Abstract
Background
Cigarette smoking is the best-established risk factor for urothelial carcinoma (UC) development, but the impact on oncologic outcomes remains poorly understood.
Objective
To analyse the effects of smoking status, cumulative exposure, and time from smoking cessation on the prognosis of patients with primary non–muscle-invasive bladder cancer (NMIBC).
Design, setting, and participants
We collected smoking data from 2043 patients with primary NMIBC. Smoking variables included smoking status, average number of cigarettes smoked per day (CPD), duration in years, and time since smoking cessation. Lifetime cumulative smoking exposure was categorised as light short term (≤19 CPD, ≤19.9 yr), light long term (≤19 CPD, ≥20 yr), heavy short term (≥20 CPD, ≤19.9 yr) and heavy long term (≥20 CPD, ≥20 yr). The median follow-up in this retrospective study was 49 mo.
Interventions
Transurethral resection of the bladder with or without intravesical instillation therapy.
Outcome measurements and statistical analysis
Univariable and multivariable logistic regression and competing risk regression analyses assessed the effects of smoking on outcomes.
Results and limitations
There was no difference in clinicopathologic factors among never (24%), former (47%), and current smokers (29%). Smoking status was associated with the cumulative incidence of disease progression in multivariable analysis (p = 0.003); current smokers had the highest cumulative incidences. Among current and former smokers, cumulative smoking exposure was associated with disease recurrence (p < 0.001), progression (p < 0.001), and overall survival (p < 0.001) in multivariable analyses that adjusted for the effects of standard clinicopathologic factors and smoking status; heavy long-term smokers had the worst outcomes, followed by light long-term, heavy short-term, and light short-term smokers. Smoking cessation >10 yr reduced the risk of disease recurrence (hazard ratio [HR]: 0.66; 95% confidence interval [CI], 0.52–0.84; p < 0.001) and progression (HR: 0.42; 95% CI, 0.22–0.83; p = 0.036) in multivariable analyses. The study is limited by its retrospective nature.
Conclusions
Smoking status and a higher cumulative smoking exposure are associated with worse prognosis in patients with NMIBC. Smoking cessation >10 yr abrogates this detrimental effect. These findings underscore the need for integrated smoking cessation and prevention programmes in the management of NMIBC patients.
Keywords: Smoking, Urothelial carcinoma, Non–muscle-invasive bladder cancer, Recurrence, Progression, Survival, Dose–response relationship
1. Introduction
Urothelial carcinoma of the bladder (UCB) is a common malignancy, with an estimated 73 510 new cases and 14 880 deaths in 2012 in the United States [1]. At initial diagnosis, >70% of patients have non–muscle-invasive bladder cancer (NMIBC), which is generally managed with endoscopic transurethral resection of the bladder (TURB) with or without intravesical therapy [2]. Recurrence rates for NMIBC range from 50% to 70%, and approximately 10–15% of tumours progress to muscle-invasive disease over a 5-yr period [2–4]. These statistics underscore the need for a continuous, costly follow-up, making UCB the most expensive malignancy per patient [5].
Cigarette smoking is a strong, established risk factor for UCB development, increasing the risk two- to four-fold [6]. Although smoking has steadily declined over the past decades, 40% of US adults are current or former smokers [7,8]. Although smoking status has been associated with more advanced tumour stage and grade as well as disease recurrence and progression in NMIBC, conclusions are limited by small sample sizes and analytical approaches of published studies [9–12]. Indeed, the relationships among smoking status, intensity, and time from smoking cessation with biologic behaviour of NMIBC remain insufficiently understood. A strong association between smoking and prognosis of NMIBC could have a significant impact on the clinical management of these patients.
We hypothesised that smoking is associated with the biologic aggressiveness of NMIBC, as reflected by pathologic factors, disease recurrence, and progression to muscle-invasive UCB. In addition, we hypothesised that there is a dose-response relationship between smoking intensity and adverse outcomes. Moreover, smoking cessation may reduce these effects. To address these hypotheses, we investigated smoking habits and intensity as well as cessation in a large, international, retrospective, multicentre cohort of patients treated with TURB for NMIBC.
2. Methods
2.1. Patient population
The study was performed with the approval and oversight of the institutional review board at each institution, with all participating sites providing the necessary data-sharing agreements prior to initiation. Templates for primary NMIBC data collection were sent out to 16 international centres. The majority of centres could not provide the requested data regarding smoking history; therefore, only six centres remained, from which data were included. In total, 3030 patients from six international centres (Hospital Motol Prague, Prague, Czech Republic; General Hospital Bolzano, Bolzano, Italy; University of Texas Southwestern Dallas, Dallas, TX, USA; University of Padua, Padua, Italy; Weill Cornell Medical Centre, New York Presbyterian Hospital, New York, NY, USA; and University of Montreal, Montreal, QC, Canada) underwent TURB for UCB between 1987 and 2007. Patients treated for recurrent NMIBC (n = 390) and those with missing variables or follow-up (n = 569) were excluded from analysis. In addition, 28 patients with pTis disease were excluded, as this group was too small for separate analyses. The data of 2043 primary NMIBC patients were frozen for analyses in January 2011.
