slow twitch muscle for assessment5,6. The tibial nerve transection model is suitable for studying the process of denervation-induced muscle atrophy in both the short term (days)7 and long term (weeks to months)4,8.
In contrast to the sciatic nerve transection model (a second model of denervation-induced muscle atrophy commonly used in rodents), tibial nerve transection induces less morbidity in the animal, making it a more attractive model. Transection of the sciatic nerve denervates all the muscles of the leg (below the knee) and foot, impairing the animal's ability to ambulate2, whereas transection of the tibial nerve leaves the peroneal and sural nerve branches of the sciatic nerve intact, thus preserving their target muscles and sensory territories. The mouse is unable to plantar flex or invert the foot, but is able to ambulate easily and the weight bears equally on both hind limbs, thereby significantly diminishing the morbidity of the model. Gait analysis studies evaluating walking patterns have been performed in rats following tibial and sciatic nerve injuries and demonstrate that footprint and weight bearing is better preserved with tibial injury9,10. In addition, in the tibial nerve transection model, the peroneal nerve can be mobilized at a later time point and transferred as a source of delayed reinnervation, if the study design requires3. In contrast, delayed reinnervation in the sciatic nerve transection model necessitates the use of a nerve graft to the sciatic nerve deficit, very significantly increasing the technical difficulty of the model and limiting its use to skilled surgeons.
While the tibial nerve transection model requires familiarity of the operator with sterile operative technique in animal surgery, both the tibial nerve and calf muscles it innervates are easily accessible and identifiable for manipulation, so that individuals who are not surgeons, or highly experienced with animal surgery, can readily master this model.
Protocol
Prior to using this model, investigators must have received approval for the surgical protocol from their institution's animal use governing body. The model is approved by the Research Ethics Board, Hamilton Health Sciences Corporation, McMaster University (AUP # 10-04-24) and is carried out in strict accordance with the recommendations of the Canadian Council on Animal Care.
1. Mouse Preparation
Weigh the mouse. Induce anesthesia with 5% isoflurane or 2% halothane. The circuit used should ensure adequate scavenging of the anesthetic to protect the surgeon. After 2-3 min the animal's respiration will slow. Ensure the blink reflex is absent and pinch the interdigital spaces in the paw to confirm surgical anesthesia (i.e. no response by the mouse). Apply ophthalmic lubricant to the eye, to prevent drying of the cornea during surgery.
Shave the lateral thigh and buttock from the sciatic notch to the knee and disinfect with proviodine. Shaving will keep the site of the incision hair free to ensure adequate visualization of the surgical field and to minimize interference with nerve dissection and transection. The sciatic notch, which is superior and posterior to the femur, can be identified by palpation.
2. Operative Procedure
Reduce inhalational isoflurane to 2% (halothane 1%) and place the mouse on its side (side intended for surgery facing up), under an operating or dissecting microscope. Alternatively surgery can be performed with surgical loupes since 3.5X magnification is satisfactory for an adult (20-25 g) mouse.
Don sterile gloves. Identify the sciatic notch by palpation. Using a scalpel, incise the skin of the lateral thigh from the sciatic notch to the knee (approximately 1 cm).
Gently spread the skin. Identify the biceps femoris muscle, which is the flat superficial muscle of the lateral thigh immediately beneath the skin. Using fine scissors, split the biceps femoris muscle along the muscle fibers and hold open with a spring retractor to expose the sciatic nerve and its branches.
Identify the sciatic nerve immediately deep to the biceps femoris muscle. It can be identified by its characteristic shiny white color and is approximately 0.8 mm in diameter. It runs from the sciatic notch to the knee, branching into the tibial, peroneal and sural nerves at the level of the popliteal fossa.
Gently separate the tibial from the peroneal and sural nerve branches with ultrafine forceps and spring microdissecting scissors. The tibial nerve is the largest branch and is usually central. It's important not to crush the nerve while separating the branches. Holding the nerve just on the outer adventicial layer with the ultrafine forceps, and keeping the nerve slack (not taught), will avoid nerve crush and traction injury.
For complete and lasting denervation, cut the tibial nerve with microdissecting scissors as distally as possible, carefully avoiding the popliteal vessels. Alternatively, for temporary denervation with expected complete reinnervation in 2-4 weeks, the tibial nerve can simply be crushed with ultrafine forceps for 15 sec instead of transected. (Peripheral nerves regrow following injury and will reinnervate target muscle.)
