Abstract
Withdrawal from a history of extended access to self-administered cocaine produces a time-dependent intensification of drug-seeking, which might relate to a cocaine-induced imbalance in the relative expression of constitutively expressed Homer1 versus Homer2 isoforms within the ventromedial aspect of the prefrontal cortex (vmPFC). Thus, we employed immunoblotting to examine the relation between cue-reinforced lever-pressing at 3 versus 30 days withdrawal from a 10-day history of extended access (6 hrs/day) to intravenous cocaine (0.25 mg/infusion) or saline (Sal6h) and the expression of Homer1b/c and Homer2a/b within the vmPFC versus the more dorsomedial aspect of this structure (dmPFC). Behavioral studies employed adeno-associated viral vectors (AAVs) to reverse cocaine-elicited changes in the relative expression of Homer1 vs. Homer2 isoforms and tested animals for cocaine prime-, and cue-induced responding following extinction training. Cocaine self-administration elevated both Homer1b/c and Homer2a/b levels within the vmPFC at 3 days withdrawal and the rise in Homer2a/b persisted for at least 30 days. dmPFC Homer levels did not change as a function of self-administration history. Reversing the relative increase in Homer2 versus Homer1 expression via Homer1c over-expression or Homer2b knock-down failed to influence cue-reinforced lever-pressing when animals were tested in a drug-free state, but both AAV treatments prevented cocaine-primed reinstatement of lever-pressing behavior. These data suggest that a cocaine-elicited imbalance in the relative expression of constitutively expressed Homer2 versus Homer1 within the vmPFC is necessary for the capacity of cocaine to reinstate drug-seeking behavior, posing drug-induced changes in vmPFC Homer expression as a molecular trigger contributing to drug-elicited relapse.
Keywords: Homer proteins, prefrontal cortex, self-administration, reinstatement, addiction, craving
Introduction
Drug craving in addiction is associated with metabolic dysfunction within prefrontal cortex (PFC), which is theorized to involve anomalies in excitatory neurotransmission (e.g., Knackstedt and Kalivas 2009; Ferrario and Wolf 2010). Repeated cocaine experience, including cocaine self-administration, produces anomalies within the corticoaccumbens pathway and neuropharmacological evidence supports dysregulated corticoaccumbens glutamate transmission as critical for the capacity of drug-associated cues and the drug itself to reinstate previously extinguished drug-seeking behavior in animal models (c.f., Knackstedt and Kalivas 2009). Homer post-synaptic scaffolding proteins regulate forebrain glutamate transmission by influencing not only extracellular levels of glutamate, but also the function of Group1 metabotropic glutamate receptors (mGluR1/5) and NMDA glutamate receptors (c.f., Shiraishi-Yamaguichi and Furuichi 2007; Szumlinski et al. 2008). Homers are encoded by 3 genes (Homer1, -2 and -3) (Brakeman et al. 1997; Kato et al. 1998) and a single nucleotide polymorphism in intron 1 of Homer1 has been associated with cocaine addiction in humans, which was posited to perhaps influence RNA stability (Dahl et al. 2005). While the majority of studies examining Homer-cocaine interactions have focused on Homer1 gene products (c.f., Szumlinski et al. 2008; see also Ghasemzadeh et al. 2011; Knackstedt et al. 2010), repeated experimenter-administered, as well as self-administered, cocaine up-regulates Homer2 expression within medial PFC (mPFC) during protracted withdrawal (Ary and Szumlinski 2007; Ben-Shahar et al. 2009, respectively). These data, coupled with earlier data from Homer1 KO mice administered IP cocaine (Lominac et al. 2005; Szumlinski et al. 2004; 2005) have led us to propose that an imbalance in the relative expression of Homer2 versus constitutively expressed Homer1 isoforms within the mPFC may be a molecular switch that promotes drug-seeking behavior.
Protracted withdrawal from extended access self-administered cocaine produces a time-dependent intensification of drug-seeking behavior (e.g., Grimm et al. 2001; Neiswander et al. 2001), which is associated with perturbed mGluR1/5 expression within the ventral aspect of the mPFC (vmPFC) (Ben-Shahar et al. 2013). As Homers are critical for the in vivo regulation of mGluR1/5 function, as well as expression (c.f., Szumlinski et al. 2008), the present study first determined whether or not an association exists between the “incubation of cocaine craving” observed during protracted withdrawal from extended-access cocaine self-administration procedures and an imbalance in Homer1/2 expression within PFC subregions. We next employed virus-mediated transgenic strategies to reverse cocaine-induced changes in vmPFC Homer1/2 expression and assayed for their relevance for drug-seeking behavior under drug-abstinent and drug-primed conditions.
Materials and Methods
Subjects, surgery and self-administration procedures
Male Srague-Dawley rats (275–325 g; Charles River Laboratories, Hollister, CA) were initially trained in standard operant chambers (Med Associates, St. Albans, VT) to lever press for 45g food pellets (Noyes, Lancaster, NH) on an FR1 reinforcement schedule and then were surgically fitted with intravenous catheters, as well as bilateral guide cannulae aimed above the vmPFC, using surgical procedures identical to those described recently by our group (e.g., Ben-Shahar et al. 2009; 2013). One week later, animals (n=12/group) were subjected to cocaine self-administration procedures (Coc6h; 0.25 mg/0.1ml/infusion; National Institute on Drug Abuse, Bethesda, MD), with control animals in our immunoblotting study trained to self-administer saline (0.1 ml/infusion) during 10 daily 6-h sessions (Sal6h). Each infusion of cocaine or saline was accompanied by a 20-sec light and tone cue (see Ben-Shahar et al. 2013). To prevent over-dose, the number of cocaine infusions permitted during the first 2 days of training was capped at 100 (day1) and 120 (day2) and rats failing to meet self-administration criterion (minimum of 50 infusions/6-h session for the last 3 days of training) were excluded from the study.
