Abstract
Vascular structures in natural systems are able to provide high mass transport through high surface areas and optimized structure. Few synthetic material fabrication techniques are able to mimic the complexity of these structures while maintaining scalability. The Vaporization of a Sacrificial Component (VaSC) process is able to do so. This process uses sacrificial fibers as a template to form hollow, cylindrical microchannels embedded within a matrix. Tin (II) oxalate (SnOx) is embedded within poly(lactic) acid (PLA) fibers which facilitates the use of this process. The SnOx catalyzes the depolymerization of the PLA fibers at lower temperatures. The lactic acid monomers are gaseous at these temperatures and can be removed from the embedded matrix at temperatures that do not damage the matrix. Here we show a method for aligning these fibers using micromachined plates and a tensioning device to create complex patterns of three-dimensionally arrayed microchannels. The process allows the exploration of virtually any arrangement of fiber topologies and structures.
Keywords: Physics, Issue 81, Biomedical Engineering, Chemical Engineering, Silicone Elastomers, Micro-Electrical-Mechanical Systems, Biomimetic Materials, chemical processing (general), materials (general), heat exchangers (aerospace applications), mass transfer, Massive microfabrication, high surface area structures, 3-dimensional micro exchange devices, biomimetics
Introduction
Natural systems use extensive vascular networks to facilitate many biological functions. Mass transport can be achieved efficiently in such systems due to high surface area to volume ratios and optimized packing structures. While many synthetic fabrication techniques can produce microvascular structures, none can produce large-scale microvasculature while maintaining complexity and compatibility with existing manufacturing methods1-5. Structures such as the avian lung provide an inspiration. How do we fabricate structures of this complexity for enhancing mass transport?
The Vaporization of a Sacrificial Component (VaSC) can produce large-scale, complex microvascular structures6-7. This method uses the thermal depolymerization and evaporative removal of poly(lactic) acid fibers to form hollow channels that are the inverse of the fiber template. This is a sacrificial technique compatible with existing manufacturing methods. Meter long, cylindrical microchannel patterns can be formed using this fabrication process. This can be used to create vascularized devices such as self-healing polymers and 3D microvascular carbon capture units7-10.
The carbon capture units were inspired by the avian lung that provides an efficient gas-exchange-to-weight ratio owing to its use in flight. The parabronchus is composed of hexagonally patterned microchannels, which provides high gas exchange rates and structurally stable gas exchange units. In order to create exchange units with microscale features aligned in three-dimensions, we developed a method of independently tensioning fibers using a custom designed tension board with guitar tuners and laser-micromachined plates. Each fiber is held in place by external tension and the pattern is set by the placement of holes in the plate through which the fibers run.
Protocol
1. Catalyzing Sacrificial Fibers
Wrap the desired amount of poly(lactic) acid fibers around the lower ¾ of customized spindle. Reduce fiber overlap to provide the maximum surface area exposure.
- Mix deionized H2O with 40 ml of Disperbyk 130 in a closed bottle and shake until a homogenous solution is obtained. Then place a 1,000 ml beaker in a water bath at 37 °C and pour trifluoroethanol into the beaker. The amount of H2O and TFE to use depends on the PLA fiber diameter used.
Fiber Diameter Amount of H2O (ml) Amount of TFE (ml) 200 400 400 300 360 440 500 320 480 Add the H2O/Disperbyk 187 solution to the beaker and stir until uniform.
Add 1 g of Malachite Green to the mixture and stir until dissolved.
Place the custom spindle with fibers in the beaker ½ inch from the bottom and attach the spindle to a digital mixer. Then start the digital mixer at 400 rpm.
Slowly add 1.3 g of tin (II) oxalate (SnOx) catalyst to the mixture. The addition of SnOx must be gradual in order to prevent large agglomerations of material from crashing out of the solution.
Adjust the pH in the mixture using NaOH until the pH is ~6.8-7.2.
Secure a lid to the beaker and increase the spindle rotation to 500 rpm for 24 hr. If an agglomeration of SnOx is observed, manually break it up within the first 2 hr.
Remove spindle and dry in oven at 35 °C overnight.
Unwrap and remove excess catalyst from the catalyzed PLA fibers.
2. Microvascular Gas Exchange Unit Fabrication
Obtain a pair of laser-cut brass patterning brass plates with the desired microvascular pattern and affix the plates on clip holders.
Cut a 10 inch length of catalyzed fiber per microchannel and remove any remaining catalyst using a thicker plate cut to the fiber diameter (draw plate).
Taper the edges of the fibers by using the tip of a hot glue gun to slowly extrude the fiber tips.