If complete denervation is required, suture the end of the transected tibial nerve to the anterior surface of the biceps femoris muscle with 10-0 Nylon and re-approximate the biceps femoris with 5-0 Vicryl to prevent aberrant re-innervation of the gastrocnemius and soleus muscles.
Close the skin with a running 5-0 Vicryl suture.
3. Post Operative Care
Turn off the inhalational anesthetic but maintain the flow of oxygen. Administer buprinorphine (or substitute) analgesic subcutaneously.
Transfer the mouse to a clean cage with no bedding while waking from anesthesia. Keep on a warming blanket within the cage and under direct observation until ambulating.
Transfer and house in soft bottom cage (not wire) with ample soft bedding.
Inspect the operative limb daily for condition of the surgical wound and the foot for the development of decubitus heel ulcers or evidence of chewing. Minor problems can be managed with topical antibiotics or antiseptics such as proviodine. Endpoint indicators requiring animal euthanasia are weight loss, evidence of poor self care (ruffled fur), and hunched posture. In addition, animals with major wound disruption or ulcers that do not heal in 1-2 weeks with topical antibiotics or appear to have pain should be sacrificed.
4. Denervated Gastrocnemius and Soleus Muscle Harvest
At the post-operative time point desired, weigh the mouse and sacrifice with CO2 overdose.
Shave the medial aspect of both the operative and contralateral control legs and clean with alcohol. Place the mouse under an operating or dissecting microscope, or alternatively use surgical viewing loupes for magnification.
On the operative limb, incise the medial calf skin with a scalpel from the ankle to the knee and circumferentially around the ankle. Gently pull the skin with forceps off the muscle and proximally toward the thigh. This exposes all the muscles of the leg. Identify the gastrocnemius muscle, which is the calf muscle that runs from the knee to the ankle on the posterior aspect of the leg and lies immediately underneath the skin. Identify the distal insertion of the biceps femoris muscle, proximal to the gastrocnemius muscle on the medial aspect of the knee. At its distal insertion the biceps femoris appears thin and filmy and overlies the most proximal portion of the gastrocnemius. Using scissors and blunt end dissection, gently separate the distal insertion of the biceps femoris from the gastrocnemius muscle.
At its distal insertion into the calcaneous, the gastrocnemius muscle tapers into the Achilles tendon. Identify the Achilles tendon, which appears white and sinewy. Hold the Achilles tendon with forceps, taking care not to hold or crush the gastrocnemius muscle, and divide the Achilles tendon from the calcaneus insertion using scissors.
Still holding the tendon, gently lift the gastrocnemius muscle (pale red) off the deep soleus (deeper red), from the distal insertion towards its origin at the knee (the soleus can be harvested separately).
Dissect the gastrocnemius off the leg by dividing the origin of the gastrocnemius from the medial and lateral femur chondyles using scissors. Very gentle traction on the muscle facilitates this process. Be careful not to crush the muscle.
The soleus will now be plainly visible, immediately under the site of the gastrocnemius. Lift the soleus from its insertion on the Achilles tendon to its origin on the posterior calf. If the soleus is inadvertently raised with the gastrocnemius, gently separate it from the harvested specimen. The gastrocnemius (light red) and soleus (dark red) remain easily identifiable in the harvested specimen due to their color differences.
Weigh the muscles separately on a precision scale.
Split the muscles vertically, half for snap freezing in liquid nitrogen (for subsequent protein and/or RNA extraction), and half for histology (i.e. morphometric assessment, immunohistology) fixing either in 10% formalin, or frozen fixation in isopentane cooled with liquid nitrogen, as desired.
Repeat from step 4.3 above on the control, un-operated, side to harvest control gastrocnemius and soleus muscles.
Representative Results
Tibial nerve transection denervates the gastrocnemius, soleus and plantaris muscles of the calf. Here we assess the development of atrophy in the gastrocnemius muscle, as a representative muscle. Gastrocnemius muscle was harvested from 2-3 months old C57Black 6 mice (Jackson Laboratories) denervated for 1, 2, or 4 weeks. Muscle weights progressively decrease (Figure 1), as does the cross-sectional area of type II fast twitch muscle fibers (Figure 2), over time. The gastrocnemius is a mixed fiber type muscle (type I and type II), but denervation induces a fiber type switch from type I to type II fibers11, and as a result an adequate number of type I fibers may not be available for measurement and robust statistical analysis.