Immunoblotting
To determine whether or not vmPFC or dmPFC Homer1/2 expression was a biochemical correlate of “incubated cocaine craving”, a series of immunoblotting experiments were conducted. As described recently (Ben-Shahar et al. 2013), tissue was collected from the ventromedial PFC (vmPFC; including infralimbic cortex and ventral portion of prelimbic cortex) and the dorsomedial PFC (dmPFC; including dorsal portion of prelimbic cortex and anterior cingulate) at 3 or 30 days withdrawal from groups of Sal6h and Coc6h rats that were either (1) tested for cue-reinforced responding during a 2-h session under extinction conditions (i.e. cues were contingent upon responding; cue-tested group), and euthanized immediately following this single extinction session or (2) remained undisturbed in their home cage during withdrawal (home cage withdrawal group). The total protein expression of Homer1b/c and Homer2a/b in the supernatant of the tissue homogenate was determined using standard immunoblotting procedures identical to those described previously (Cozzoli et al. 2009; 2012). As described in greater detail in Ben-Shahar et al. (2013), immunoblotting was first conducted between saline- and cocaine-experienced rats tested for cue-reinforced behavior, separately at each withdrawal time-point and these data were analyzed using t-tests for saline-cocaine differences. If significant saline-cocaine differences were indicated by this cursory analysis, the immunoblotting study for cue-tested animals was replicated and the tissue from both cocaine- and saline-experienced animals, sacrificed at both withdrawal time-points, was directly compared on the same membrane. The follow-up study of protein changes in home-cage withdrawal animals did not involve a cursory analysis and directly compared tissue from both saline- and cocaine-experienced animals, sacrificed at both 3 and 30 days withdrawal. For these latter two studies, the data were analyzed using a Self-administration × Time ANOVA (α=0.05).
Virus-mediated gene transfer and tests for cocaine-seeking
To reverse the effects of long-term cocaine withdrawal upon vmPFC Homer expression, groups of cocaine self-administering rats (n=10/group) were infused intra-vmPFC with adeno-associated virus (AAV) vectors carrying shRNA against Homer2b (shRNA-H2b; see Ary et al. 2013; Cozzoli et al. 2009), cDNA for Homer1c (cDNA-H1c; see Klugmann et al. 2005; Lominac et al. 2005), or an AAV carrying human renilla green fluorescent protein (GFP; Control subjects) on Day 1 of withdrawal from cocaine self-administration training. Homer1c and Homer2b were targeted in this study as they are the major isoforms found in rodents (Soloviev et al. 2000). The procedural details concerning in vivo delivery of these vectors can be found elsewhere (e.g., Szumlinski et al. 2006). At 30 days post-AAV infusion, all rats were subjected to 3 sessions of testing for cue-reinforced lever-pressing behavior under extinction conditions, conducted across 3 days. On days 1 and 2, rats were tested over a 2-h period in a cocaine-free state to assay both within- and between-session extinction, as imbalances in Homer1/2 expression might underpin mGluR1/5 anomalies in the vmPFC that impair the consolidation of extinction memories (Ben-Shahar et al. 2013). On day 3, rats were tested for cocaine-primed reinstatement of cocaine-seeking behavior. For this, cue-reinforced behavior was first assessed in a cocaine-free state for 1 h. Then, rats were injected intraperitoneally (IP) with saline (1 ml/kg) and behavior assessed for another 30 min. Finally, rats were injected IP with 15 mg/kg cocaine and behavior recorded for an additional hour. Histological verification of AAV transduction was then confirmed by either immunoblotting or by immunocytochemistry as described previously (e.g., Ary et al. 2013). The data were analyzed by a multi-variate ANOVA with repeated measures on the Lever factor, as well as on the Within-Session Time and/or Between-Session factors, depending upon the analysis (α=0.05).
Results
A history of cocaine self-administration alters the Homer1:Homer2 ratio within vmPFC during long-term withdrawal
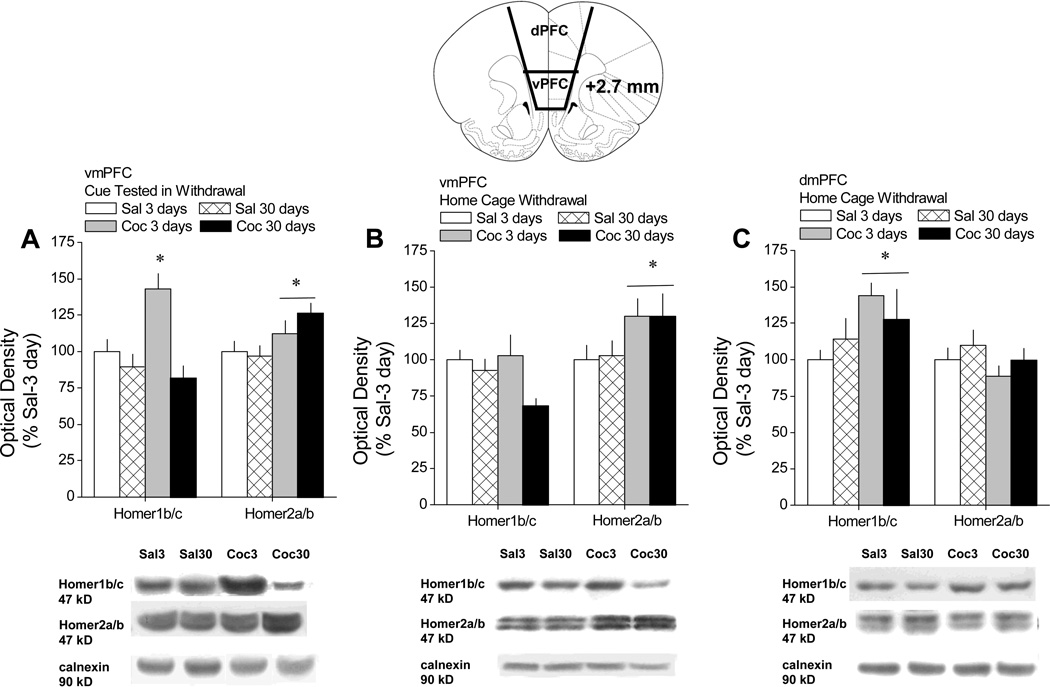