2.2. Transurethral resection of the bladder and instillation therapy
All patients had cystoscopically proven primary UCB and underwent complete TURB according to guideline recommendations [2,13]. A re-resection was performed according to guideline recommendations and at surgeons' discretion within 2–6 wk after initial treatment based on pathologic and intraoperative findings. Immediate single-dose postoperative instillation chemotherapy (40 mg mitomycin or 80 mg epirubicin or 50 mg doxorubicin), adjuvant intravesical mitomycin C (MMC) chemotherapy, or bacillus Calmette-Guérin (BCG) immunotherapy was administered to 787 (39%), 77 (3.8%), and 328 (16.1%) patients, respectively. The first adjuvant instillation was generally given within 7–21 d of the diagnostic TURB and repeated once weekly for 6 wk. One hundred ninety (47%) patients received maintenance therapy. None of the patients had upper tract urothelial carcinoma (UTUC) at diagnosis.
2.3. Pathologic evaluation
All surgical specimens were processed according to standard pathologic procedures. Genitourinary pathologists assigned tumour grade according to the 1973 World Health Organisation grading system. Pathological stage was reassigned according to the 2002 American Joint Committee on Cancer TNM staging system. The presence of concomitant carcinoma in situ (CIS) was defined as the presence of CIS in conjunction with another tumour.
2.4. Smoking assessment
Smoking history was routinely assessed at the time of diagnosis through self-report. Patients were considered ever smokers if they had smoked 100 cigarettes during their lifetimes. Data on cigarette smoking included smoking status (current, former, or never smoker), average number of cigarettes per day (CPD; ie, quantity—1–9, 10–19, 20–29, ≥30), duration in years (≤9.9, 10–19.9, 20–29.9, 30–39.9, ≥40 yr), and years since smoking cessation to TURB in former smokers (≤4.9, 5-9.9, ≥10 yr). Patients reporting smoking cessation within 1 yr before TURB were considered current smokers. Tobacco use other than cigarette smoking (eg, tobacco chewing, cigars, pipes) was not assessed.
2.5. Follow-up regimen
Patients were generally followed every 3–6 mo during the first 2 yr after TURB, biannually up to 5 yr, and annually thereafter [2,13]. Follow-up consisted of a history, physical examination, urinary cytology, cystoscopy, and biopsy of suspicious lesions. Radiographic evaluation of the upper urinary tract to rule out UTUC was generally done at NMIBC diagnosis in every patient and yearly or in case of disease recurrence or suspicion, such as positive cytology during follow-up. When disease recurrence was detected, the tumour was resected. When disease recurrence was not detected but urinary cytology was positive, bladder and prostatic urethra biopsies in addition to upper urinary tract workup were performed. Disease recurrence was defined as first tumour relapse in the bladder regardless of tumour stage. Progression was defined as tumour relapse at tumour stage T2 or higher in the bladder. In case of death, cause of death was determined by treating physicians, by chart review corroborated by death certificates, or by death certificates alone [14]. Tumour recurrence in the upper urinary tract was not considered tumour recurrence but rather as a second primary tumour.
2.6. Statistical analysis
For statistical analyses, tumour size (<3 cm vs ≥3 cm) and number of tumours (single vs multifocal) were categorised based on previous stratifications [4]. Smoking quantity (never vs ≤19 vs ≥20 CPD), duration (never vs ≤19 vs ≥20 yr), and years since cessation (never vs ≤9.9 vs ≥10 vs current smoking) were categorised based on previous reports [15,16]. Based on their smoking quantity and duration, we divided ever smokers into four categories of lifetime cumulative smoking exposure: light short term (≤19 CPD and ≤19.9 yr), light long term (≤19 CPD and ≥20 yr), heavy short term (≥20 CPD and ≤19.9 yr), and heavy long term (≥20 CPD and ≥20 yr). To identify cut-offs for stratification, we ran incidence analyses using the original categories to get an impression of the distribution of our data. Contingency tables were constructed and used to compare the data regarding disease recurrence and progression. Finally, we categorised a composite variable in which former smokers were stratified into those who quit <10 yr ago and those who quit ≥10 yr ago.
Descriptive statistics were calculated for patient and disease characteristics by smoking status. Associations among categoric variables were assessed using the χ2 test, whereas the Kruskal-Wallis test was used for continuous variables. Follow-up time was calculated from the date of TURB. Three end points were investigated in this study: disease recurrence, progression, and overall survival (OS). OS probabilities were estimated using the Kaplan-Meier method, in which patients still alive were censored at the date of their last follow-up. The log-rank test determined differences in survival function among groups. To assess the impact of smoking on disease recurrence and progression, competing risk analyses were performed, because smoking is an established risk factor for common health problems that increase the risk of death [17]. The cumulative incidence was estimated, where patients who died without experiencing the event of interest were treated as a competing event. Gray's test was used to determine differences in cumulative incidence function among groups [18].
We next fit two sets of multivariable models. First, we estimated the impact of smoking status (never, former, current) with disease recurrence, progression, and overall mortality. Next, we limited the dataset to ever smokers and investigated the association between both cumulative smoking exposure and smoking status (current vs former) at concurrent consideration of time from cessation in former smokers with regard to disease recurrence, progression, and mortality.
