Abstract
Background
Higher tissue transcript levels of immune-related markers, including the recently discovered viral restriction factor interferon-induced transmembrane protein (IFITM) which inhibits viral entry and replication, have been reported in the prefrontal cortex in schizophrenia. Interestingly, mouse models of neuroinflammation have higher IFITM levels and deficits in GABA-related markers that are similar to findings in schizophrenia, suggesting that a shared pathogenetic process may underlie diverse cortical pathology in the disorder. However, the cell types that overexpress IFITM mRNA in schizophrenia are unknown, and it is unclear whether higher IFITM mRNA levels are associated with lower GABA-related marker levels in the same schizophrenia subjects.
Methods
We used quantitative PCR and in situ hybridization with film and grain counting analyses to quantify IFITM mRNA levels in prefrontal cortex area 9 of 57 schizophrenia and 57 healthy comparison subjects and in antipsychotic-exposed monkeys.
Results
Quantitative PCR and in situ hybridization film analysis revealed markedly elevated IFITM mRNA levels (+114% and +117%, respectively) in prefrontal gray matter in schizophrenia. Interestingly, emulsion-dipped, Nissl-stained sections from schizophrenia and comparison subjects revealed IFITM mRNA expression in pia mater and blood vessels. IFITM grain density over blood vessels was 71% higher in schizophrenia. IFITM mRNA levels were negatively correlated with GABA-related mRNAs in the same schizophrenia subjects.
Conclusions
The finding that schizophrenia subjects with higher IFITM mRNA levels in cortical blood vessels have greater disturbances in cortical GABA neurons suggests that these cell-type distinct pathological disturbances may be influenced by a shared upstream insult that involves immune activation.
Keywords: IFITM, immune, inflammation, GABA, GAD67, parvalbumin
Introduction
Immune- and inflammation-related abnormalities have been implicated in the pathophysiology of schizophrenia. For example, genome-wide association studies have identified associations between single-nucleotide polymorphisms in genes involved in immune and inflammatory signaling pathways and schizophrenia (1-5). Furthermore, maternal exposure to infection (6,7) and elevated serum cytokine levels during pregnancy (8) have been associated with an increased risk of schizophrenia in offspring (9). In adult schizophrenia subjects, elevated levels of proinflammatory cytokines such as IL-6 have been consistently reported in the peripheral serum (10,11), including subjects with first-episode psychosis (12), and have also been found in the prefrontal cortex (13).
Additional immune-related markers have been reported to be abnormally expressed in schizophrenia. For example, two microarray studies reported higher mRNA levels for interferon-induced transmembrane protein (IFITM) in the prefrontal cortex gray matter homogenates in schizophrenia (14,15). However, the cellular localization of IFITM in human prefrontal cortex has not been determined, and the cell types that overexpress IFITM mRNA in the prefrontal cortex in schizophrenia remain unknown. In addition, it is unclear whether higher IFITM mRNA levels in schizophrenia are related to long-term treatment with antipsychotic medications. Interestingly, more recent studies have found that IFITM plays a unique role as a viral restriction factor in preventing viral replication prior to fusion of the viral and cellular membranes (16) of many different viruses (17-22). Furthermore, IFITM expression is induced by interferons and cytokines including IL-6 (16,19,23). A mouse model of mild chronic neuroinflammation (i.e. mice with deficits in a nuclear factor-κB site-binding protein which normally suppresses production of cytokines such as IL-6) has been reported to have higher IFITM levels in cortex (24). Of particular interest, mice with chronic neuroinflammation have also been reported to have deficits in GABA neuron-related markers, including the calcium-binding protein parvalbumin and the GABA synthesizing enzyme GAD67 (24). Similar disturbances in prefrontal GABA neurons have been commonly reported in schizophrenia (25-38). However, it is unclear whether the same schizophrenia subjects that have higher IFITM mRNA levels also have deficits in GABA neuron-related markers which would suggest a shared underlying pathogenetic process that is related to immune activation.
Consequently, to further investigate the role of IFITM in the pathophysiology of schizophrenia, we first replicated the finding of higher IFITM mRNA levels in the prefrontal cortex in schizophrenia using quantitative PCR in a large new cohort (n=57) of subjects. We next used in situ hybridization to identify the cell types that overexpress IFITM mRNA in schizophrenia. We then determined whether IFITM mRNA levels are affected by antipsychotic medications by quantifying IFITM mRNA levels in the prefrontal cortex of monkeys chronically exposed to olanzapine, haloperidol, or placebo. Finally, we determined whether elevated IFITM mRNA levels are associated with larger deficits in GABA neuron-related markers in the same schizophrenia subjects which may indicate a shared pathogenetic origin in the disorder.