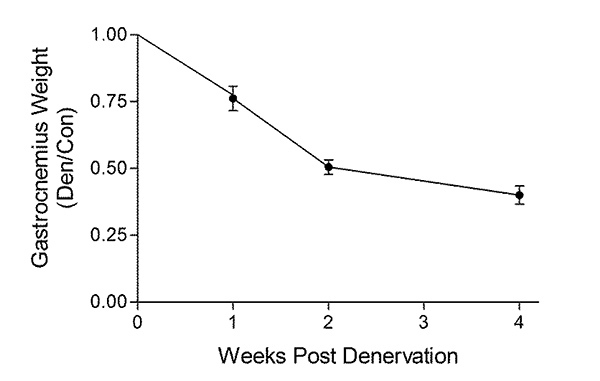 Figure 1. Denervated gastrocnemius muscle demonstrates progressive atrophy. C57Black 6 mice underwent transection of the right tibial nerve. Gastrocnemius muscle was harvested from the denervated (right) and contralateral control (left) hindlimbs at 1, 2, or 4 weeks following nerve transection. Gastrocnemius muscles were weighed, and the weight of the denervated muscle is expressed as a ratio of the contralateral control innervated muscle. Denervation induces a progressive loss of muscle mass.
Figure 1. Denervated gastrocnemius muscle demonstrates progressive atrophy. C57Black 6 mice underwent transection of the right tibial nerve. Gastrocnemius muscle was harvested from the denervated (right) and contralateral control (left) hindlimbs at 1, 2, or 4 weeks following nerve transection. Gastrocnemius muscles were weighed, and the weight of the denervated muscle is expressed as a ratio of the contralateral control innervated muscle. Denervation induces a progressive loss of muscle mass.
 Figure 2. Denervated gastrocnemius muscle demonstrates progressive decrease in myofiber cross section area (A) Denervated and control gastrocnemius muscles were formalin fixed, cut on cross section at the muscle mid-section and immunostained for anti-skeletal muscle myosin, fast twitch isoform (My-32, Sigma, 1:500 dilution) followed by biotinylated secondary antibody and streptavidin-HRP/DAB as described7. Hematoxylin was used as a counterstain. Fast twitch type II fibers stain brown and slow twitch type I fibers stain light purple. The cross-sectional area (CSA) of the fibers was measured using ImageJ software (Bethesda, NIH) as described7,12. The fast twitch type II fibers demonstrate progressive atrophy. Too few type I fibers are present in denervated gastrocnemius to permit a statistically valid evaluation of fiber size. (n = 6 to 9 mice/group. A minimum of 200 myofibers were measured per muscle by a reviewer blinded to operative phenotype. Data are presented as the mean +/- SD. Scale bar equals 100 μm). Click here to view larger figure.
Figure 2. Denervated gastrocnemius muscle demonstrates progressive decrease in myofiber cross section area (A) Denervated and control gastrocnemius muscles were formalin fixed, cut on cross section at the muscle mid-section and immunostained for anti-skeletal muscle myosin, fast twitch isoform (My-32, Sigma, 1:500 dilution) followed by biotinylated secondary antibody and streptavidin-HRP/DAB as described7. Hematoxylin was used as a counterstain. Fast twitch type II fibers stain brown and slow twitch type I fibers stain light purple. The cross-sectional area (CSA) of the fibers was measured using ImageJ software (Bethesda, NIH) as described7,12. The fast twitch type II fibers demonstrate progressive atrophy. Too few type I fibers are present in denervated gastrocnemius to permit a statistically valid evaluation of fiber size. (n = 6 to 9 mice/group. A minimum of 200 myofibers were measured per muscle by a reviewer blinded to operative phenotype. Data are presented as the mean +/- SD. Scale bar equals 100 μm). Click here to view larger figure.