When tested for cue-reinforced lever-pressing behavior in the absence of any further cocaine/saline, cocaine-experienced rats responded at significantly higher levels than saline controls, and exhibited an incubation of craving (i.e., significantly higher levels of responding at 30 vs. 3 days of withdrawal; Grimm et al., 2001) as presented in Ben-Shahar et al. (2013). For rats tested for cue-reinforced behavior, the results of the cursory immunoblotting study conducted on vmPFC tissue indicated saline-cocaine differences at 3 days withdrawal for both Homer1b/c [t(17)=2.40, p=0.03] and Homer2a/b [t(17)=3.56, p=0.002], with cocaine-experienced animals exhibiting elevated protein expression (data not shown). At 30 days withdrawal, saline-cocaine differences were not apparent for Homer1b/c (p=0.53), while Homer2a/b levels were elevated in cocaine versus saline rats [t(21)=3.20, p=0.004] (data not shown). As these data suggested time-dependent changes in the expression of Homer1b/c, but not Homer2a/b, in cue-tested, cocaine-experienced rats, the tissue from both withdrawal time-points were directly compared in a replicate immunoblotting study. Indeed, when the time-course of protein changes was assayed directly in animals tested for cue-reinforced responding, the effects of cocaine withdrawal upon vmPFC Homer1b/c levels varied as a function of time, with cocaine-experienced animals exhibiting a significant increase in protein expression at 3, but not 30, days following the last self-administration session, relative to saline controls (Fig.1A) [Self-administration × Time: F(1,36)=8.01, p=0.008; LSD post-hoc tests]. In contrast, the elevation in vmPFC Homer2a/b expression exhibited by cocaine-experienced animals subjected to cue testing did not vary as a function of withdrawal duration (Fig.1A) [Self-administration effect: F(1,35)=7.93, p=0.008; interaction: p=0.25]. Thus, the expression of both Homer1b/c and Homer2a/b was elevated at 3 days withdrawal from cocaine; however, only Homer2a/b expression was elevated at 30 days withdrawal from cocaine, resulting in an altered ratio or imbalance in the expression of Homer 2a/b versus Homer 1b/c within the vmPFC.
Figure 1. A history of extended access to cocaine produces a time-dependent imbalance in CC-Homer1 versus –Homer2 isoforms within the vmPFC.
Top: Summary of the change in protein expression within the vmPFC exhibited by rats with a history of extended access (6 h/day) to intravenous saline (Sal) or cocaine (Coc) at 3 and 30 days withdrawal, expressed as a percent change from protein levels exhibited by Sal animals at 3 days withdrawal. Bottom: Representative immunoblots of the 4 treatment groups for Homer1b/c, Homer2a/b and the calnexin loading control. A, Immunoblotting results for vmPFC tissue obtained immediately upon completion of a 2-hr behavioral test conducted at 3 versus 30 days withdrawal, in which lever-pressing behavior was reinforced by the presentation of the saline/cocaine-associated cues. n=11 for Sal-3day, Sal-30day and Coc-30day; n=7 for Coc-3day. B, Immunoblotting results for vmPFC tissue of Sal and Coc animals left undisturbed in their home cage for 3 versus 30 days withdrawal. n=13 for Sal groups; n=15–16 for Coc groups. C, Immunoblotting results for dmPFC tissue of Sal and Coc animals left undisturbed in their home cage for 3 versus 30 days withdrawal. n=13 for Sal groups; n=15–16 for Coc groups. All data represent the mean ± SEM. *p<0.05 vs. respective 3 day data point, a priori t-tests.
Interestingly, the temporal pattern and magnitude of cocaine-induced changes in vmPFC Homer2a/b expression of home-cage withdrawal animals was nearly identical to that exhibited by animals tested for cue-reinforced behavior (Fig.1B) [Self-administration: F(1,50)=5.09, p=0.03; no Time effect or interaction]. However, the pattern of cocaine-elicited changes in vmPFC Homer1b/c of home-cage withdrawal animals was distinct from that of cue-tested animals (Fig.1A vs. 1B). For one, the statistical analysis of the data for home-cage withdrawal rats failed to indicate either a significant Self-administration effect or Self-administration × Time interaction (p>0.05), although the Time effect was statistically reliable [F(1,53)=7.84, p=0.0072]. As is clear from an inspection of Fig.1B, this statistical result appeared to be driven by the relatively large reduction in Homer1b/c levels exhibited by cocaine-experienced animals. Thus, rats withdrawn from self-administered cocaine in the home cage exhibited an imbalance in Homer 2a/b versus Homer 1b/c within the vmPFC, but this imbalance was apparent at both withdrawal time-points.
The cursory analysis of dmPFC tissue failed to suggest any effects of cocaine withdrawal upon Homer protein expression in animals tested for cue-reinforced behavior when the tissue of saline-experienced and cocaine-experienced rats was compared separately at either withdrawal time-point (n=11–12; t-tests, p’s>0.35; data not shown). Thus, we had no scientific rationale to conduct the replicate immunoblotting study to examine for cocaine by withdrawal interactions in Homer expression within dPFC tissue. Interestingly, an examination of Self-administration × Time interactions in home-cage withdrawal animals indicated a time-independent increase in Homer1b/c within the dmPFC (Fig.1C) [Cocaine effect: F(1,56)=4.27, p=0.04; Cocaine × Withdrawal, p=0.29], but no cocaine-induced change in the expression of Homer2a/b at either time-point (Self-administration × Time ANOVA, all p’s>0.05).