Methods and Materials
Human subjects
Brain specimens were obtained during routine autopsies conducted at the Allegheny County Medical Examiner's Office after consent was obtained from next-of-kin. An independent committee of experienced research clinicians made consensus DSMIV (39) diagnoses for each subject using structured interviews with family members and review of medical records (40). The absence of a psychiatric diagnosis was similarly confirmed in healthy comparison subjects. To control for experimental variance, subjects with schizophrenia or schizoaffective disorder (n=57) were matched individually to one healthy comparison subject for sex and as closely as possible for age (Supplement: Table S1) as previously described (33,40,41). Samples from subjects in a pair were processed together throughout all stages of the study. Fourteen subject pairs had previously been studied for IFITM mRNA levels by microarray (14). The mean age, postmortem interval, freezer storage time, and RNA integrity number (RIN; Agilent Bioanalyzer) did not differ between subject groups (Table 1; t(112) ≤ 1.06, p ≥ .29), and each subject had a RIN ≥ 7.0. Mean brain pH was slightly lower in schizophrenia (6.6 ± 0.3) relative to healthy comparison subjects (6.7 ± 0.2; t(112)=2.09, p=.04); each subject had a brain pH ≥ 5.9. All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research Involving the Dead and Institutional Review Board.
Table 1.
Summary of demographic and postmortem characteristics of human subjects
| Parameter | Healthy Comparison | Schizophrenia |
|---|---|---|
| N | 57 | 57 |
| Sex | 42M / 15F | 42M / 15F |
| Race | 47W / 10B | 43W / 14B |
| Age (years) | 47.8 ± 13.9 | 46.9 ± 12.9 |
| Postmortem Interval (hours) | 18.4 ± 5.6 | 18.9 ± 8.5 |
| Freezer Storage Time (months) | 111.0 ± 51.8 | 109.5 ± 53.4 |
| Brain pH | 6.7 ± 0.2 | 6.6 ± 0.3 |
| RNA Integrity Number | 8.2 ± 0.6 | 8.1 ± 0.6 |
| Medications At Time of Death | ||
| Antipsychotic | - | 48/57 |
| Antidepressant | - | 25/57 |
| Benzodiazepine/Anticonvulsant | - | 21/57 |
| NSAID | 11/57 | 13/57 |
| Cause of Death | ||
| Cardiopulmonary-Related | 42/57 | 19/57 |
| Infection/Inflammation-Related | 1/57 | 5/57 |
| Suicide | - | 16/57 |
| Other | 14/57 | 17/57 |
For age, postmortem interval, freezer storage time, brain pH, and RNA integrity number, values are group means ± standard deviation. For medications at time of death and cause of death, number of subjects in each applicable category are provided. NSAID – non-steroidal anti-inflammatory drug.
Quantitative PCR
RNA was isolated from prefrontal cortex area 9 from each subject and used to synthesize cDNA, as previously described (26,42) (Supplemental Methods). Primer sets were designed to quantify the three relevant variants of IFITM mRNA (IFITM1, IFITM2, IFITM3; IFITM4 is a pseudogene and IFITM5 is only found in osteoblasts (16)) (Supplement: Table S2). Due to the very high sequence similarity between IFITM2 and IFITM3 in humans, one primer set targeted a homologous region in IFITM2 and IFITM3 (termed IFITM2/3; Supplement: Table S2). All primer pairs (Supplement: Table S2) demonstrated high amplification efficiency (>96%) across a wide range of cDNA dilutions, and dissociation curve analysis[notdef]of amplified products revealed melting temperatures nearly identical to that predicted by online oligonucleotide calculator software programs. Quantitative PCR was performed using the comparative cycle threshold (CT) method with Power SYBR Green dye and the ViiA-7 Real-Time PCR System (Applied Biosystems), as previously described (26,42) (Supplemental Methods). Based on their stable relative expression levels between schizophrenia and comparison subjects (42), three reference genes (beta actin, cyclophilin A, and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were used to normalize target mRNA levels. The difference in CT (dCT) for each target transcript was calculated by subtracting the geometric mean CT for the three reference genes from the CT of the target transcript (mean of four replicate measures). Because dCT represents the log2-transformed expression ratio of each target transcript to the reference genes, the relative level of the target transcript for each subject is reported as 2−dCT (41,43,44).