Thread the fibers through matching holes in the brass patterning plate pairs.
Screw the plates onto a molding box. Make sure the fibers are not twisted when attaching the plates.
String the fiber tips through the tuning pegs of the custom tensioning board.
Tension the PLA fibers until taut. Be careful not to over-tension and snap the fibers.
Remove excess particulates from the fiber pattern using compressed air.
Mix polydimethylsiloxane (PDMS) base with curing agent in a 10:1, v:v ratio.
Degas the mixture under vacuum in a desiccator jar for 10 min.
Pour the PDMS mixture into the mold box. Do not pour directly over the fibers in order to reduce the trapping of air bubbles.
Using a 26 G needle, remove any bubbles within the molding box or between the fibers.
Cure the PDMS mixture at 85 °C for 30 min.
Unfasten the brass plates from the mold box, making sure not to bend the plates or pull too hard. Remove the cured 1st stage from the mold box.
Thread the fibers through an RTV end-cap by puncturing holes in the end-cap with a hypodermic needle. Depending on fiber size, use a needle gauge that has at least 2x the inner diameter of the outer diameter of your fiber. Maintain a similar pattern as the brass patterning plate, but more widely spread out.
Fasten the end-caps to the ends of a larger mold box and pour a 2nd stage of PDMS.
Remove any remaining gas bubbles and cure at 85 °C for 30 min.
Cut any excess PLA fibers from the sample and place in a vacuum oven at 210 °C for 24 hr, or until the PLA fibers have been mostly evacuated.
If any PLA cannot be removed, gently dissolve out of the microchannels using an injection of 1 ml of chloroform.
Representative Results
This procedure provides a method of fabricating microvascular structures embedded within a resin. These structures can conform to a variety of patterns (Figure 2). The structure of the microvascular network is only limited by the structures that can be formed with the sacrificial fibers.
Using a parallel arrangement of microvascular channels, gas transport between fluid streams is facilitated as gases traverse a permeable inter-channel membrane. These devices can be fabricated in a scalable manner without the need for lithography (Figure 3). The microchannels formed are completely hollow and can be separated by less than 50 μm.
It is possible for both leaks and plugs to appear within the microchannels (Figure 4). The formation of a plug will prevent any fluid flow through the microchannel and must be removed manually. A leak between channels can form when the fibers are not thoroughly cleaned and tensioned.
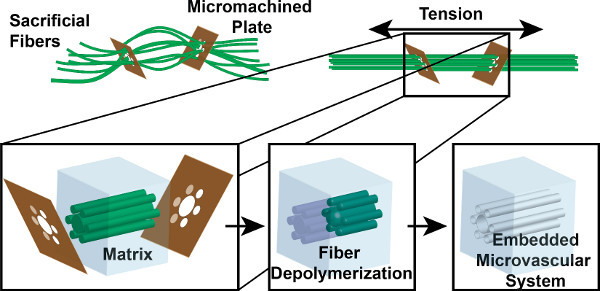 Figure 1. VaSC fabrication process overview. Sacrificial PLA fibers are threaded through micromachined guide plates. The fibers are strung until taut to create a parallel arrangement. The fibers are then embedded within a matrix. Heat and vacuum are then used to depolymerize the fibers into gaseous monomers. The end result is a hollow set of microchannels where the fibers once were.
Figure 1. VaSC fabrication process overview. Sacrificial PLA fibers are threaded through micromachined guide plates. The fibers are strung until taut to create a parallel arrangement. The fibers are then embedded within a matrix. Heat and vacuum are then used to depolymerize the fibers into gaseous monomers. The end result is a hollow set of microchannels where the fibers once were.
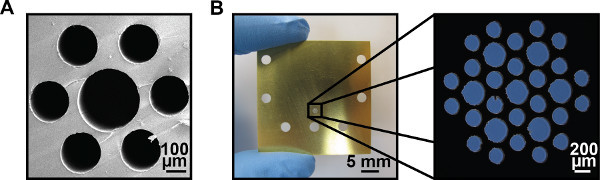 Figure 2. Sample patterns. (A) SEM image of a single hexagonal pattern of 200 μm and 300 μm diameter channels. (B) Guide plate for a hexagonally packed pattern of 200 μm and 300 μm diameter microchannels.
Figure 2. Sample patterns. (A) SEM image of a single hexagonal pattern of 200 μm and 300 μm diameter channels. (B) Guide plate for a hexagonally packed pattern of 200 μm and 300 μm diameter microchannels.