Preventing the cocaine-induced imbalance in vmPFC Homer1/2 isoforms blocks cocaine-primed reinstatement, without influencing cue-induced responding
We next determined the functional relevance of an imbalance in vmPFC Homer1 versus Homer2 expression for cocaine-seeking during protracted withdrawal by infusing intra-vmPFC shRNA-Homer2b and cDNA-Homer1c to “reverse” the Homer1/2 imbalance. All AAV treatment groups exhibited equivalent cocaine self-administration behavior prior to AAV delivery and withdrawal (Table 1; univariate ANOVAs, p’s>0.60; n=9–10).
Table 1.
Comparison of the average number of lever-presses emitted, and cocaine infusions earned, by the different AAV treatment groups during the last 3 days of self-administration training, prior to AAV delivery and withdrawal. No group differences in either variable were indicated (one-way ANOVAs, p>0.05). Samples sizes are indicated in parentheses.
| GFP (9) | cDNA-Homer1c (10) | shRNA-Homer2b (10) | |
|---|---|---|---|
| Lever-presses | 181.9 ± 41.1 | 139.4 ± 11.1 | 164.6 ± 41.3 |
| Infusions | 105.0 ± 6.7 | 106.4 ± 7.8 | 100.7 ± 3.8 |
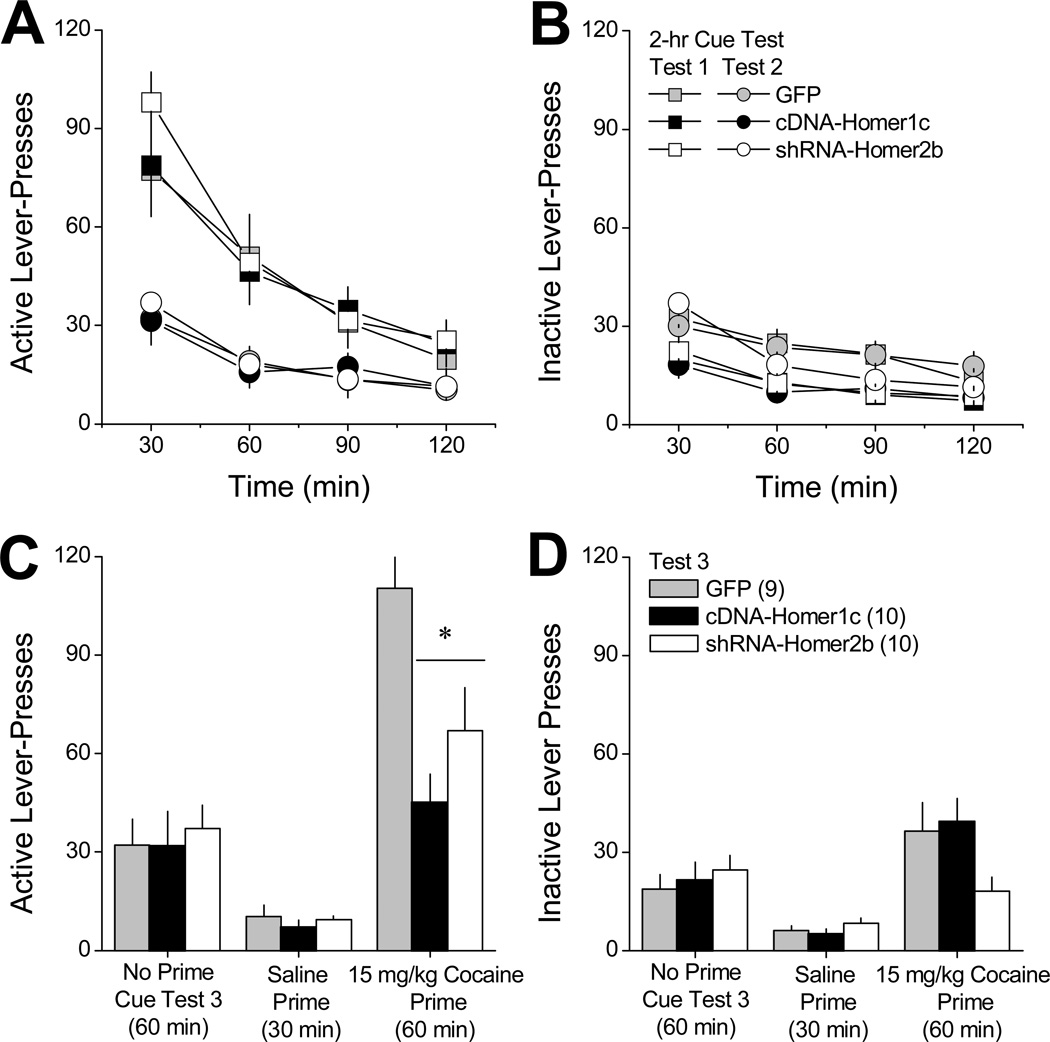
Fig.2A and 2B summarize, respectively, the time-courses of active and inactive lever-pressing behavior exhibited by GFP controls, cDNA-Homer1c and shRNA-Homer2b animals on two consecutive, 2-h cue tests (i.e., lever-pressing resulted in the presentation of the saline/cocaine-paired light-tone cue), spaced 1 day apart. During these cue tests, behavioral responding was selective for the active (cue-reinforced) lever and we observed no AAV effects on lever-pressing behavior, either across time within each cue test (i.e., intra-session extinction) or between the two 2-hr cue tests (i.e., inter-session extinction) [n=9–10; Lever × Session × Time: F(6,78)=36.97, p<0.0001; no main effects or interactions with the AAV factor, all p’s>0.05].