In Situ Hybridization
A 266–base pair fragment corresponding to bases 145-410 of the human IFITM2 gene (NM_006435), a region with high homology in all three human IFITM variants, was PCR-amplified, and nucleotide sequencing confirmed 100% homology for the amplified fragment to the reported IFITM2 sequence. Sense and antisense 35S-labeled riboprobes were generated by in vitro transcription, and hybridization procedures were performed using 3 tissue sections from each subject. Tissue sections were then exposed to Biomax MR film (Kodak), coated with nuclear emulsion, developed, and counterstained with cresyl violet, as previously described (26,29,45) (Supplemental Methods). A Microcomputer Imaging Device (MCID) system (Imaging Research Inc, London, Ontario, Canada) was employed to measure optical density in the gray matter and white matter using film autoradiographs, as previously described (45,46) (Supplemental Methods). IFITM mRNA expression was then evaluated at the cellular level using the MCID system coupled to a microscope equipped with a motor-driven stage. Silver grains generated by the 35S-labeled riboprobe in emulsion-dipped sections were counted over traced outlines of blood vessels excluding the lumen when present. Blood vessels were identified as distinct clusters of packed cells with at least two elongated nuclei and the occasional presence of a lumen or branching point, in two 500 μm-wide gray matter traverses in each of three sections per subject. The mean (SD) number of blood vessels sampled in each subject was 70 (10) for control subjects and 73 (10) for schizophrenia subjects.
Antipsychotic-exposed Monkeys
Young adult, male, long-tailed monkeys (Macaca fascicularis) received oral doses of haloperidol, olanzapine or placebo (n=6 monkeys per group) twice daily for 17–27 months, as previously described (47). RNA was isolated from prefrontal cortical area 9, and qPCR was conducted for the same three reference genes and IFITM (Supplement: Table S2) with all monkeys from a triad processed together on the same plate. All animal studies followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Statistical Analysis
The ANCOVA model we report includes mRNA level (i.e. normalized expression level, optical density, or grain density) as the dependent variable, diagnostic group as the main effect, subject pair as a blocking factor, and postmortem interval, brain pH, RIN, and freezer storage time as covariates. Subject pairing may be considered an attempt to account for the parallel processing of tissue samples from a pair and to balance diagnostic groups for sex and age, and not a true statistical paired design. Therefore, a second ANCOVA model without subject pair as a blocking factor and including sex and age as covariates was also used, and both models produced similar results. Subsequent analyses of differences in mRNA levels between schizophrenia subjects grouped by predictors and indicators of disease severity, psychotropic medications, nicotine use, and cause of death were conducted using the unpaired ANCOVA models with α=.05. For the antipsychotic-exposed monkey study, an ANOVA model with mRNA level as the dependent variable, treatment group as the main effect, and triad as a blocking factor was employed.
Results
Quantitative PCR Analysis of IFITM mRNA Levels in the Prefrontal Cortex in Schizophrenia
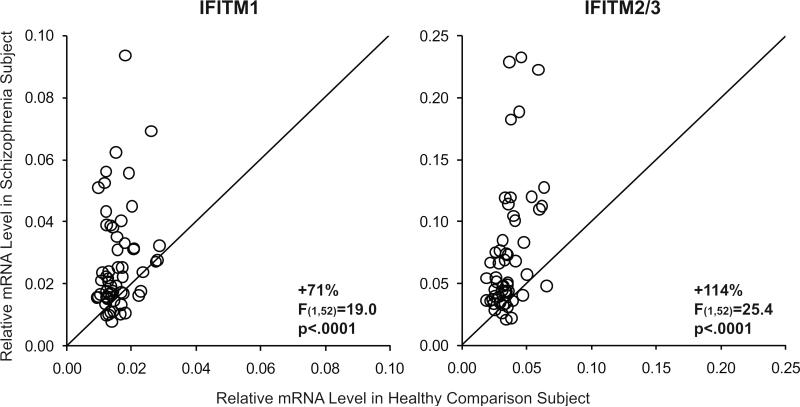
Mean mRNA levels in schizophrenia subjects were markedly higher for IFITM1 (+71%; F(1,52)=19.0, p<.0001) and IFITM 2/3 (+114%; F(1,52)=25.4, p<.0001) relative to healthy comparison subjects (Figure 1). Furthermore, IFITM2/3 mRNA levels were higher in the schizophrenia subject relative to the healthy comparison subject in 86% of the subject pairs, and IFITM2/3 mRNA levels were at least two-fold higher in 40% of schizophrenia subjects relative to healthy comparison subjects (Figure 1). IFITM mRNA levels were previously studied in 14 of the 57 schizophrenia subjects included in the present study (14). In the newly studied 43 schizophrenia subjects alone, we also found higher mRNA levels for IFITM1 (+75%; F(1,38)=13.2, p=.001) and IFITM 2/3 (+116%; F(1,38)=15.8, p<.001). Because 1) mRNA levels for IFITM1 and IFITM2/3 are highly correlated across all subjects (r=0.74, p<.0001) and are similarly over-expressed in schizophrenia and 2) the different IFITM variants have largely similar roles as restriction factors for the same viruses (17,19,20), we focused on IFITM2/3 mRNA levels for subsequent data analyses.
Figure 1. Quantitative PCR analysis of IFITM mRNA levels in the prefrontal cortex in schizophrenia.
