Abstract
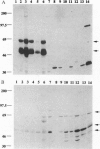
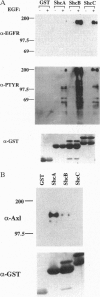
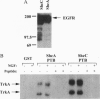
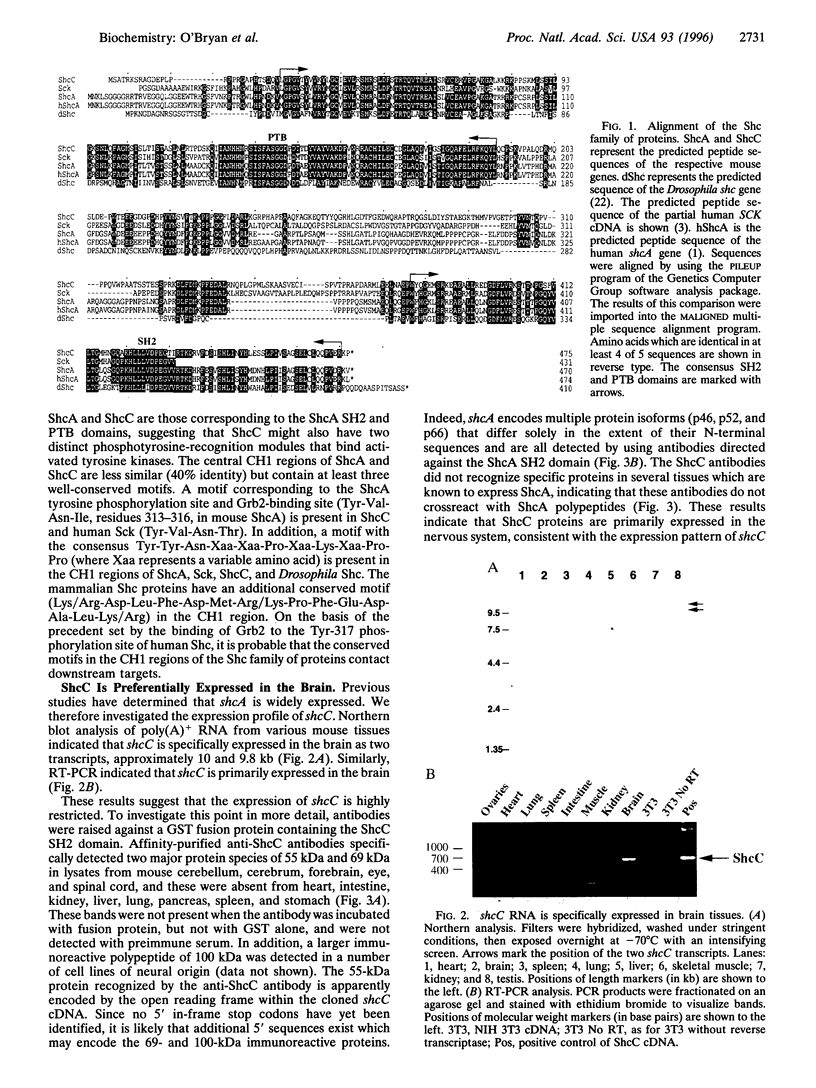
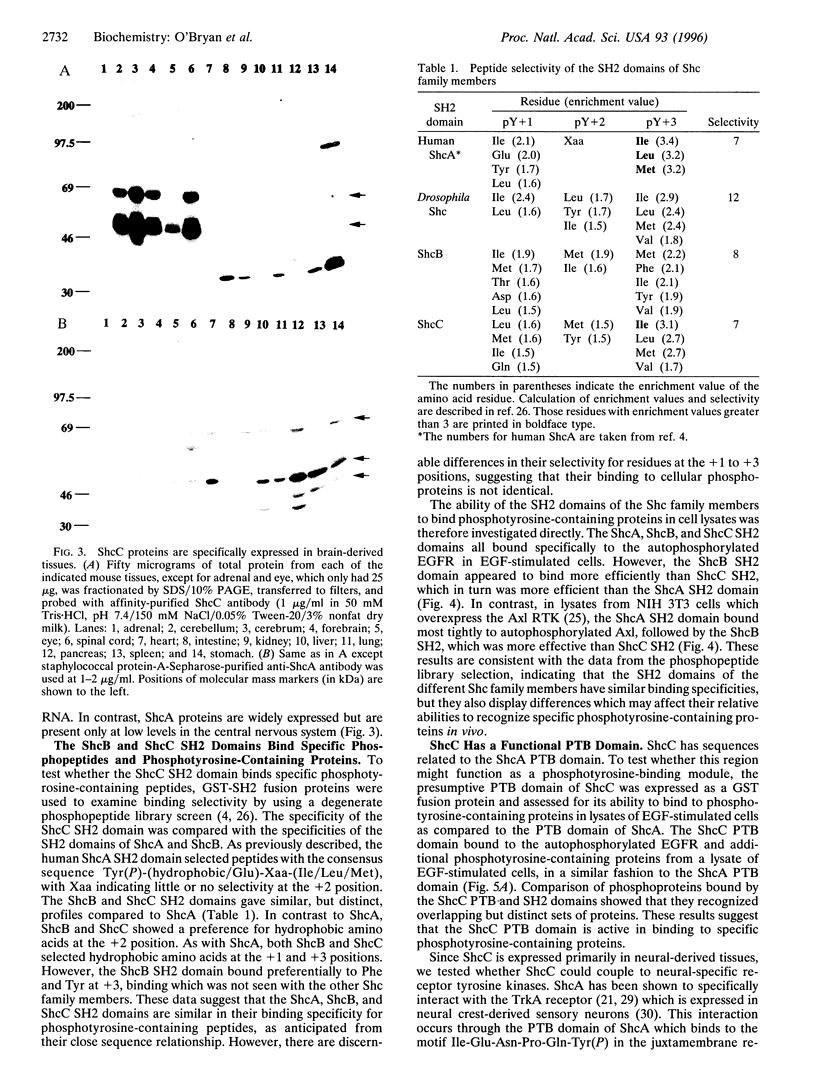
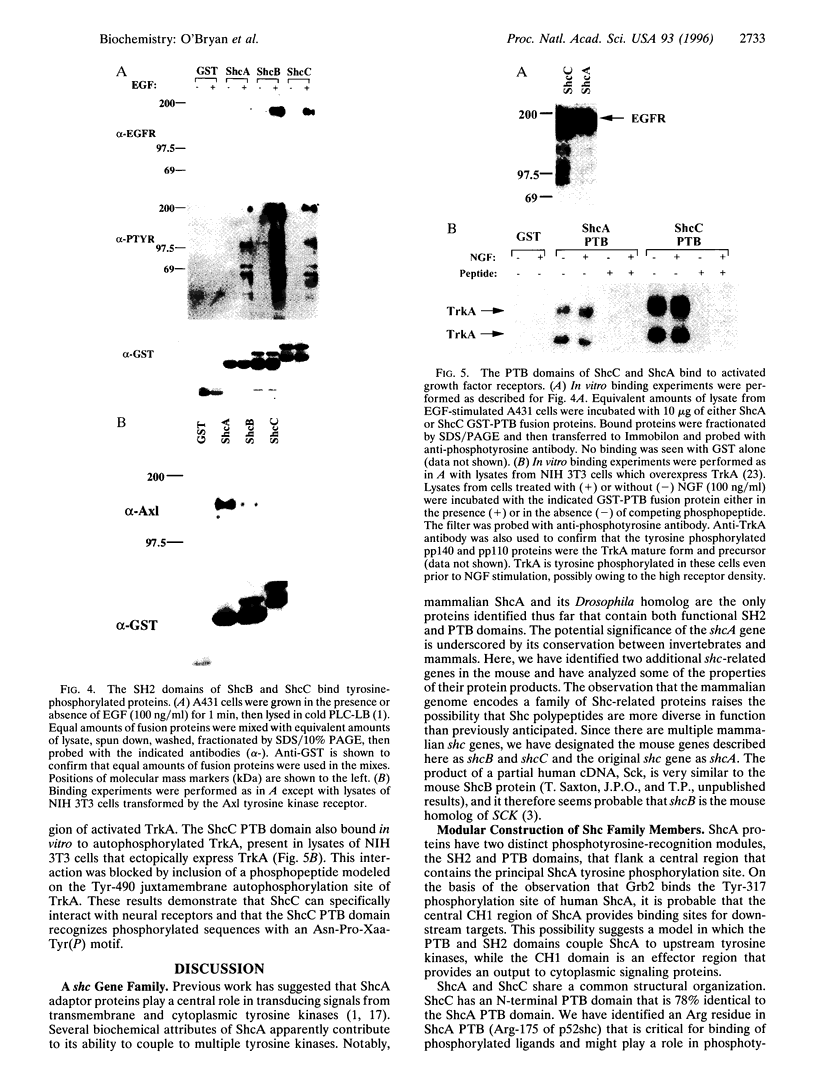
The Shc adaptor protein, hereafter referred to as ShcA, possesses two distinct phosphotyrosine-recognition modules, a C-terminal Src homology 2 (SH2) domain and an N-terminal phosphotyrosine-binding (PTB) domain, and is itself phosphorylated on tyrosine in response to many extracellular signals. Phosphorylation of human ShcA at Tyr-317 within its central (CH1) region induces binding to the Grb2 SH2 domain and is thereby implicated in activation of the Ras pathway. Two shc-related genes (shcB and shcC) have been identified in the mouse. shcB is closely related to human SCK, while shcC has not yet been found in other organisms. The ShcC protein is predicted to have a C-terminal SH2 domain, a CH1 region with a putative Grb2-binding site, and an N-terminal PTB domain. The ShcC and ShcB SH2 domains bind phosphotyrosine-containing peptides and receptors with a specificity related to, but distinct from, that of the ShcA SH2 domain. The ShcC PTB domain specifically associates in vitro with the autophosphorylated receptors for nerve growth factor and epidermal growth factor. These results indicate that ShcC has functional SH2 and PTB; domains. In contrast to shcA, which is widely expressed, shcC RNA and proteins are predominantly expressed in the adult brain. These results suggest that ShcC may mediate signaling from tyrosine kinases in the nervous system, such as receptors for neurotrophins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzer A. G., Rotin D., Ureña J. M., Skolnik E. Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994 Aug;14(8):5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaikie P., Immanuel D., Wu J., Li N., Yajnik V., Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem. 1994 Dec 23;269(51):32031–32034. [PubMed] [Google Scholar]
- Burns L. A., Karnitz L. M., Sutor S. L., Abraham R. T. Interleukin-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J Biol Chem. 1993 Aug 25;268(24):17659–17661. [PubMed] [Google Scholar]
