SUMMARY
Resistin is a cytokine that induces low-grade inflammation by stimulating monocytes in human. Resistin-mediated chronic inflammation can lead to obesity, atherosclerosis and other cardiometabolic disease. Nevertheless, the receptor for human resistin has not yet been clarified. Here, we identified adenylyl cyclase-associated protein 1(CAP1) as a functional receptor for human resistin and clarified its intracellular signaling pathway to modulate inflammatory action of monocytes. We found that human resistin directly binds to CAP1 in monocytes and up-regulates intracellular cAMP concentration, PKA activity and NF-kB-related transcription of inflammatory cytokines. Over-expression of CAP1 in monocytes enhanced resistin-induced increased activity of cAMP-dependent signaling pathway. Moreover, CAP1-over-expressed monocytes aggravated adipose tissue inflammation in transgenic mice that express human resistin from their monocytes. In contrast, suppression of CAP1 expression abrogated the resistin-mediated inflammatory activity both in vitro and in vivo. Our results highlight CAP1 as the bona fide receptor for resistin leading to inflammation in human.
INTRODUCTION
Resistin was first identified as a mediator of insulin resistance in obese mice(Steppan et al., 2001). It is secreted from mature adipocytes in rodents and a few studies have implicated murine resistin in the pathogenesis of obesity-mediated insulin resistance and of type 2 diabetes(Li et al., 2009; Nakata et al., 2007; Steppan et al., 2001). However, human resistin is quite different from murine resistin. Human resistin is primarily expressed in and secreted from monocytic cells(Patel et al., 2003). Moreover, the function of resistin in insulin resistance and obesity remains inconclusive in humans(McTernan et al., 2002; Savage et al., 2001; Utzschneider et al., 2005). Interestingly, human resistin seems to be involved in the recruitment of other immune cells and in the secretion of pro-inflammatory factors(Bokarewa et al., 2005; Silswal et al., 2005) and increasing evidence links resistin with inflammation and atherogenesis(Burnett et al., 2005; Jung et al., 2006; Reilly et al., 2005). As we report previously(Cho et al., 2011), resistin directly aggravates atherosclerosis by stimulating monocytes to induce vascular inflammation. In terms of the increasing role for inflammation in metabolic disease, the receptor and its intracellular signaling of resistin in human monocytes can be the therapeutic target of chronic inflammation leading to cardiometabolic disease.
Although the inflammatory functions of human resistin appear to be regulated by activation of nuclear factor kappa B (NF-κB) transcription factor(Silswal et al., 2005), understanding the biological function of human resistin has been slowed by lack of information about its corresponding receptor and signaling mechanisms. Recent reports have suggested isoform of decorin(Daquinag et al., 2011), mouse receptor tyrosine kinase-like orphan receptor (ROR)1(Sanchez-Solana et al., 2012), and toll-like receptor (TLR)4(Tarkowski et al., 2010) as potential receptors for resistin. However, both decorin and ROR1 are only putative receptors for murine resistin and none of these has been shown to mediate the inflammatory effects of resistin in humans. In addition, there was no evidence of the direct interaction between TLR4 and human resistin with a lack of biochemical binding assay.
Here, we report the identification of adenylyl cyclase-associated protein 1 (CAP1) as a protein that directly binds to human resistin and elicits inflammatory effects in cultured human monocytes and in white adipose tissue (WAT) in humanized resistin mice in vivo. The gain- and loss-of-function study implicates CAP1 as a bona fide functional receptor for human resistin.
RESULTS
Identification of a human resistin binding protein
Human resistin was conjugated with mouse Fc (denoted as mFc-hResistin) to detect potential receptor proteins using immuno-affinity methods (Figure 1A). The amino terminus of resistin was selected for conjugation with mFc (Figure 1B) because resistin molecules exist as multimeric complexes of coiled-coil trimers that form tail-to-tail hexamers via disulfide bonding near their amino termini(Patel et al., 2004). We hypothesized that the carboxyl terminal globular region constitutes the receptor binding site of resistin; similar to what has been observed for the adiponectin globular domain(Yamauchi et al., 2003).