Transcript levels for each schizophrenia subject relative to the matched healthy comparison subject are indicated by open circles. Data points to the left of the unity line indicate higher mRNA levels in the schizophrenia subject relative to the healthy comparison subject. Mean mRNA levels (± SD) in schizophrenia subjects were statistically significantly higher for IFITM1 (0.027 ± 0.017) and IFITM2/3 (0.078 ± 0.058) relative to healthy comparison subjects (IFITM1: 0.016 ± 0.005; IFITM2/3: 0.036 ± 0.011).
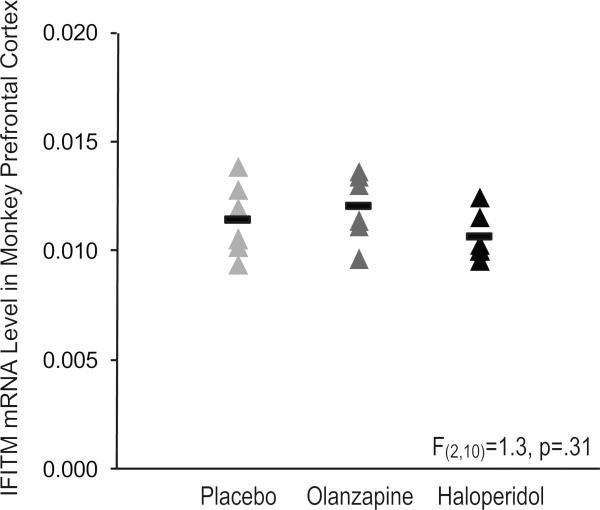
Among schizophrenia subjects, IFITM mRNA levels did not differ as a function of factors that predict a more severe course of illness (male sex, a diagnosis of schizophrenia rather than schizoaffective disorder, first-degree relative with schizophrenia, early age at illness onset [≤18 years of age]) or measures of illness severity (suicide, no history of marriage, low socioeconomic status as measured by the Hollingshead Index of Social Position, living dependently at the time of death) (all F≤.37, p≥.55; Supplement: Figure S1). We also found no relationship between use of antipsychotic, antidepressant, benzodiazepine, or smoking at time of death and IFITM mRNA levels in schizophrenia subjects (all F≤0.56, p≥0.46; Supplement: Figure S1). IFITM mRNA levels also did not differ in the prefrontal cortex of monkeys chronically exposed to haloperidol, olanzapine, or placebo (F(2,10)=1.3, p=.31; Figure 2). These data suggest that elevated IFITM mRNA levels in schizophrenia are not associated with disease severity and are not attributable to the effects of psychotropic medication.
Figure 2. IFITM mRNA levels in prefrontal area 9 of antipsychotic-exposed monkeys.
Quantitative PCR analysis revealed no statistically significant differences in IFITM mRNA levels (mean ± standard deviation) in monkeys chronically exposed to either olanzapine (0.0120 ± 0.0016; dark gray triangles) or haloperidol (0.0107 ± 0.0011; black triangles) compared to placebo (0.0115 ± 0.0017; light gray triangles). Mean values are shown as horizontal black bars.
We next sought to determine whether higher IFITM mRNA levels in schizophrenia may be related to infection/inflammation-related cause of death. The most common cause of death in our community-based, middle-aged cohort collected from autopsies is cardiopulmonary-related (~54%; Table 1), and only ~5% of subjects died of infection/inflammation-related causes (i.e. peritonitis, myocarditis, pneumonia, and anaphylaxis; Supplement: Table S1). Only including subjects with cardiopulmonary-related deaths revealed that IFITM mRNA levels were still much higher in schizophrenia (+76%; 0.061± 0.030; F(1,54)=32.9, p<.0001) relative to comparison subjects (0.035 ± 0.010), suggesting that higher IFITM mRNA levels in schizophrenia cannot be explained by infection/inflammation-related cause of death. Furthermore, excluding all subjects exposed to non-steroidal anti-inflammatory drugs at time of death (either prescribed or over-the-counter use indicated by presence of NSAIDs in toxicology screens of blood and/or urine; Table 1, Supplement: Table S1) revealed that IFITM mRNA levels remained much higher in subjects with schizophrenia (+110%; 0.078 ± 0.054; F(1,83)=22.9, p<.0001) relative to healthy subjects (0.037 ± 0.011). Finally, excluding subjects with overdose as cause of death (Supplement: Table S1) also revealed that IFITM mRNA levels continued to be much higher in subjects with schizophrenia (+115%; 0.078 ± 0.057; F(1,95)=26.8, p<.0001) relative to healthy subjects (0.036 ± 0.011).