- Crowe A. J., McGlade J., Pawson T., Hayman M. J. Phosphorylation of the SHC proteins on tyrosine correlates with the transformation of fibroblasts and erythroblasts by the v-sea tyrosine kinase. Oncogene. 1994 Feb;9(2):537–544. [PubMed] [Google Scholar]
- Cutler R. L., Liu L., Damen J. E., Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993 Oct 15;268(29):21463–21465. [PubMed] [Google Scholar]
- Damen J. E., Liu L., Cutler R. L., Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993 Oct 15;82(8):2296–2303. [PubMed] [Google Scholar]
- Kavanaugh W. M., Turck C. W., Williams L. T. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995 May 26;268(5214):1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Williams L. T. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994 Dec 16;266(5192):1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Lai K. M., Olivier J. P., Gish G. D., Henkemeyer M., McGlade J., Pawson T. A Drosophila shc gene product is implicated in signaling by the DER receptor tyrosine kinase. Mol Cell Biol. 1995 Sep;15(9):4810–4818. doi: 10.1128/mcb.15.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfrancone L., Pelicci G., Brizzi M. F., Aronica M. G., Casciari C., Giuli S., Pegoraro L., Pawson T., Pelicci P. G., Arouica M. G. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995 Mar 2;10(5):907–917. [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Marengere L. E., Pawson T. Identification of residues in GTPase-activating protein Src homology 2 domains that control binding to tyrosine phosphorylated growth factor receptors and p62. J Biol Chem. 1992 Nov 15;267(32):22779–22786. [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L. F. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 1990 May;4(5):683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade J., Cheng A., Pelicci G., Pelicci P. G., Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan J. P., Frye R. A., Cogswell P. C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M. M., Earp H. S., Liu E. T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A., Bradshaw R. A., Seedorf K., Choidas A., Schlessinger J., Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO J. 1994 Apr 1;13(7):1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A., Lammers R., Wiesmüller