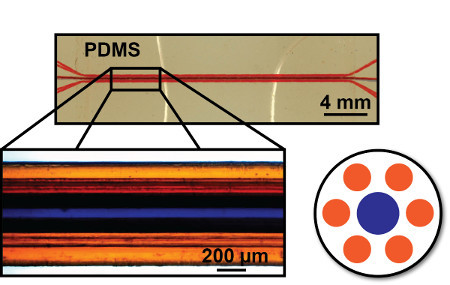 Figure 3. Representative gas exchange unit. The central portion of the unit contains a hexagonally arrangement of 200 μm and 300 μm diameter microchannels. A secondary structure spreads out and allows for easier access to the microchannels. Microchannels are loaded with blue and orange dye for visual clarity.
Figure 3. Representative gas exchange unit. The central portion of the unit contains a hexagonally arrangement of 200 μm and 300 μm diameter microchannels. A secondary structure spreads out and allows for easier access to the microchannels. Microchannels are loaded with blue and orange dye for visual clarity.
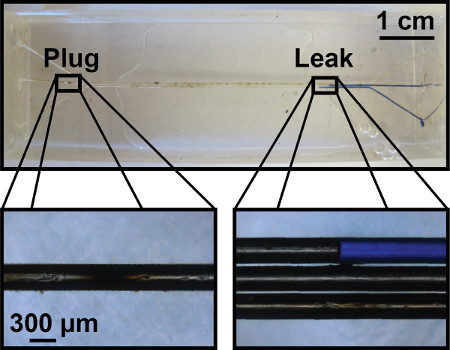 Figure 4. Representative failed gas exchange unit. Both plug and leak formations are represented. The formation of a plug can often be cleared with the use of a solvent, such as chloroform, to remove any remaining PLA. Leaks can develop between channels can form which are undesirable for the purposes of controlled gas exchange.
Figure 4. Representative failed gas exchange unit. Both plug and leak formations are represented. The formation of a plug can often be cleared with the use of a solvent, such as chloroform, to remove any remaining PLA. Leaks can develop between channels can form which are undesirable for the purposes of controlled gas exchange.
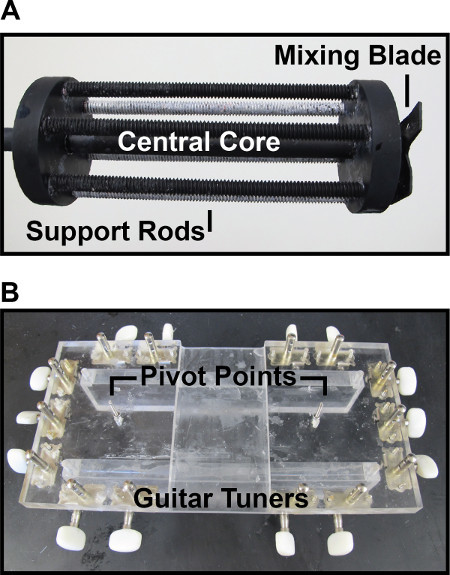 Figure 5. Custom devices for fabrication. (A) Custom spindle. Six supporting rods surround a central core. PLA fibers are wrapped around the supporting rods to maximize contact with the catalytic solution. A mixing blade is positioned at the bottom of the spindle to introduce chaotic flow. (B) Custom tensioning board. Guitar tuners are positioned along the edges of an acrylic board to tension the PLA fibers. Pivot points are positioned so that the angle between the fibers and guide plates remains close to perpendicular.
Figure 5. Custom devices for fabrication. (A) Custom spindle. Six supporting rods surround a central core. PLA fibers are wrapped around the supporting rods to maximize contact with the catalytic solution. A mixing blade is positioned at the bottom of the spindle to introduce chaotic flow. (B) Custom tensioning board. Guitar tuners are positioned along the edges of an acrylic board to tension the PLA fibers. Pivot points are positioned so that the angle between the fibers and guide plates remains close to perpendicular.
Discussion
The introduction of the SnOx catalyst into the PLA fibers allows the fibers to depolymerize at a lower temperature. This prevents the degradation of the embedding resin, in this case PDMS. A custom spindle is required to properly mix the treatment solution (Figure 5A). The spindle is composed of six supporting rods surrounding a central core which attaches to a digital mixer. The fibers are wrapped around the support rods so that the surface area of the wrapping fibers in contact with the catalytic solution was maximized. The bottom of the spindle contained a set of blades to introduce chaotic flow. The chaotic flow prevents the agglomeration of the catalyst.