Multivariable Cox regression and competing risks regression models were adjusted a priori for the effects of age, gender, pathologic T-stage and grade, number of tumours, tumour size, and intravesical therapy. Disease recurrence models were additionally adjusted for the effect of perioperative chemotherapy, as it was found to be associated with smoking status and disease recurrence in univariable analysis [19]. In exploratory analyses, we examined the impact of smoking among patients receiving intravesical adjuvant BCG therapy. Because variable distributions and outcomes differed by centre, additional adjustment was made for study centre in all models. Potential interactions were tested using the likelihood ratio test. All p values are two sided, and statistical significance was defined as p < 0.05. Statistical analyses were conducted using SAS v.9.2 software (SAS Institute, Cary, NC, USA) and R v.2.11.0 (R Development Core Team), including the Survival and Cmprsk packages.
3. Results
3.1. Association of smoking with clinicopathologic characteristics
Table 1 summarises clinicopathologic characteristics stratified by smoking status. Of 2043 patients, 494 (24%), 956 (47%), and 593 (29%) were never, former, and current smokers at the time of TURB, respectively. There were no differences in clinicopathologic factors among the three groups, except the use of immediate postoperative chemotherapy (former smokers receiving immediate postoperative chemotherapy more commonly; p = 0.032). Among ever smokers, current smokers smoked for a longer duration than former smokers (p < 0.001) and consequently were more frequently light long-term or heavy long-term smokers (p < 0.001).
Table 1. Descriptive characteristics of 2043 patients with primary non–muscle-invasive bladder cancer treated with transurethral resection of the bladder with or without intravesical therapy according to smoking status.
| Smoking status | |||||
|---|---|---|---|---|---|
|
|
|||||
| Overall (n = 2043) | Never (n = 494; 24%) | Former (n = 956; 47%) | Current (n = 593; 29%) | p value* | |
|
|
|||||
| Age, yr, median (IQR) | 67 (59–74) | 68 (59–75) | 67 (59–74) | 67 (58–75) | 0.917 |
| Gender, no. (%): | 0.441 | ||||
| Female | 435 (21.3) | 115 (23.3) | 195 (20.4) | 125 (21.1) | |
| Male | 1608 (78.7) | 379 (76.7) | 761 (79.6) | 468 (78.9) | |
| Pathologic stage, no. (%): | 0.270 | ||||
| pTa | 1246 (61.0) | 316 (64.0) | 578 (60.5) | 352 (59.4) | |
| pT1 | 797 (39.0) | 178 (36.0) | 378 (39.5) | 241 (40.6) | |
| Pathologic grade, no. (%): | 0.260 | ||||
| G1 | 482 (23.6) | 113 (22.9) | 220 (23.0) | 149 (25.1) | |
| G2 | 691 (33.8) | 184 (37.2) | 324 (33.9) | 183 (30.9) | |
| G3 | 870 (42.6) | 197 (39.9) | 412 (43.1) | 261 (44.0) | |
| Concomitant CIS, no. (%): | 0.249 | ||||
| Yes | 87 (4.3) | 15 (3.0) | 42 (4.4) | 30 (5.1) | |
| No | 1956 (95.7) | 479 (97.0) | 914 (95.6) | 563 (94.9) | |
| No. of tumours (%): | 0.137 | ||||
| 1 | 1424 (69.7) | 356 (72.1) | 672 (70.3) | 396 (66.8) | |
| 2-7 | 334 (16.3) | 73 (14.8) | 145 (15.2) | 116 (19.6) | |
| ≥8 | 285 (14.0) | 65 (13.2) | 139 (14.5) | 81 (13.6) | |
| Tumour size in cm, no. (%): | 0.063 | ||||
| <3 | 1529 (74.8) | 379 (76.7) | 727 (76.0) | 423 (71.3) | |
| ≥3 | 514 (25.2) | 115 (23.3) | 229 (24.0) | 170 (28.7) | |
| Adjuvant intravesical therapy, no. (%): | 0.147 | ||||
| Yes | 405 (19.8) | 101 (20.4) | 173 (18.1) | 131 (22.1) | |
| No | 1638 (80.2) | 393 (79.6) | 783 (81.9) | 462 (77.9) | |
| Type of adjuvant intravesical therapy, no. (%): | 0.115 | ||||