Figure 2. Reversing the cocaine-induced imbalance in vmPFC Homer1 versus Homer2 isoforms attenuates cocaine-primed reinstatement of drug-seeking.
Summary of the lever-pressing behavior emitted by rats with a 10-day history of intravenous cocaine (6h/day) that were infused intra-vPFC with AAVs carrying a GFP control vector (GFP), cDNA to over-express Homer1c (cDNA-Homer1c) or a shRNA to knock-down Homer2b (shRNA-Homer2b) prior to a 30-day withdrawal period. A and B, Time-course of active and inactive lever-pressing (in 30-min blocks) exhibited by the 3 AAV groups during an initial test for cue-reinforced responding, conducted at 30 days withdrawal (Test 1; squares) and during a subsequent cue test session, conducted the next day (Test 2; circles). C and D, Mean number of active and inactive lever-presses exhibited by the 3 AAV groups during a final test for cue-reinforced responding, in which rats were first allowed to lever-press under extinction conditions for 60 min in the absence of any manipulation (Extinction). Rats were then injected intraperitoneally with saline and allowed to respond for an additional 30 min (Saline), followed by a 15 mg/kg intraperitoneal injection of cocaine and an additional 60 min of testing (Cocaine). All data represent the mean ± SEM of the number of animals indicated in parentheses in panel B. *p<0.05 vs. GFP (LSD post-hoc tests).
We next assessed whether or not our AAV manipulations might influence the capacity of a cocaine priming injection to reinstate the now extinguished active lever-pressing behavior. As illustrated in Fig.2C and 2D, the effect of intra-vmPFC AAV treatment was differentially expressed across levers and across the different testing conditions [AAV × Lever × Test: F(4,52)=3.86, p=0.008]. Deconstruction of the 3-way interaction along the Lever factor indicated significant AAV × Test interactions for both levers [active: F(4,52)=3.84, p=0.002; inactive: F(4,52)=3.17, p=0.02]. As depicted in Fig.2C, the AAVs did not influence active lever-presses during either a 1-h cue test, prior to which animals received no priming injection, or a 30-min cue test, prior to which animals were injected with a saline prime (saline-prime test; univariate ANOVAs, p’s>0.05). However, both vmPFC cDNA-Homer1c and shRNA-Homer2 significantly attenuated active lever-pressing on a 1-h cue test, prior to which animals received a 15 mg/kg cocaine priming injection [cocaine-primed test; F(2,28)=6.48, p=0.005; post-hoc tests]. As was observed for active lever-presses, the AAVs did not influence inactive lever-pressing in the absence of cocaine (Fig.2D; univariate ANOVAs, p>0.05). While the data suggested that shRNA-Homer2b lowered the number of inactive lever-presses emitted during the cocaine-primed test for reinstatement, this effect was not statistically reliable (Fig. 2D) [F(2,28)=2.90, p=0.07].
Verification of AAV transduction within vmPFC
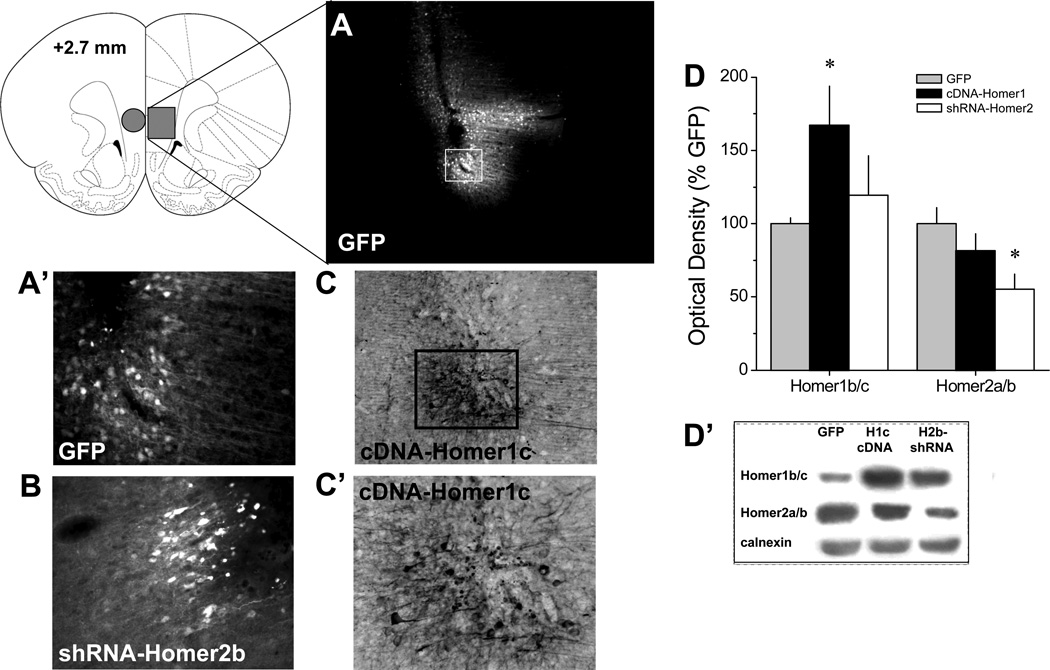
Immunoblotting conducted on a small subset of animals (n=5/AAV) supported in vivo efficacy of our cDNA-Homer1c and shRNA-Homer2b vectors for altering protein expression within vmPFC of drug-naïve subjects (Fig.3A, B). A priori comparisons between GFP controls and their respective experimental AAVs revealed that cDNA-Homer1c elevated vmPFC Homer1b/c levels by approximately 70% [t(8)=2.26, p=0.05], without influencing Homer2a/b expression (p=0.5), while shRNA-Homer2b reduced vmPFC Homer2a/b levels by approximately 45% [t(8)=3.36, p=0.01], but did not significantly influence Homer1b/c expression (p=0.28). Consistent with earlier reports (e.g., Ary et al. 2013; Cozzoli et al. 2009; 2012; Szumlinski et al. 2006), immunohistochemical examination of the microinjection sites revealed neuronal transduction by our different AAV constructs that was restricted to within 1–1.5 mm of the microinjection tip within the vmPFC. As illustrated in Fig.3C, the pattern of transduction did not differ in any obvious manner across the different constructs, with staining of both cell bodies and processes apparent whether transduction was detected using fluorescent microscopy to visualize the GFP reporter (for GFP control, shRNA-Homer1c) or using an antibody against the hemagluttanin (HA) tag (cDNA-Homer2b). Importantly, there was absolutely no indication of neuronal transduction within the over-lying dmPFC (Fig.3A) negating the possibility that any of the observed behavioral effects of our AAV manipulations are related to some back-flow into more dorsal aspects of the PFC.