In Situ Hybridization for IFITM mRNA in Schizophrenia
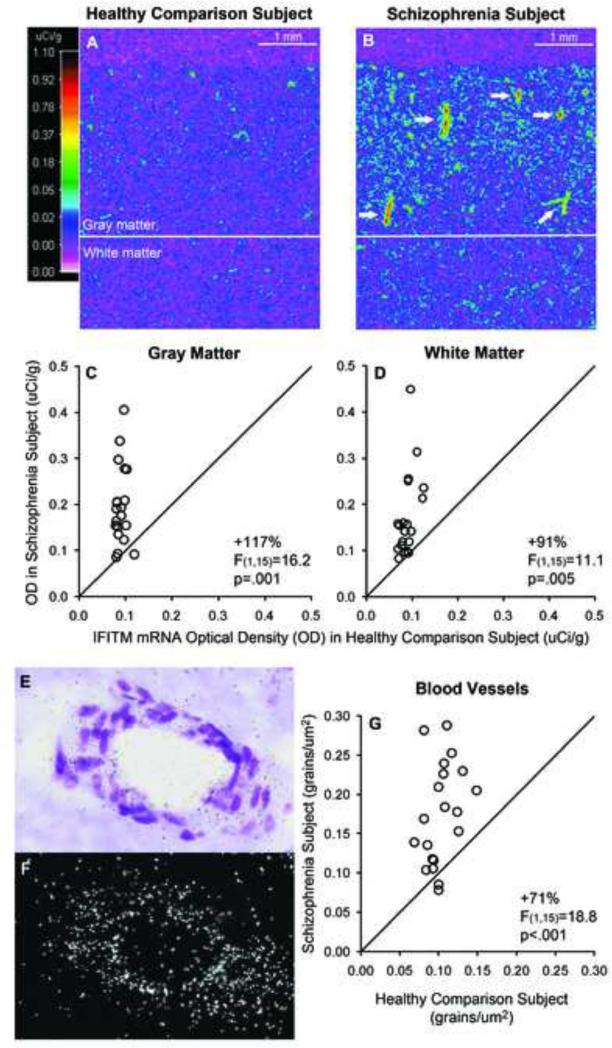
The cellular localization of IFITM in human prefrontal cortex has not been previously reported, and the cell types that overexpress IFITM mRNA in the prefrontal cortex in schizophrenia remain unknown. Therefore, we validated the finding of higher IFITM mRNA levels in schizophrenia using in situ hybridization in a limited number of subjects (i.e. 20 subject pairs that had a sufficient number of available tissue sections; Supplement: Table S1). Optical density analysis of film autoradiographs revealed that mean IFITM mRNA levels were higher in the gray matter (+117%; F(1,15)=16.2, p=.001) and white matter (+91%; F(1,15)=11.1, p=.005) in schizophrenia (Figure 3A-D). Furthermore, gray matter IFITM mRNA levels quantified by in situ hybridization and by qPCR were correlated in the same subjects (r=.72, p<.0001), which indicates the reliability of the mRNA quantification techniques and the reproducibility of the results. Interestingly, pseudocolor film autoradiographs from schizophrenia subjects (Figure 3B) revealed small structures with intense signal that were linear, round, and/or had branch points, which is consistent with labeling of blood vessels. Consequently, we exposed the same tissue sections to nuclear emulsion and developed and stained the sections for Nissl substance to conduct a grain counting analysis. Dense silver grain clusters indicating IFITM mRNA expression were present over distinct clusters of packed cells with elongated nuclei that often contained a lumen or branching point. These cell clusters had an either linear or round orientation, consistent with longitudinal or transverse profiles of blood vessels, respectively (Figure 3E-F). Grain clusters were also consistently observed over pia mater (not shown). In contrast, qualitative inspection of all schizophrenia and healthy comparison subjects revealed an absence of distinct grain clusters over neurons or glia (Supplement: Figure S2). In schizophrenia subjects, the mean grain density over blood vessels was higher in schizophrenia subjects (+71%; F(1,15)=18.8, p<.001; Figure 3G) relative to comparison subjects and was highly correlated with gray matter optical density measures (r=.73, p<.0001). However, we were not able to quantify grain density over pia mater due to the limited presence of pia mater in the tissue sections.
Figure 3. In situ hybridization for IFITM mRNA in the prefrontal cortex of schizophrenia and healthy comparison subjects with film and grain counting analyses.