| None | 1638 (80.2) | 393 (79.6) | 783 (81.9) | 462 (77.9) | |
| BCG | 328 (16.1) | 76 (15.4) | 141 (14.8) | 111 (18.7) | |
| MMC | 77 (3.7) | 25 (5.1) | 32 (3.3) | 20 (3.4) | |
| Immediate postoperative intravesical chemotherapy, no. (%): | 0.032 | ||||
| Yes | 787 (38.5) | 177 (35.8) | 397 (41.5) | 213 (35.9) | |
| No | 1256 (61.5) | 317 (64.2) | 559 (58.5) | 380 (64.1) | |
| No. of cigarettes per day (%): | 0.140 | ||||
| 1–9 | 453 (29.3) | N/A | 270 (28.2) | 183 (30.9) | |
| 10–19 | 547 (35.3) | N/A | 352 (36.8) | 195 (32.9) | |
| 20–29 | 290 (18.7) | N/A | 186 (19.5) | 104 (17.5) | |
| ≥30 | 259 (16.7) | N/A | 148 (15.5) | 111 (18.7) | |
| Years of smoking, no. (%): | <0.001 | ||||
| <10 | 150 (9.7) | N/A | 118 (12.3) | 32 (5.4) | |
| 10–19.9 | 278 (17.9) | N/A | 214 (22.4) | 64 (10.8) | |
| 20–29.9 | 421 (27.2) | N/A | 279 (29.2) | 142 (23.9) | |
| 30–39.9 | 513 (33.1) | N/A | 340 (35.6) | 173 (29.2) | |
| ≥40 | 187 (12.1) | N/A | 5 (0.5) | 182 (30.7) | |
| Years since cessation, no. (%): | N/A | ||||
| ≥10 | 375 (39.2) | N/A | 375 (39.2) | N/A | |
| 5–9.9 | 319 (33.4) | N/A | 319 (33.4) | N/A | |
| 1–4.9 | 262 (27.4) | N/A | 262 (27.4) | N/A | |
| Cumulative smoking exposure, no. (%): | <0.001 | ||||
| Light short | 293 (18.9) | N/A | 227 (23.7) | 66 (11.1) | |
| Light long | 707 (45.7) | N/A | 395 (41.3) | 312 (52.6) | |
| Heavy short | 135 (8.7) | N/A | 105 (11.0) | 30 (5.1) | |
| Heavy long | 414 (26.7) | N/A | 229 (24.0) | 185 (31.2) | |
IQR = interquartile range; CIS = carcinoma in situ; BCG = bacillus Calmette-Guérin; MMC = mitomycin C; N/A = not applicable.
P value from χ2 test when categoric and Kruskal-Wallis test when continuous; p value for cigarettes per day, years of smoking, and cumulative smoking exposure compares former and current smokers.
3.2. Association of smoking with outcomes
The median follow-up for patients who were alive at last follow-up was 49 mo (interquartile range [IQR]: 18–93); 1791 (88%) patients had a follow-up >24 mo, and 1117 (55%) had a follow-up >60 mo. During the follow-up period, 41% (n = 835) of the study population experienced disease recurrence, 7% (n = 143) experienced disease progression, 21% (n = 421) died of any cause, and 5% (n = 117) died of UCB.
Figure 1 displays the cumulative incidence of disease recurrence and progression as well as OS probabilities stratified by smoking status (Fig. 1a, c, and e) and cumulative smoking exposure (Fig. 1b, d, and f). Smoking status was significantly associated with the cumulative incidence of disease recurrence (Fig. 1a; p = 0.044) and progression (Fig. 1c; p < 0.001), with current smokers having the highest cumulative incidence for both end points. There was no difference between current and former smokers regarding disease recurrence and former and never smokers regarding disease progression. Smoking status was not associated with OS (Fig. 1e; p = 0.66).
Fig. 1.
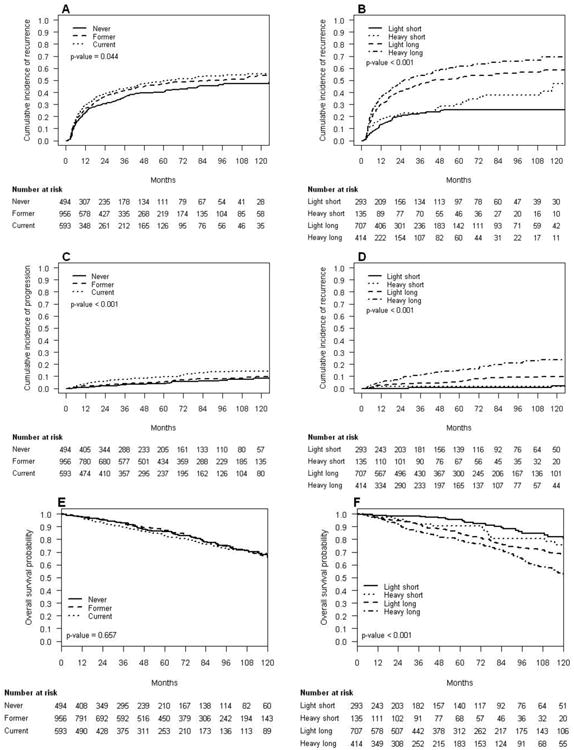
Outcomes of 2043 patients with non-muscle-invasive bladder cancer treated with transurethral resection of the bladder according to cigarette smoking status and cumulative smoking exposure: Cumulative incidence of disease recurrence by (a) smoking status and (b) cumulative smoking exposure; cumulative incidence of disease progression by (c) smoking status and (d) cumulative smoking exposure; Kaplan-Meier estimates of overall survival by (e) smoking status and (f) cumulative smoking exposure.
OS = overall survival.