Figure 3. Verification of the in vivo transduction efficacy of the AAVs constructs within vmPFC.
A, Micrograph (4 × magnification of tissue section) of immunofluorescence for the GFP reporter in the vmPFC of Rat #14. This rat exhibited the greatest lateral and dorsal spread of AAV from the microinjector tip of the 30 rats infused with AAV. Despite this, there was little evidence for AAV spread into the dmPFC. A’, 20 × magnification of the immunofluorescence from the GFP reporter on our control AAV from Panel A. B, 20 × magnification of the immunofluorescence from the GFP reporter on shRNA-Homer2b. C, 10× magnification of immunostaining for the HA tag on cDNA-Homer1c, demonstrating the typical pattern of AAV spread from the microinjector tip. C’, 20 × magnification of the immunostaining for the HA Tag from Panel C. D, Summary of the effects of the AAV-GFP (GFP), cDNA-Homer1c or shRNA-Homer2b upon the protein expression of Homer1b/c and Homer2a/b within vmPFC of drug-naïve rats (tissue punch indicated by circle in coronal section of brain). The data represent the mean ± SEM of 5 rats/AAV, expressed as a percentage of GFP controls. *p<0.05 vs. GFP controls (LSD post-hoc tests). D’, Representative immunoblots for Homer1b/c, Homer2a/b and calnexin from AAV-infused animals.
Discussion
Cocaine addiction is associated with metabolic and glutamatergic anomalies within PFC, theorized to underpin impaired volitional control over behavior and drug craving (e.g., Kalivas and Volkow 2011). As Homer2 is an important regulator of both pre- and postsynaptic aspects of excitatory neurotransmission in vivo (c.f., Szumlinski et al., 2008), we hypothesized that a cocaine-induced increase in the relative expression of Homer2 versus Homer1 isoforms within the PFC (Ary and Szumlinski 2007) may contribute to the enduring nature of addiction and more specifically, the propensity to engage in heightened drug-seeking behavior during protracted withdrawal (Grimm et al. 2001; Ben-Shahar et al. 2013).
Extended cocaine access differentially alters Homer1 and Homer2 expression within vmPFC
Consistent with previous data (Ary and Szumlinski 2007), cocaine-experienced rats in early withdrawal exhibited elevated vmPFC Homer2a/b that persisted for at least 30 days (Fig.1). While our earlier immunoblotting study of tissue from cocaine animals self-administering drug under, comparable, long-access procedures described a moderate (~30%) rise in Homer2a/b within medial PFC, this effect was not statistically reliable (Ben-Shahar et al. 2009). Of note, the tissue employed in this previous study included anterior cingulate, prelimbic, and infralimbic cortex. As we observed no cocaine-induced changes in dmPFC Homer2a/b expression in the present study (e.g., Fig.1C), it is likely that the more moderate cocaine effects observed in our earlier report resulted from incorporating all subregions of the mPFC into the tissue sample.
Along these lines, we observed a very large (50%) increase in vmPFC Homer1b/c at 3 days withdrawal in cocaine-experienced animals tested for cue-reinforced behavior that dissipated by the 30-day time-point (Fig.1A). While these data are reminiscent of our earlier results for cocaine-experienced animals left undisturbed in the home cage during early withdrawal (Ben-Shahar et al. 2009), home-cage withdrawal (i.e., cue test-naïve) cocaine subjects in the present study failed to exhibit the Homer1b/c increase in vmPFC (Fig.1B). However, consistent with studies conducted in cocaine-injected rodents (Ghasemzadeh et al. 2009), cue test-naive subjects exhibited a time-independent increase in dmPFC Homer1b/c expression (Fig.1C), which was not apparent in animals tested for cue-reinforced behavior. Thus, the increase in mPFC Homer1b/c expression reported earlier in rats with extended cocaine access (Ben-Shahar et al. 2009) was likely driven by the strong cocaine effect within more dorsal aspects of the PFC. Thus, it is clear from the relatively few immunoblotting studies conducted to date that there appears to exist subregional specificity to the changes in different Homer isoforms observed within the PFC of cocaine-experienced animals. Of note, the tissue dissection procedures employed in the 2 studies that have attempted to more carefully examine this issue by grossly subdividing the PFC into more dorsal versus ventral regions (Ghasemzadeh et al. 2009; present study) still fall short on neuroanatomical specificity and future studies will more carefully examine for cocaine-elicited alterations in Homer1 versus Homer2 expression within anterior cingulate, dorsal prelimbic, ventral prelimbic and infralimbic cortices as each of these major PFC subdivisions in rodents has been implicated in mediating different aspects of cognitive, attentional, and motivational processing of relevance to addiction (e.g., Lasseter et al. 2010).