A-B. Pseudocolored film autoradiographs of tissue sections from a matched pair of healthy comparison (A) and schizophrenia (B) subjects processed by in situ hybridization with an 35S-riboprobe for IFITM mRNA are shown. Solid white line indicates the gray matter/white matter border; calibration bar = 1 mm. While the comparison subject has relatively low levels (colder colors; scale) of IFITM mRNA throughout the gray and white matter, the schizophrenia subject has distinct small structures (white arrows) with intense signal (warmer colors) that are linear, round, and/or have branch points, which is consistent with the anatomical appearance of blood vessels. IFITM mRNA levels in the gray matter (C) and white matter (D) of prefrontal cortical area 9 for schizophrenia subjects and matched control subjects in a pair are indicated by open circles. Data points to the left of the unity line indicate higher mRNA levels in the schizophrenia subject relative to the healthy comparison subject. Mean (± SD) IFITM mRNA levels measured by optical density in schizophrenia subjects were statistically significantly higher in the gray matter (0.197 ± 0.85) and white matter (0.172 ± 0.091) relative to healthy comparison subjects (0.091 ± 0.010 and 0.090 ± 0.016, respectively). E-F. Photomicrograph of a prefrontal cortex section processed by in situ hybridization with an 35S-riboprobe for IFITM mRNA that was subsequently exposed to nuclear emulsion, developed, and Nissl-stained. E. A representative brightfield image from the same tissue section shown in frame B of Figure 5 illustrates a cluster of packed cells with elongated nuclei comprising a round structure with a lumen that is consistent with the transverse profile of a blood vessel. The darkfield image (F) reveals the accumulation of silver grains representing IFITM mRNA labeling over the blood vessel. Mean grain density per profile area (μm2) of blood vessel (i.e. traced outline of blood vessel excluding the lumen when present) was significantly higher in schizophrenia subjects (0.175 ± 0.064) relative to healthy comparison subjects (0.103 ± 0.020) (G).
Relationship between IFITM mRNA Levels and GABA Neuron-Related Markers in Schizophrenia
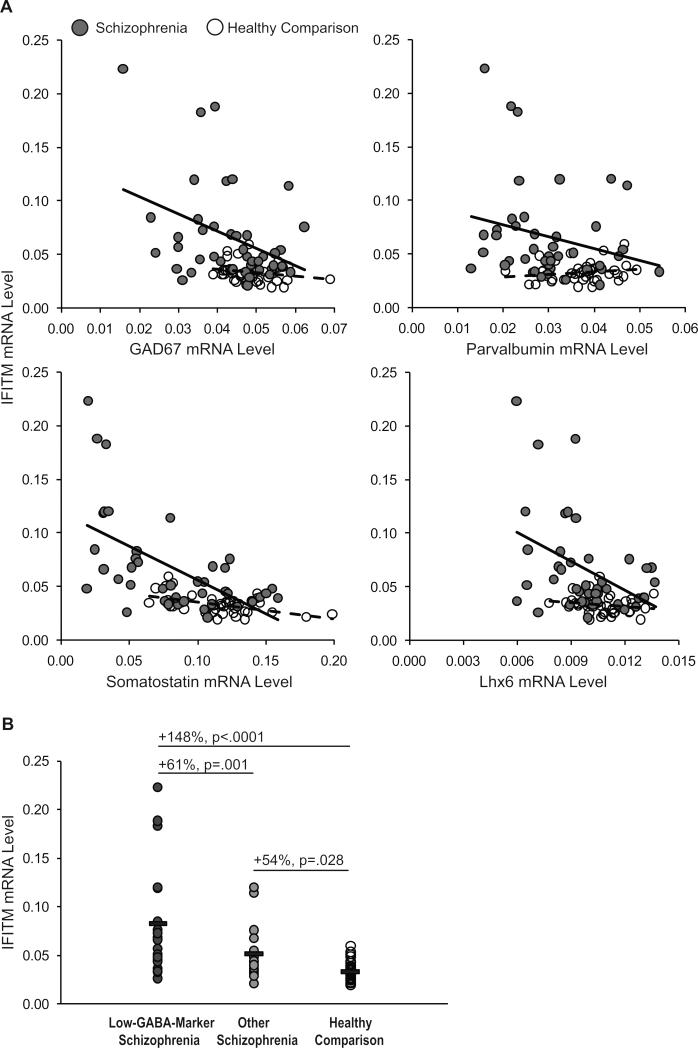
Disturbances in prefrontal inhibitory neurons have been commonly reported in schizophrenia (25-38). In a recent study of 42 of the schizophrenia subjects included in the present study, we reported lower mRNA levels for the GABA synthesizing enzyme GAD67, neuronal subpopulation markers parvalbumin and somatostatin, and the GABA neuron-specific transcription factor Lhx6 (37). Interestingly, in schizophrenia subjects (Figure 4), IFITM mRNA levels were inversely correlated with mRNA levels for GAD67 (r=−.38, p=.01), somatostatin (r=−.56, p<.0001), and Lhx6 (r=−.42, p=.005) but did not reach statistical significance for parvalbumin (r=−.24, p=.13). In contrast, in healthy subjects, IFITM mRNA levels were not correlated with GAD67, parvalbumin, or Lhx6 (for all, r<.23, p>.15) but were inversely correlated with somatostatin (r=−.51, p=.001). Furthermore, we had previously identified a “low-GABA-marker” subset of schizophrenia subjects that had the most severe deficits in GAD67, parvalbumin, somatostatin, and Lhx6 mRNA levels (37). IFITM mRNA levels were even higher (F(2,76)=15.6, p<.0001) in the low-GABA-marker schizophrenia subjects (n=20) relative to other schizophrenia subjects (+61%, p=.001; n=22) and to healthy subjects (+148%, p<.0001; n=42).