Among ever smokers, cumulative smoking exposure was significantly associated with disease recurrence (Fig. 1b; p < 0.001), progression (Fig. 1d; p < 0.001), and OS (Fig. 1f; p < 0.001). Heavy long-term smokers had the worst outcomes, followed by light long-term smokers, heavy short-term smokers, and light short-term smokers.
We also tested for an interaction between smoking status, including consideration of time from cessation, and cumulative smoking exposure among ever smokers. The associations between cumulative smoking exposure and disease recurrence, progression, and mortality were similar for current smokers, former smokers who stopped <10 yr prior to NMIBC diagnosis, and former smokers ≥10 yr (all p > 0.05).
3.3. Risk factor analyses
In multivariable analyses that adjusted for the effects of standard clinicopathologic factors, smoking status was not associated with disease recurrence (p = 0.120), but it was associated with disease progression (p = 0.003; Table 2). As compared to never smokers, current smokers had a 2.09 times (95% confidence interval [CI], 1.29–3.39) increased risk of disease progression. The risk of disease progression did not differ between never and former smokers (Table 2).
Table 2. Multivariable regression analyses predicting disease recurrence, disease progression, and overall mortality in 2043 patients with primary non–muscle-invasive bladder cancer treated with transurethral resection with or without intravesical therapy.
| Disease recurrence | Disease progression | Overall mortality | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR* (95% CI) | p value | HR* (95% CI) | p value | HR** (95% CI) | p value | |
| Age, yr | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.00–1.03) | 0.027 | 1.08 (1.07–1.09) | <0.001 |
| Gender: | 0.980 | 0.960 | 0.005 | |||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 1.00 (0.84–1.19) | 0.99 (0.65–1.50) | 0.69 (0.53–0.89) | |||
| Pathologic stage: | 0.490 | 0.150 | 0.456 | |||
| pTa | 1.00 | 1.00 | 1.00 | |||
| pT1 | 0.88 (0.61–1.26) | 0.59 (0.29–1.21) | 0.82 (0.48–1.39) | |||
| Pathologic grade: | <0.001 | <0.001 | 0.130 | |||
| G1 | 1.00 | 1.00 | 1.00 | |||
| G2 | 1.48 (1.22–1.80) | 1.99 (1.13–3.51) | 1.18 (0.91–1.54) | |||
| G3 | 1.77 (1.21–2.58) | 5.74 (2.46–13.39) | 1.71 (0.98–2.97) | |||
| Tumour multifocality: | <0.001 | 0.041 | 0.193 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.56 (1.34–1.82) | 1.44 (1.02–2.03) | 1.15 (0.93–1.43) | |||
| Tumour size, cm: | <0.001 | 0.019 | 0.367 | |||
| <3 | 1.00 | 1.00 | 1.00 | |||
| ≥3 | 1.36 (1.15–1.59) | 1.54 (1.07–2.22) | 1.11 (0.89–1.38) | |||
| Intravesical therapy: | 0.950 | 0.570 | 0.670 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.99 (0.81–1.22) | 0.86 (0.5–1.46) | 0.93 (0.66–1.31) | |||
| Perioperative chemotherapy: | <0.001 | N/A | N/A | |||
| No | 1.00 | N/A | N/A | |||
| Yes | 0.68 (0.56–0.83) | N/A | N/A | |||
| Smoking status: | 0.120 | 0.003 | 0.690 | |||
| Never | 1.00 | 1.00 | 1.00 | |||
| Former | 1.12 (0.94–1.34) | 1.29 (0.79–2.09) | 1.10 (0.86–1.41) | |||
| Current | 1.22 (1.01–1.48) | 2.09 (1.29–3.39) | 1.12 (0.85–1.47) | |||
HR = hazard ratio; CI = confidence interval; N/A = not applicable.
HR estimated for
competing risks regression and
Cox regression. All estimates additionally adjusted for study centre.
Table 3 displays the results of multivariable analyses performed in ever smokers to simultaneously account for the effect of cumulative smoking exposure in addition to smoking status under consideration of time from cessation. After adjusting for the effects of standard clinicopathologic factors, smoking status categorised as current versus former <10 yr versus former ≥10 yr was significantly associated with disease recurrence (p < 0.001) and progression (p = 0.036) but not overall mortality (p = 0.98). Compared to current smokers, patients who quit smoking ≥10 yr prior to TURB had a 0.66 times (95% CI, 0.52–0.84) lower risk of disease recurrence and 0.42 times (95% CI, 0.22–0.83) lower risk of disease progression. In addition, cumulative smoking exposure was an independent predictor of all three outcomes (disease recurrence and progression p values <0.001; overall mortality p = 0.002). Compared to heavy long-term smokers, light short-term smokers had the lowest hazard ratios (HRs) of disease recurrence, progression, and mortality.