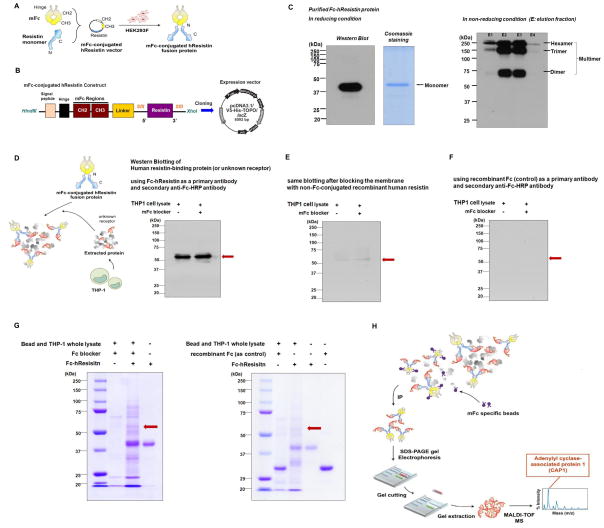
Figure 1. Identification of a human resistin binding protein.
(A,D,H) Schematic representation of methodology to detect putative resistin receptor(s) using an mFc-conjugated hResistin fusion protein(mFc-hResistin). (B) Schematic construct of mFc-hRe sistin shows that mFc region was conjugated at N-terminal of human resistin. (C) The expression and purification of mFc-hResistin were confirmed by Western blotting with an anti-mFc-HRP antibody and by Coomassie staining, respectively. Western analysis in non-reducing condition indicates that the purified mFc-hResistin forms a multimeric assembly, not a monomer. (D–F) Western blotting applying mFc-hResistin as a primary antibody identified an approximately 55 kDa protein that bound specifically to mFc-hResistin (red arrow) and that was undetectable following blocking with non-Fc-conjugated recombinant human resistin. (G) Representative immunoprecipitation findings. The incubation mixture of mFc-hResistin and human monocytic leukemia (THP-1) cell extracts was precipitated using mFc-specific beads. The red arrow indicates a putative human resistin receptor at approximately 55 kDa.
See also Figure S1.
The mFc-hResistin fusion was expressed and purified to homogeneity after transfecting mFc-hResistin DNA into HEK293F cells. The Western blot analysis which was performed in non-reducing condition during purification indicated that the purified mFc-hResistin forms a multimeric assembly, not a monomer (Figure 1C).
By Western blotting applying the multimeric mFc-hResistin as a ligand (or a primary antibody) to human monocytic leukemia (THP-1) cell lysate, we were able to identify a protein with molecular mass of about 55 kDa (Figure 1D). This protein nearly disappeared in the presence of an abundant non-Fc-conjugated recombinant human resistin protein (rhResistin) (Figure 1E). Also, when we used just “recombinant Fc” as a primary antibody instead of Fc-hResistin, we could not detect any protein (Figure 1F). These findings suggested that THP-1 cells expressed a protein with molecular mass of about 55 kDa capable of specifically binding with human resistin.
To identify the 55kDa-sized molecule which believed to be a receptor for human resistin, we incubated the purified mFc-hResistin with THP-1 cell lysates and then re-purified mFc-hResistin by immunoprecipitation using mFc specific beads. As expected, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) chromatography detected a specific band corresponding to an approximately 55 kDa protein (Figure 1G). Then, we excised the gel band and performed matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) (Figure 1H). This analysis identified the binding protein as CAP1 (NCBI accession number Q01518). Figure S1 depicted the full amino acid sequence of CAP1 in Homo sapiens and the sequences of matched peptides profiled by MALDI as underlined and in bold face.
CAP1 characterization
Human CAP1 is an actin-binding protein of 475 amino acids with a molecular mass of 51.9 kDa. CAP1 originally was isolated as a component of the Saccharomyces cerevisiae adenylyl cyclase complex(Field et al., 1990), and it is highly conserved from yeast to humans(Matviw et al., 1992). In yeast, the CAP1 homologue plays a key role in the cyclic AMP pathway. We analyzed the expression of CAP1 mRNA in various human cell lines and multiple organ tissues of rabbits and transgenic mice expressing macrophage-specific human resistin, compared to general C57BL/6 mice (Figure 2A). In our previous study(Cho et al., 2011), rabbits serve as an established model for studying the physiology of human resistin rather than general mice. CAP1 gene was detectable in most tissues but enriched in peripheral blood mononuclear cells (PBMCs) and bone marrow. The expression pattern of CAP1 was similar to that of resistin (Figure 2A) particularly in human cells, rabbits and the transgenic mice, which is consistent with the hypothesis that both molecules contribute to inflammatory processes in human monocytes.