Figure 4. Relationship between IFITM mRNA levels and GABA neuron-related markers in schizophrenia.
A. Quantitative PCR analysis revealed that in schizophrenia subjects (grey circles; black linear regression lines, n=42 subjects), IFITM mRNA levels were inversely correlated with mRNA levels for GAD67 (r=−.38, p=.01), somatostatin (r=−.56, p<.0001), Lhx6 (r=−.42, p=.005), but did not reach statistical significance for parvalbumin (r=−.24, p=.13). In contrast, in healthy subjects (open circles, dashed linear regression lines, n=42 subjects), IFITM mRNA levels were not correlated with GAD67, parvalbumin, or Lhx6 (for all, r<−.23, p>.15), but were inversely correlated with somatostatin (r=−.51, p=.001). B. IFITM mRNA levels were even higher in the low-GABA-marker schizophrenia subjects (dark grey circles; n=20) relative to other schizophrenia subjects (light grey circles; n=22) and to healthy comparison subjects (open circles; n=42). IFITM mRNA levels were also higher in the schizophrenia subjects not included in the low-GABA-marker subset relative to healthy comparison subjects.
Discussion
In this study, we found marked elevations in mRNA levels for the viral restriction factor IFITM in the prefrontal cortex of a large cohort of schizophrenia subjects. The consistent reports of higher IFITM mRNA levels across different schizophrenia subject cohorts (14,15) and in the vast majority of schizophrenia subjects relative to healthy comparison subjects in the presently studied cohort suggests that higher IFITM mRNA levels are a common feature in schizophrenia. Furthermore, higher IFITM mRNA levels in schizophrenia did not appear to be attributable to antipsychotic medications, smoking, or an immune- or inflammation-related cause of death. One particularly unique finding of this study is that in schizophrenia, IFITM mRNA levels were markedly elevated in cortical blood vessels, which may indicate a more systemic immune/inflammatory disturbance in the disorder. Finally, we found an association between higher IFITM mRNA levels in cortical blood vessels and deficits in cortical GABA neuron markers in schizophrenia, suggesting that these cell-type distinct disturbances may be affected by a shared upstream insult that is related to immune activation.
Elevated IFITM mRNA levels may be related to neuroinflammatory processes in schizophrenia. For example, elevated serum levels of proinflammatory cytokines, including cytokines known to induce IFITM expression such as IL-6 (16,19,23), have been commonly reported in schizophrenia (10,11); however, peripheral serum was not available for any subjects in the present study. Interestingly, elevated serum cytokine levels have also been reported in drug-naive and first-episode psychosis subjects (11,12), suggesting that higher cytokine levels are not a consequence of treatment or chronic illness. Interestingly, higher mRNA levels of proinflammatory cytokines such as IL-6 were recently found to be associated with lower mRNA levels for GAD67, parvalbumin, and somatostatin in the prefrontal cortex of another cohort of schizophrenia subjects (13). Furthermore, IL-6 reduces levels of GAD67 and parvalbumin in cell culture (48), and overexpression of IL-6 leads to fewer parvalbumin-immunoreactive neurons in the hippocampus (49-51). In addition, administration of an immune stimulating agent to neonatal mouse pups has been reported to greatly elevate IFITM immunoreactivity in hippocampus (52). Taken together, these findings suggest that neuroinflammation, in part through elevated levels of IL-6, may also contribute to GABA neuron dysfunction and IFITM mRNA overexpression in schizophrenia. Consistent with this hypothesis, a mouse model of mild chronic neuroinflammation (i.e. mice with a complete loss of the nuclear factor-κB site-binding protein Schnurri-2 which normally suppresses production of cytokines such as IL-6) has been reported to have lower parvalbumin and GAD67 levels and higher IFITM levels in the cortex and hippocampus (24).
Several lines of evidence suggest that altered immune/inflammatory processes may even be present at very early stages of life in schizophrenia. For example, prenatal exposure to immune activation has been associated with an increased risk of developing schizophrenia later in life. Furthermore, the population attributable risk for schizophrenia due to maternal infection has been estimated to be ~30% (9), indicating that maternal infection is a relatively common in utero exposure in offspring who eventually develop schizophrenia. Animal models using the viral mimic and immune stimulant poly I:C have shown that maternal immune activation raises levels of multiple cytokines in fetal brain (53-55) and induces higher peripheral cytokine levels that persist into adulthood (56), including cytokines known to induce IFITM expression such as IL-6 (16,19,23). Maternal immune activation has also been shown to lower parvalbumin immunoreactivity and GAD67 mRNA levels in the prefrontal cortex of adult mice (57,58), similar to findings in schizophrenia (25-38). Thus, while further proof-of-principle testing is needed, it is possible that maternal immune activation could contribute to long-lasting elevations in IFITM mRNA levels and deficits in GABA-related mRNAs in adult brain through multiple mechanisms that disrupt early fetal development by elevating cytokine levels in fetal brain (53) and through enduring epigenetic modifications at gene promoter regions (59).