K. H., Jung G., Schlessinger J., Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3'-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993 Nov 5;268(31):22963–22966. [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. S., Lee K. K., Songyang Z., Cantley L. C., Burn P., Burakoff S. J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993 Nov 5;262(5135):902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Salcini A. E., McGlade J., Pelicci G., Nicoletti I., Pawson T., Pelicci P. G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994 Oct;9(10):2827–2836. [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Loeb D. M., Copeland T. D., Pawson T., Greene L. A., Kaplan D. R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994 Mar;12(3):691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Turner S. L., Jenkins F. J. Use of deoxyinosine in PCR to improve amplification of GC-rich DNA. Biotechniques. 1995 Jul;19(1):48–52. [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Yokote K., Mori S., Hansen K., McGlade J., Pawson T., Heldin C. H., Claesson-Welsh L. Direct interaction between Shc and the platelet-derived growth factor beta-receptor. J Biol Chem. 1994 May 27;269(21):15337–15343. [PubMed] [Google Scholar]
- Zhou M. M., Meadows R. P., Logan T. M., Yoon H. S., Wade W. S., Ravichandran K. S., Burakoff S. J., Fesik S. W. Solution structure of the Shc SH2 domain complexed with a tyrosine-phosphorylated peptide from the T-cell receptor. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7784–7788. doi: 10.1073/pnas.92.17.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Wiley S., Gish G. D., Lai V. K., Stephens R., White M. F., Kaplan D., Pawson T. Identification of residues that control specific binding of the Shc phosphotyrosine-binding domain to phosphotyrosine sites. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):963–968. doi: 10.1073/pnas.93.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Wiley S., Lai V. K., Olivier J. P., Gish G. D., Stephens R., Kaplan D., Shoelson S., Pawson T. A conserved amino-terminal Shc domain binds to phosphotyrosine motifs in activated receptors and phosphopeptides. Curr Biol. 1995 Apr 1;5(4):404–412. doi: 10.1016/s0960-9822(95)00081-9. [DOI] [PubMed] [Google Scholar]