Table 3. Multivariable regression analyses predicting disease recurrence, disease progression, and overall mortality in 1549 ever-smokers with primary non–muscle-invasive bladder cancer treated with transurethral resection with or without intravesical therapy.
| Disease recurrence | Disease progression | Overall mortality | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR* (95% CI) | p value | HR* (95% CI) | p value | HR** (95% CI) | p value | |
| Age, yr | 1.00 (0.99–1.01) | 0.760 | 1.00 (0.98–1.02) | 0.980 | 1.08 (1.07–1.10) | <0.001 |
| Gender: | 0.920 | 0.820 | 0.004 | |||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 0.99 (0.82–1.20) | 1.05 (0.69–1.6) | 0.65 (0.49–0.87) | |||
| Pathologic stage: | 0.550 | 0.220 | 0.436 | |||
| pTa | 1.00 | 1.00 | 1.00 | |||
| pT1 | 0.89 (0.60–1.32) | 0.63 (0.30–1.32) | 0.79 (0.44–1.43) | |||
| Pathologic grade: | <0.001 | <0.001 | 0.300 | |||
| G1 | 1.00 | 1.00 | 1.00 | |||
| G2 | 1.65 (1.33–2.06) | 2.27 (1.20–4.27) | 1.10 (0.82–1.47) | |||
| G3 | 1.92 (1.27–2.90) | 5.64 (2.29–13.90) | 1.63 (0.88–3.01) | |||
| Tumour multifocality: | <0.001 | 0.059 | 0.073 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.49 (1.26–1.77) | 1.44 (0.99–2.10) | 1.25 (0.98–1.59) | |||
| Tumour size, cm: | <0.001 | 0.067 | 0.087 | |||
| <3 | 1.00 | 1.00 | 1.00 | |||
| ≥3 | 1.40 (1.18–1.67) | 1.45 (0.97–2.14) | 1.24 (0.97–1.60) | |||
| Intravesical therapy: | 0.790 | 0.680 | 0.363 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.03 (0.83–1.27) | 0.89 (0.50–1.58) | 0.83 (0.55–1.25) | |||
| Perioperative chemotherapy: | 0.001 | N/A | N/A | |||
| No | 1.00 | N/A | N/A | |||
| Yes | 0.69 (0.55–0.85) | N/A | N/A | |||
| Smoking status (including cessation status): | <0.001 | 0.036 | 0.980 | |||
| Current | 1.00 | 1.00 | 1.00 | |||
| Former <10 yr | 1.30 (1.09–1.53) | 0.99 (0.65–1.50) | 1.02 (0.79–1.30) | |||
| Former ≥10 yr | 0.66 (0.52–0.84) | 0.42 (0.22–0.83) | 0.98 (0.72–1.34) | |||
| Cumulative smoking exposure: | <0.001 | <0.001 | 0.002 | |||
| Heavy long | 1.00 | 1.00 | 1.00 | |||
| Light long | 0.91 (0.77–1.07) | 0.43 (0.29–0.63) | 0.67 (0.52–0.85) | |||
| Heavy short | 0.43 (0.30–0.60) | 0.12 (0.03–0.44) | 0.81 (0.51–1.27) | |||
| Light short | 0.35 (0.26–0.47) | 0.05 (0.01–0.19) | 0.54 (0.37–0.80) | |||
HR = hazard ratio; CI = confidence interval; N/A = not applicable.
HR estimated for
competing risks regression and
Cox regression; all estimates additionally adjusted for study centre.
3.4. Influence of smoking on bacillus Calmette-Guérin response
Among the 328 patients who received adjuvant intravesical BCG immunotherapy, 133 (41%) patients experienced disease recurrence and 16 (5%) experienced disease progression. The median follow-up in patients who received adjuvant intravesical BCG therapy was 42 mo (IQR: 15–73). Smoking status was significantly associated with disease recurrence after adjusting for the effect of study centre (p = 0.024). Current smokers had an increased risk of disease recurrence compared to never smokers (HR: 1.63; 95% CI, 1.02–2.61), although there was no difference between former and never smokers (HR: 1.02; 95% CI, 0.62–1.67). After adjusting for the effects of standard clinicopathologic factors in multivariable analysis, the magnitude between smoking status and disease recurrence was 1.06 (95% CI, 0.651.71) between former and never smokers and 1.62 (95% CI, 1.00–2.60) between current and never smokers, but the p value was no longer statistically significant (p = 0.059). The effect of smoking on disease progression and overall mortality in patients who received BCG immunotherapy was not assessed because of the low number of events.
4. Discussion
We found that continuous smoking is associated with poor outcomes in patients with primary NMIBC. The high rate of current (29%) and former (47%) smokers in our cohort of patients with NMIBC supports the association of smoking with UCB development [6,20,21]. Although previous studies reported that smoking is associated with disease recurrence [9,10,20], we found that smoking is associated with disease progression, which is a more important end point in the management of NMIBC. Tumour grade was also associated with oncologic outcomes. Indeed, smoking has been shown to affect the biologic aggressiveness of UCB [9,11,20,21]. Cigarette smoke contains >60 carcinogenic substances that can induce genetic and epigenetic changes and instabilities, leading to new and more aggressive UCB clones [22,23]. Sequential genetic changes that are known to accumulate during multistage progression of UCB may be induced or promoted by smoking-related carcinogens, causing more virulent UCB [24]. Similarly, in lung cancer, smoking has been associated with increasing rates of molecular alterations, leading not only to cancer genesis but also tumour progression [25]. The failure to find an independent association between smoking status and disease recurrence in our study might be influenced by the use of immediate or adjuvant intravesical therapy use, which has been shown to modify the risk of disease recurrence [4,19]. Similar to previous studies, we found that clinicopathologic factors such as tumour grade, size, and multifocality were more powerful predictors of disease recurrence than smoking status [2,20]. Furthermore, other inherent or genetic, environmental, behavioural, or lifestyle factors that our study could not adjust for could influence the association between smoking and NMIBC prognosis (eg, second-hand smoking, different genetic alterations in tumours of never smokers) [26]. In addition, controlling for intermediate variables (eg, clinicopathologic variables) can result in overadjustment, which usually biases estimates towards the null [27].