These findings may also provide insight into preventative and treatment strategies for schizophrenia. Preventative strategies might involve maternal prenatal care to reduce infection rates and potentially reduce risks for developing schizophrenia in at-risk offspring. For example, prevention of the maternal infection via vaccinations or personal health practice or rapid treatment of maternal infection may help mitigate the deleterious effects of maternal immune activation (9). In addition, chronic neuroinflammation may be targeted by immune-modulating agents such as non-steroidal anti-inflammatory drugs. Indeed, adjunctive treatment with the cyclooxygenase-2 inhibitor celecoxib has been reported to improve schizophrenia-related symptoms (60,61). Finally, another clinical approach may involve targeting the JAK-STAT (Janus-activated kinase and signal transducer and activator of transcription) pathway which is involved in signal transduction for a broad range of pro-inflammatory cytokines and IFITM (62). JAK-STAT inhibitors, such as ruxolitinib which was recently FDA-approved for the treatment of myelofibrosis (63), may potentially limit neuroinflammation and lower IFITM mRNA expression. Thus, further investigation into the role of immune- and inflammatory-related processes in producing cortical inhibitory circuitry disturbances in schizophrenia may help inform novel preventative and treatment strategies for the disorder.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (MH084016 to DWV and MH043784 and MH084053 to DAL) and the Hamilton Family Award for Basic Neuroscience Research in Psychiatry (DWV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and serves as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb and Concert Pharmaceuticals. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Schaefer CA, Quesenberry CP, Jr., Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 8.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2013;31:90–95. doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 14.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen LM, et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7:e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang IC, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7:e1001258. doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Pan Q, Rong L, He W, Liu SL, Liang C. The IFITM proteins inhibit HIV-1 infection. J Virol. 2011;85:2126–2137. doi: 10.1128/JVI.01531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YK, Huang IC, Farzan M. IFITM proteins restrict antibody-dependent enhancement of dengue virus infection. PLoS One. 2012;7:e34508. doi: 10.1371/journal.pone.0034508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li K, Markosyan RM, Zheng YM, Golfetto O, Bungart B, Li M, et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9:e1003124. doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey CC, Huang IC, Kam C, Farzan M. Ifitm3 limits the severity of acute influenza in mice. PLoS Pathog. 2012;8:e1002909. doi: 10.1371/journal.ppat.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, et al. Deficiency of Schnurri-2, an MHC Enhancer Binding Protein, Induces Mild Chronic Inflammation in the Brain and Confers Molecular, Neuronal, and Behavioral Phenotypes Related to Schizophrenia. Neuropsychopharm. 2013;38:1409–1425. doi: 10.1038/npp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney Jr WE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 26.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma- aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 27.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 28.Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 31.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon WC. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatry Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2011;69:71–79. doi: 10.1016/j.biopsych.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharm. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: Evidence for incomplete developmental trajectories. Submitted. 2013 doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association . DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- 40.Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167:1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 2011;22:1215–1223. doi: 10.1093/cercor/bhr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34.1–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volk DW, Siegel BI, Verrico CD, Lewis DA. Endocannabinoid metabolism in the prefrontal cortex in schizophrenia. Schizophr Res. 2013;147:53–57. doi: 10.1016/j.schres.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beneyto M, Morris HM, Rovensky KC, Lewis DA. Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology. 2012;62:1598–1605. doi: 10.1016/j.neuropharm.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharm. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 48.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- 52.Ibi D, Nagai T, Nakajima A, Mizoguchi H, Kawase T, Tsuboi D, et al. Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice. Glia. 2013;61:679–693. doi: 10.1002/glia.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arrode-Bruses G, Bruses JL. Maternal immune activation by poly(I:C) induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J Neuroinflammation. 2012;9:83. doi: 10.1186/1742-2094-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basta-Kaim A, Szczesny E, Leskiewicz M, Glombik K, Slusarczyk J, Budziszewska B, et al. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring - the effect of antipsychotic drugs. Pharmacol Rep. 2012;64:1400–1410. doi: 10.1016/s1734-1140(12)70937-4. [DOI] [PubMed] [Google Scholar]
- 57.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal Immune Activation Induces Maturation-Dependent Alterations in the Prefrontal GABAergic Transcriptome. Schizophr Bull. 2013 doi: 10.1093/schbul/sbs195. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 60.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18:3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.