A custom tensioning board is used to create the parallel set of fibers (Figure 5B). This consists of a board with guitar tuning pegs along the edges of the board. The dimensions of the board are unimportant as long as enough tuning pegs are present to tension all of the fibers used in the pattern. The addition of pivot points for the fibers is helpful to prevent the fibers from bending at too large of an angle at the guide plate interface. The most challenging part of the fabrication procedure is likely the threading of the fibers for larger patterns. It is important to remain organized when threading the fibers, ensuring that there is ample space in threading the next fiber.
For patterns with microchannel separations under 50 μm, it is possible to break the guide plates. Care must be taken in removing excess catalyst from the fibers as the excess is often larger in diameter than the plate pattern holes. When removing the plates, PDMS that has leaked through the plates must also be removed as it can produce additional stress on the plates when they are removed.
The guide plates were fabricated using laser micromachining. This process produces a slight taper to the hole of the plate, resulting in one side having a slightly larger opening than the other. It is important to have the smaller opening facing towards the mold box. If the smaller end faces away from the mold box, the increased resistance during the removal process can also break the plate.
It is possible for plugs of PLA to remain in the microchannels after longer periods of evacuation. Frequent oven and vacuum cleaning can help alleviate this. Remaining plugs can be removed with chloroform as long as the plug is of a short length. Longer microvascular structures can also help reduce plug formations as they often appear towards the ends of a microchannel. The extra length can allow the plug to be cut off from the device.
This fabrication process is non lithographic and can be adapted to a variety of existing manufacturing methods. The use of a sacrificial template allows for the creation of complex three dimensional microfluidic devices. The close positioning of the microvascular pattern was used for the transport of gas between microchannels in a gas permeable matrix, but it is not the only potential application. With the intimate contact between microchannels, microfluidic heat exchange in three dimensions also becomes accessible on a large scale using this fabrication process. It is also possible to purposely join the microchannels to induce chemical reactivity. This fabrication process allowed for the creation of biomimetic systems and can be used for as wide of a variety of applications as those performed by natural microvascular systems.
Disclosures
We have filed for a provisional patent on this technology und US patent U.S. Provisional Application Serial No. 61/590,086.
Acknowledgments
This work was supported by the AFOSR Young Investigator Program under FA9550-12-1-0352 and a 3M Non-Tenured Faculty Award. The authors would like to thank Lalisa Stutts and Janine Tom for helpful discussion relating to this project. The authors thank the Calit2 Microscopy Center and Laser Spectroscopy Facility at the University of California, Irvine for allowing use of its facilities. Hodge Harland and the UCI Physical Sciences Machine Shop are acknowledged for the fabrication of tools. Poly(lactic) acid fibers were generously provided by Teijin Monofilament.
References
- Bellan LM, Singh SP, Henderson PW, Porri TJ, Craighead HG, Spector JA. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;5(7):1354. [Google Scholar]
- Bellan LM, Strychalski EA, Craighead HG. Nano-channels fabricated in polydimethylsiloxane using sacrificial electrospun polyethylene oxide nanofibers. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2008;26(5):1728. [Google Scholar]
- Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication of three-dimensional engineered Scaffolds. Tissue Eng. 2007;13(8):1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- Wu H, Odom TW, Chiu DT, Whitesides GM. Fabrication of complex three-dimensional microchannel systems in PDMS. J. Am. Chem. Soc. 2003;125(2):554–559. doi: 10.1021/ja021045y. [DOI] [PubMed] [Google Scholar]
- Trask RS, Bond IP. Biomimetic self-healing of advanced composite structures using hollow glass fibres. Smart Mater. Struct. 2006;15(3):704–710. [Google Scholar]
- Dong H, Esser-Kahn AP, et al. Chemical treatment of poly(lactic acid) fibers to enhance the rate of thermal depolymerization. ACS Appl. Mater. Interfaces. 2012;4(2):503–509. doi: 10.1021/am2010042. [DOI] [PubMed] [Google Scholar]
- Esser-Kahn AP, Thakre PR, et al. Three-dimensional microvascular fiber-reinforced composites. Adv. Mater. 2011;23(32):3654–3658. doi: 10.1002/adma.201100933. [DOI] [PubMed] [Google Scholar]
- White SR, Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR. Self-healing polymers and composites. Am. Sci. 2011;99(5):392. [Google Scholar]
- Nguyen DT, Leho YT, Esser-Kahn AP. A three-dimensional microvascular gas exchange unit for carbon dioxide capture. Lab Chip. 2012;12(7):1246. doi: 10.1039/c2lc00033d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DT, Leho YT, Esser-Kahn AP. The effect of membrane thickness on a microvascular gas exchange unit. Adv. Funct. Mater. 2012. [DOI] [PMC free article] [PubMed]