We found a significant dose-response relationship between cumulative smoking exposure, which combines smoking quantity and duration, and clinical outcomes of primary NMIBC patients. This is in accordance with studies that reported an association between smoking quantity and duration and the risk of UCB development and aggressiveness [6,11,23]. Indeed, increasing the number of CPD and years of smoking has been shown to be associated with more advanced and higher-grade tumours [11,12,23]. Interestingly, the HRs indicate a stronger effect of smoking duration than quantity on clinical outcomes. The exact biologic and molecular mechanisms of smoking-induced urothelial carcinogenesis and progression remain poorly understood [20]. Further work is necessary to understand the molecular correlates of these associations to allow modification of the detrimental effect of smoking on health.
We found that smoking cessation >10 yr prior to NMIBC diagnosis lowered the risk of disease recurrence and progression by a statistically and clinically significant margin. Similar to other pathologies related to smoking, long-term smoking cessation is necessary to reduce the detrimental effect of smoking on NMIBC outcomes [28]. The beneficial effect of long-term smoking cessation may be the result of a decrease in the field damage effects, improved repair mechanisms, or recovery of defence mechanisms [20,28]. Because the majority of NMIBC patients die from cardiovascular events and not UCB, smoking cessation could benefit by decreasing the individual risk for UCB recurrence and progression as well as that for reducing cardiovascular and respiratory morbidity and mortality [20,21].
We found a significant detrimental effect of smoking on the recurrence rate in patients treated with intravesical BCG immunotherapy, the most effective adjuvant therapy for patients with high-risk NMIBC [2,29]. Although former and never smokers had a similar risk of disease recurrence after BCG immunotherapy, current smokers had a reduced BCG response after adjusting for the effects of standard clinicopathologic factors. One recent study did not find any effect of smoking on BCG response but was limited by the lack of control for smoking dose and duration [30]. Intravesical BCG instillations induce a cytokine-transmitted immune response, leading to an apoptosis enhancement in UCB tissue [31]. Active cigarette smoking is suggested to impair the cytokine activity, B- and T-cell response, and natural killer cell activation, which might explain the reduced response to BCG therapy in our study [30,32].
Our study is not devoid of limitations inherent to its multicentre and retrospective study design. We did not control for treatment delay, effect of repeat TURB, quality of the TURB, and prognostic factors such as lymphovascular invasion. In particular, given the heterogeneous treatment pattern across centres, it is difficult to ascertain whether patients and which patients have received re-TURB, a treatment that is instrumental in the proper management of some high-risk NMIBC, and how many patients completed adjuvant intravesical instillation therapy. In addition, we could not adjust for the number and experience of surgeons and pathologists at each institution; no central pathology review was performed. However, all surgeons and pathologists operated at tertiary care centres with extensive experience in urothelial carcinoma. Comorbidities might have influenced the decision-making regarding surgical therapy, introducing a selection bias. Another limitation may be the failure to control for other tobacco products and different forms of tobacco exposure (eg, second-hand smoke). Smoking history was self-reported and therefore subject to recall bias. If current smokers reported themselves as former smokers, estimates would be biased towards the null, thereby obscuring associations. Future studies using biochemical verification of smoking status are needed to determine the extent of misreporting among NMIBC patients. Smoking variables could not be analysed as continuous variables, because self-reported smoking data were assessed in categorised fashion, possibly reducing the power of statistical analyses. Finally, the cancer-specific deaths' event rate was low in our study cohort, and thus we could not analyse the impact of smoking on cancer-specific mortality in primary NMIBC. These limitations clearly indicate that our findings need to be confirmed in robust, prospective studies or future clinical trials, specifically if smoking information and intervention are to be included in daily clinical decision-making of NMIBC.
5. Conclusions
We confirmed the detrimental effects of cigarette smoking and benefits of smoking cessation on oncologic outcomes of patients with primary NMIBC. Current and heavy long-term smokers are at the greatest risk for disease recurrence and progression. A high cumulative smoking intensity is also associated with an increased risk for overall mortality. Moreover, smoking negatively affected the response to intravesical BCG therapy regarding disease recurrence. Long-term smoking cessation (>10 yr) seems to abrogate the detrimental effect of cigarette smoking on oncologic outcomes. Taken together, these results seem to underscore the need for smoking cessation and counselling programs. General health care practitioners and urologists may play an important role in informing smokers regarding their risk of UCB development and progression as well as the benefits of smoking cessation.
Take-home message.