Together, these immunoblotting data also indicate that a history of cocaine self-administration elicits an enduring and robust increase in vmPFC Homer2a/b that is not affected by the opportunity to seek cocaine in the presence of drug-paired cues, and thus, likely reflects a pharmacodynamic response to cocaine and/or cocaine-withdrawal. Alternatively, given obvious group differences in the amount of reinforced responding exhibited by the saline-versus cocaine-experienced animals during the self-administration sessions (see Ben-Shahar et al., 2013), it is also possible that the persistent rise in the relative expression of Homer2 within the vmPFC of the 2 cocaine groups in this study reflects neuroplasticity related to their more extensive training in the operant procedures. Although studies involving a non-drug reinforcer that engenders relatively high rates of responding (e.g., sucrose) were not included in the experimental design to address this possibility, previous immunoblotting data derived from studies of rats and mice subjected to repeated bolus cocaine dosing (30 mg/kg/day, IP) indicate that a persistent increase in PFC Homer2 expression can be produced in the absence of any operant training (Ary and Szumlinski 2007). Thus, while operant learning could certainly contribute to the persistent rise in vmPFC Homer2 expression observed following withdrawal in cocaine self-administering animals, it does not appear to be necessary for this neuroadaptation. The data to date (Ben-Shahar et al. 2009; present study) also indicate a short-lived increase in vmPFC Homer1b/c only in cocaine self-administering subjects exposed to extinction testing. Such findings support an important role for non-pharmacological factors in regulating the effects of cocaine and/or cocaine withdrawal upon vmPFC Homer1b/c expression, an interpretation supported by the negative results of prior studies using non-contingent, bolus, cocaine injection regiments (Ary and Szumlinski 2007; Swanson et al. 2001). Regardless of the precise mechanism(s) underpinning changes in vmPFC Homer expression, animals with a history of cocaine self-administration exhibit an imbalance between Homer1b/c and Homer 2a/b expression within vmPFC that manifests in protracted withdrawal, which we hypothesized might be relevant to either the incubation of cocaine craving that occurs with the passage of time following a history of drug intake (e.g., Grimm et al. 2001).
Reversing the imbalance in vmPFC Homer1 vs. Homer2 expression does not impact cue-induced cocaine-seeking
Protracted withdrawal from extended cocaine self-administration heightens cue-reinforced responding and impairs the capacity of animals to extinguish this drug-seeking (e.g., Ben-Shahar et al. 2013; Grimm et al. 2001). Thus, we posited that if the observed imbalance in vmPFC Homer1/2 expression contributed in any way to these behavioral phenomena, then reversing the imbalance should attenuate cue-reinforced behavior and/or facilitate extinction upon repeated testing. The results of the present behavioral study are contrary to our hypothesis (Fig.2). The amount of cue-reinforced lever-pressing behavior exhibited during initial testing by all 3 groups of our cocaine-experienced rats (~225 lever-presses/2 hrs or ~90 lever-pressing/30 min) is consistent with the high levels of responding reported previously for rats in protracted withdrawal from a history of daily, 6-h access to IV cocaine (e.g., Ben-Shahar et al. 2013; Grimm et al. 2001). While shRNA-Homer2b animals tended towards higher responding during the very first 30-min of initial cue testing following 30 days withdrawal, an examination of the time-courses of responding across two subsequent 2-hr cue-test sessions revealed nearly identical patterns of responding across the 3 AAV groups and there were no group differences apparent for within- or between-session extinction across the 3 cue test sessions (Fig.2). Further, the level of lever-responding was equivalent across the 3 AAV groups on the 3rd day of testing when rats were injected systemically with saline prior to an opportunity to behave (Fig.2B). Together, the above results fail to support any significant role for cocaine-elicited changes in vmPFC Homer1/2 expression in: (1) the reinforcing properties of cocaine-paired cues; (2) learning to suppress behavioral responding for cocaine-conditioned stimuli; or (3) the consolidation of extinction memory, at least when animals are tested in a cocaine-free state. The transduction efficiency of our AAV constructs as revealed by immunohistochemistry in our cocaine-experienced subjects (Fig.3A-A”’) is consistent with that of our earlier reports in mice and in rats (e.g., Ary et al., 2013; Cozzoli et al. 2009; Goulding et al. 2011; Szumlinski et al 2006) and quantification of AAV-mediated changes in Homer2 expression in drug-naïve rats by immunoblotting (Fig.3B-B’) yielded results akin to those reported in recent studies of the nucleus accumbens and PFC of mouse (e.g., Ary et al., 2013; Goulding et al. 2011). This, coupled with the fact that our AAV constructs influence behavioral responsiveness to a cocaine prime (see below), argues against anomalies in transduction efficacy to account for the lack of AAV effects upon cue-reinforced responding in the absence of cocaine (i.e., under extinction conditions).
vmPFC Homer1/2 expression contributes to cocaine-primed reinstatement of cocaine-seeking
Although “reversing” the cocaine-elicited imbalance in vmPFC Homer1/2 expression failed to influence cue-reinforced cocaine-seeking when animals were tested in a cocaine-free state, vmPFC Homer2b knock-down or Homer1c over-expression (both treatments aimed at maintaining the balance between Homer 1b/c versus Homer 2a/b within the vmPFC during protracted cocaine withdrawal) produced a marked attenuation in the capacity of a non-contingent cocaine priming injection to reinstate cue-reinforced lever-pressing in behaviorally extinguished animals (Fig.2C). Thus, an increase in the relative expression of Homer2b versus Homer1c within vmPFC appears to facilitate the triggering of relapse to cocaine-seeking by a cocaine prime. While not tested directly in this study, the attenuation of cocaine-primed reinstatement by our AAVs is not likely related to their effects upon cocaine-induced psychomotor activity or sensitization as these manipulations had no effect on responding on the inactive lever, or on behavioral responding during the initial extinction testing and similar manipulations in mice to not impact cocaine-induced locomotion or locomotor sensitization (Ary et al. 2013).