Discussion
The tibial nerve transection model of denervation-induced skeletal muscle atrophy is a commonly employed and well validated model in rats. We have adapted this model for use in mice, which allows the investigator to take advantage of the existence of genetically engineered mice and study the process of muscle atrophy in vivo in the absence of proteins crucial to the regulation of muscle mass7,8. The gastrocnemius and soleus muscles, both denervated in this model, can be easily and rapidly dissected with minimal handling, thus providing excellent quality mRNA and protein for subsequent molecular analyses. Similarly, because of the size of the muscles, they can be split, providing tissue from the same animal for concomitant histologic and morphometric analyses. If hindlimb functional assessment is required, walking track analysis can be serially performed. The feet are dipped in ink and the mouse is walked through a enclosure with paper on the bottom. Characteristics of the prints can be reliably measured and scored to indicate the extent of neuromuscular disability and gait compromise, since footprint characteristics reflect the functional muscle groups13,14. While originally developed and validated in rat13, walking track analysis can also be performed in mice15.
Tibial nerve transection is generally extremely well tolerated by the mice. Only a single dose of analgesic is necessary in the immediate postoperative period. With the use of proper sterile technique, soft tissue infection is rare. While tibial nerve transection does induce sensory paraesthesia on the plantar aspect of the foot, in our experience C57black6 and knockout or transgenic mice derived on this line do not tend to auto-mutilate. However, the mice must be inspected daily for signs of auto-mutilation, heel pressure ulcers, as well as point of care endpoints. While we have negligible mortality with the model, we find that approximately 2-5% of mice must be euthanized due to self-mediated injury to, or pressure ulcers developing on, the operated hind limb. The use of soft bedding post-operatively is crucial to ensure the animal's comfort and helps to prevent the development of pressure ulcers on the operated side. Sciatic nerve ligation as well as the SNI model of ligation (where the tibial and common peroneal branches of the sciatic are ligated, but the sural is left intact) serve as models of neuropathic pain16,17. Thus, allodynia and thermal hyperalgesia could occur in the foot in our model as well, but we have not seen overt pain behavior in the mice with normal daily activity on soft bedding.
The tibial nerve of only one hindlimb is transected and since the mice weight bear almost equally on both hindlimbs, the musculature from the contralateral un-operated limb can be used as an internal control within each animal7-10. This is not necessarily the case in the sciatic transection model, where more significant abnormalities of gait can induce a hypertrophic response in the contralateral limb muscle. In the tibial nerve transection model, we typically use the gastrocnemius and soleus muscle from the un-operated limb as our control muscle7,8. If the investigator chooses to use separate animals from which to harvest control muscle, then sham surgery should be performed. Sham surgery would consist of the administration of anesthesia, splitting of the skin to expose the tibial nerve, but no transection. Skin would simply be closed following nerve exposure.
In some peripheral nerve transection models, errant reinnervation from the proximal stump to the target muscle contaminates the planned denervation. In this model, securing the proximal end of the transected tibial nerve to the superficial surface of the biceps femoris muscle, thus closing the muscle interface, inhibits the errant reinnervation. As such, it is a critical and essential step in the model. Errant reinnervation is rare in this model.
Similarly, careful handling of the nerve during surgery is essential. The sural and peroneal nerve branches must be gently separated from the tibial nerve prior to tibial transection, and not crushed or stretched in the process. Rough handling of these nerves will compromise their function, partially denervating other hindlimb musculature. If this occurs the animal's gait will be differentially influenced compared to those mice undergoing sole tibial nerve transection, and the variable muscle loading may contaminate the experimental results. Similarly, care must be taken when dissecting the denervated musculature. Muscle should be handled by the tendon, and not grasped directly to avoid crush artifact that will affect histology, muscle fiber morphometric analyses and possibly gene expression.
We typically employ this model in mice 20-24 g (2-3 months of age), as the animal is mature and the sciatic and tibial nerves are both of adequate size to be easily handled. The surgery can be performed on younger, smaller animals if desired, but the limiting factor here will be the prowess of the operating surgeon. This may be an issue if the investigator is interested in studying the satellite cell response in denervated muscle. The satellite cell regenerative potential diminishes in older, compared to younger, animals18 and therefore younger animals may be required in the experimental design, presenting a technical challenge for a less experienced operator.