Current and heavy long-term cigarette smoking is significantly associated with unfavourable outcomes in primary non–muscle-invasive bladder cancer. Long-term smoking cessation mitigates these detrimental effects. These findings should encourage urologists and general health care practitioners to more actively support smoking cessation programmes.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Shahrokh F. Shariat had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shariat, Rink, Furberg, Zabor.
Acquisition of data: Shariat, Babjuk, Pycha, Lotan, Novara, Robinson.
Analysis and interpretation of data: Shariat, Rink, Furberg, Zabor.
Drafting of the manuscript: Rink, Shariat, Zabor, Furberg, Xylinas.
Critical revision of the manuscript for important intellectual content: Rink, Furberg, Zabor, Xylinas, Babjuk, Pycha, Lotan, Karakiewicz, Novara, Robinson, Montorsi, Chun, Scherr, Shariat.
Statistical analysis: Zabor, Rink, Shariat, Furberg.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Shariat.
Other (specify): None.
Financial disclosures: Shahrokh F. Shariat certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Dr Michael Rink is a speaker for Pfizer Pharma; Dr Giacomo Novara is a speaker and advisory board member for Astellas, Dendreon, GlaxoSmithKline, Lilly, Pierre Fabre Pharma, Recordati, and Takeda; Dr Shahrokh F. Shariat is an advisory board member for Ferring Pharma. In addition, Dr Rink is supported by The Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, et al. European Association of Urology (EAU). EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182:2195–203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. discussion 475-7. [DOI] [PubMed] [Google Scholar]
- 5.Stenzl A, Hennenlotter J, Schilling D. Can we still afford bladder cancer? Curr Opin Urol. 2008;18:488–92. doi: 10.1097/MOU.0b013e32830b8925. [DOI] [PubMed] [Google Scholar]
- 6.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630–9. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. World Health Organization. Global Health Risks. 2009 [Google Scholar]
- 8.Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–12. [PubMed] [Google Scholar]
- 9.Fleshner N, Garland J, Moadel A, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86:2337–45. [PubMed] [Google Scholar]
- 10.Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non–muscle-invasive bladder cancer. Eur Urol. 2011;60:713–20. doi: 10.1016/j.eururo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Sturgeon SR, Hartge P, Silverman DT, et al. Associations between bladder cancer risk factors and tumor stage and grade at diagnosis. Epidemiology. 1994;5:218–25. doi: 10.1097/00001648-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Thompson IM, Peek M, Rodriguez FR. The impact of cigarette smoking on stage, grade and number of recurrences of transitional cell carcinoma of the bladder. J Urol. 1987;137:401–3. doi: 10.1016/s0022-5347(17)44048-1. [DOI] [PubMed] [Google Scholar]
- 13.NCCN clinical practice guidelines in oncology: bladder cancer. National Comprehensive Cancer Network; 2011. Web site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 14.Rink M, Fajkovic H, Cha EK, et al. Death certificates are valid for the determination of cause of death in patients with upper and lower tract urothelial carcinoma. Eur Urol. 2012;61:854–5. doi: 10.1016/j.eururo.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–55. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States—recent progress and opportunities. CA Cancer J Clin. 2009;59:352–65. doi: 10.3322/caac.20037. [DOI] [PubMed] [Google Scholar]
- 18.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 19.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 2435. [DOI] [PubMed] [Google Scholar]
- 20.Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90:228–39. doi: 10.1046/j.1464-410x.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 21.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180:31–7. doi: 10.1016/j.juro.2008.03.045. discussion 37. [DOI] [PubMed] [Google Scholar]
- 22.Hirao Y, Kim WJ, Fujimoto K. Environmental factors promoting bladder cancer. Curr Opin Urol. 2009;19:494–9. doi: 10.1097/MOU.0b013e32832eb4ef. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Castelao JE, Yuan JM, et al. Cigarette smoking and subtypes of bladder cancer. Int J Cancer. 2012;130:896–901. doi: 10.1002/ijc.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reznikoff CA, Sarkar S, Julicher KP, et al. Genetic alterations and biological pathways in human bladder cancer pathogenesis. Urol Oncol. 2000;5:191–203. doi: 10.1016/s1078-1439(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 25.Begum S. Molecular changes in smoking-related lung cancer. Expert Rev Mol Diagn. 2012;12:93–106. doi: 10.1586/erm.11.84. [DOI] [PubMed] [Google Scholar]
- 26.Zeegers MP, Kellen E, Buntinx F, van den Brandt PA. The association between smoking, beverage consumption, diet and bladder cancer: a systematic literature review. World J Urol. 2004;21:392–401. doi: 10.1007/s00345-003-0382-8. [DOI] [PubMed] [Google Scholar]
- 27.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100:281–6. doi: 10.1111/j.1464-410X.2007.06873.x. discussion 286. [DOI] [PubMed] [Google Scholar]
- 29.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 30.Sfakianos JP, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guerin therapy for urothelial carcinoma not invading muscle of the bladder. BJU Int. 2011;108:526–30. doi: 10.1111/j.1464-410X.2010.09874.x. [DOI] [PubMed] [Google Scholar]
- 31.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 32.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–7. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]