How precisely an imbalance in vmPFC Homer2 versus Homer1 expression might promote drug-primed relapse remains an on-going topic of investigation in our laboratory. Reinstatement of cocaine-seeking induced by a drug prime is known to depend upon glutamate activity within the vmPFC (Peters et al., 2008; LaLumiere et al., 2010). Manipulating the expression of Homer proteins within the vmPFC of drug-naïve animals (by infusing cDNA-Homer2b, shRNA-Homer1c, or shRNA Homer2b into the vmPFC) alters basal glutamate levels within this brain area. Importantly, these manipulations also alter cocaine-induced glutamate release within the vmPFC (Ary et al. 2013). Interestingly, rats during early withdrawal from an extensive history of IV cocaine self-administration show increased vmPFC Homer1bc expression that is paralleled by decreased basal glutamate levels within this brain area (Ben-Shahar et al. 2009, 2012, 2013). As Homer1/2 proteins are capable of regulating PFC extracellular glutamate (Ary et al. 2013; Lominac et al., 2005), a time-dependent change in their relative expression might engender distinct extracellular glutamate profiles within vmPFC during short-term versus protracted withdrawal that contributes to increased sensitivity to cocaine.
Cocaine-primed reinstatement of cocaine-seeking is also known to be highly dependent upon a rise in glutamate content within the nucleus accumbens (NAC) derived from glutamatergic projections from the mPFC (Kalivas and Volkow, 2011; McFarland et al., 2003; Ping et al., 2008). Not only do manipulations of vmPFC Homer protein expression produce local effects upon glutamate transmission, but vmPFC Homer1/2 expression also impacts indices of glutamate transmission within the NAC (Ary et al., 2013). In fact, mimicking the cocaine withdrawal-elicited imbalance in vmPFC Homer1/2 expression (Fig.1A; Ary and Szumlinski 2007; Ben-Shahar et al. 2009) is sufficient to effect reduced basal extracellular glutamate content, as well as increased cocaine-elicited glutamate release within the NAC (Ary et al. 2013). From the two in vivo studies to date (present study; Ary et al. 2013), it would appear that constitutively expressed Homer1 and Homer2 isoforms may either be competitively antagonistic at some site or may recruit different interacting partners whose functions may be antagonistic. In support of the former possibility, Homer1b and Homer2b are reported to compete for binding to mGluR1 in transfected neurons and, Homer1b expression can partially reverse the ability of Homer2b to occlude mGluR1-mediated inhibition of N-type calcium conductance (Kammermeier et al. 2000). Based on these data, on-going studies in our laboratory focus on testing the hypothesis that the time-dependent imbalance in vmPFC Homer1/2 expression produced during protracted cocaine withdrawal instigates compensatory alterations in glutamate transmission within the NAC, that increase the capacity of cocaine primes to induce drug-seeking behavior directed at drug-paired stimuli.
In summary, a history of extended access to cocaine produces a time-dependent increase in the relative expression of Homer2 versus Homer1 isoforms within the vmPFC that is independent of the self-administration context. AAV-mediated “reversal” of the vmPFC Homer1/2 imbalance fails to impact behavioral responding for cocaine-paired cues under extinction conditions but attenuates cocaine-primed reinstatement of cue-reinforced behavior in behaviorally extinguished animals. Such findings are timely given the reported association between cocaine addiction and single nucleotide polymorphisms in Homer1 that might influence RNA stability (Dahl et al. 2003). If relevant to humans, these data pose idiopathic or drug-elicited changes in vmPFC Homer expression, as well as its consequent effects upon glutamate transmission both within vmPFC and its major subcortical projections, in mediating the well-documented ability of cocaine re-exposure to precipitate relapse in abstinent addicts.
Acknowledgements
This work was funded in part by NIH grant DA024038 (KKS), NARSAD (KKS), funds from the Academic Senate of the University of California at Santa Barbara (KKS and OBS), as well as an Australian Research Council Future Fellowship to MK. The authors would like to thank Ms. Christina Shin and Ms. Megan McCloskey for their technical assistance during the preparation of this report, Dr. Tod E. Kippin for his assistance with the microscopy and Dr. Aaron Ettenberg for assistance with operant chamber availability.
Footnotes
Conflict of Interest
None of the authors have any conflict of interest to declare with respect to the research presented in this report.
References
- Ary AW, Lominac KD, Williams AR, Wroten MG, Ben-Shahar O, Klugmann M, et al. Imbalances in prefrontal cortex CC-Homer1 versus –Homer2 expression promote cocaine-seeking behavior. J Neurosci. doi: 10.1523/JNEUROSCI.1727-12.2013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495a–506a. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, et al. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Thompson AB, Wroten M, et al. Accumbens shell metabotropic glutamate receptor 5-associated signaling regulates binge alcohol drinking: Evidence from Drinking-in-the-Dark studies. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu J-H, Ary AW, et al. Binge drinking up-regulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JP, Kampman KM, Oslin DW, Weller AE, Lohoff FW, Ferraro TN, et al. Association of a polymorphism in the Homer1 gene with cocaine dependence in an African American population. Psychiatr Genet. 2005;15:277–283. doi: 10.1097/00041444-200512000-00010. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Targeting genes and proteins in the analysis of learning and memory: caveats and future directions. Rev Neurosci. 2000;11:15–26. doi: 10.1515/revneuro.2000.11.1.15. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res. 2011;1413:60–71. doi: 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, et al. Accumbens Homer2-mediated signaling: A factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011;10:111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff Shank, a novel family of postsynaptic density that binds to the NMDA receptor/PSD-95/GKAP and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–182. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T. Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci. 2003;22:188–201. doi: 10.1016/s1044-7431(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. MousebrainandmuscletissuesconstitutivelyexpresshighlevelsofHomerproteins. Eur J Biochem. 2000;267:634–639. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]