The tibial nerve transection model can be adapted from simply a model of denervation-induced muscle atrophy, to one of delayed muscle reinnervation (>4 weeks), if desired and if experienced surgical operators are available1,3. Following a period of denervation specified by the investigator, the mouse can be reoperated and the peroneal nerve mobilized to reinnervate the denervated musculature. The distal stump of the transected tibial nerve is identified, trimmed and the peroneal nerve is mobilized at its distal end and microsurgically repaired to the tibial nerve stump. The peroneal nerve will grow at a rate of approximately 1 mm/day into the tibial nerve stump to reinnervate the gastrocnemius and soleus muscles. The tibial nerve transection model provides an advantage over the sciatic nerve transection model in that a nerve graft is not necessary in the reinnervation procedure2 because of the availability of the peroneal nerve. However, it should be noted that nerve reanastomoses requires the precision of a skilled and experienced operator.
In summary, here we demonstrate the tibial nerve transection model in mice, as an easy, robust, well validated and reproducible model of denervation-induced skeletal muscle atrophy.
Disclosures
No conflicts of interest are declared.
Acknowledgments
This work was supported by grants from the CIHR Neuromuscular Research Partnership (JNM – 90959; to J.A.E.B).
References
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J. Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WM. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001. pp. 103–503. [DOI] [PubMed]
- Batt J, Bain J, Goncalves J, Michalski B, Plant P, Fahnestock M, Woodgett J. Differential gene expression profiling of short and long term denervated muscle. FASEB J. 2006;20:115–117. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- Sher J, Cardasis C. Skeletal muscle fiber types in the adult mouse. Acta Neurol. Scand. 1976;54:45–56. doi: 10.1111/j.1600-0404.1976.tb07619.x. [DOI] [PubMed] [Google Scholar]
- Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Plant PJ, Correa J, Bain A, Takeda M, Kawabe H, Rotin D, Bain JR, Batt JA. The ubiquitin ligase nedd4-1 participates in denervation-induced skeletal muscle atrophy in mice. PLoS ONE. 2012;7:e46427. doi: 10.1371/journal.pone.0046427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant PJ, Bain JR, Correa JE, Woo M, Batt J. Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J. Appl. Physiol. 2009;107:224–234. doi: 10.1152/japplphysiol.90932.2008. [DOI] [PubMed] [Google Scholar]
- Varejao AS, Meek MF, Ferreira AJ, Patricio JA, Cabrita AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J. Neurosci. Methods. 2001;108:1–9. doi: 10.1016/s0165-0270(01)00378-8. [DOI] [PubMed] [Google Scholar]
- Willand MP, Holmes M, Bain J, Fahnestock M, de Bruin H. Electrical muscle stimulation after immediate nerve repair reduces muscle atrophy without affecting reinnervation. Muscle Nerve. 2013;48:219–225. doi: 10.1002/mus.23726. [DOI] [PubMed] [Google Scholar]
- Sterne GD, Coulton GR, Brown RA, Green CJ, Terenghi G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J. Cell Biol. 1997;139:709–715. doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant PJ, North ML, Ward A, Ward M, Khanna N, Correa J, Scott JA, Batt J. Hypertrophic airway smooth muscle mass correlates with increased airway responsiveness in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2012;46:532–540. doi: 10.1165/rcmb.2011-0293OC. [DOI] [PubMed] [Google Scholar]
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- Hare GM, Evans PJ, Mackinnon SE, Best TJ, Midha R, Szalai JP, Hunter DA. Walking track analysis: utilization of individual footprint parameters. Ann. Plast. Surg. 1993;30:147–153. doi: 10.1097/00000637-199302000-00009. [DOI] [PubMed] [Google Scholar]
- McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knock-out mice. J. Neurosci. 2002;22:5481–5491. doi: 10.1523/JNEUROSCI.22-13-05481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner M, Bjerrum OJ, Nykjaer A, Vaegter CB. The spared nerve injury (SNI) model of induced mechanical allodynia in mice. J. Vis. Exp. 2011. p. e3092. [DOI] [PMC free article] [PubMed]
- Rogoz K, Lagerstrom MC, Dufour S, Kullander K. VGLUT2-dependent glutamatergic transmission in primary afferents is required for intact nociception in both acute and persistent pain modalities. Pain. 2012;153:1525–1536. doi: 10.1016/j.pain.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:22–27. doi: 10.1097/MCO.0b013e3283412260. [DOI] [PubMed] [Google Scholar]