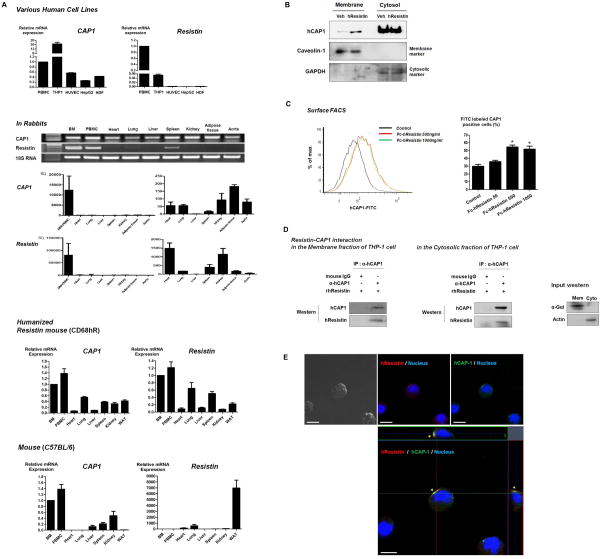
Figure 2. CAP1 characterization.
(A) The expression of CAP1 mRNA in various human cell lines and multiple organ tissues of rabbits, humanized resistin mice (transgenic mice with macrophage-specific expression of human resistin) compared to general C57BL/6 mice. Reverse-transcriptase polymerase chain reaction of resistin in various tissues indicated robust expression of CAP1 and resistin in peripheral blood mononuclear cells (PBMC). The error bars represent standard error of the mean (SEM). (B) Representative Western blotting detecting human CAP1 in the membrane fraction of THP-1 cells as well as cytosol. Importantly, the exogenously applied resistin elicited a change in CAP1 localization to membrane surface. (C) Also, the surface flow cytometric analyses (FACS) demonstrated that the number of membrane CAP1-positive cells were increased significantly after the stimulation with Fc-hResistin (n=3, *p< 0.001). The error bars represent SEM. (D) Co-immunoprecipitation of human resistin and CAP1 in the membrane fraction as well as in the cytosolic fraction of human monocytic leukemia (THP-1) cells. (E) Co-localization of human resistin and CAP1 as determined by immune-fluorescence double-staining in THP-1 cells. Scale=10 μm. HUVEC = human umbilical vein endothelial cells, HepG2 = human hepatocellular liver carcinoma cells, HDF = human dermal fibroblasts, WAT = white adipose tissue, hCAP1 = human CAP1, hResistin = human resistin, Fc-hResistin = mFc-conjugated human resistin fusion protein.
While CAP1 was considered to be a cytoplasmic protein in Dictyostelium discoideum, CAP1 is localized near the plasma membrane of resting cells(Noegel et al., 1999). Recently CAP1 was found to be mainly associated with plasma membrane in human THP-1 cells(Wakeel et al., 2009). Indeed, we were able to detect CAP1 not only in the cytosol, but in the membrane fraction of THP-1 cells by Western blotting (Figure 2B). Also, we found that the exogenously applied resistin elicited a change in CAP1 localization to membrane surface (Figure 2B and Figure 2C). Furthermore, the co-immuneprecipitation assay demonstrated the direct binding interaction between resistin and CAP1 particularly in the membrane fraction, as well as in the cytosolic fraction of THP-1 cells (Figure 2D). Through double-immuno-fluorescent staining of THP-1 cells treated with rhResistin using antibodies against resistin and CAP1, the strongest co-localization was observed between resistin and CAP1, which exhibited similar distribution and fluorescent intensity around the cell membrane (Figure 2E).
Human resistin binds directly to CAP1
To address whether resistin binds directly to CAP1, whole cell extracts of THP-1 cells were immuno-precipitated with anti-hCAP1 antibodies and immuno-blotted with anti-hResistin antibodies, and vice versa. We observed co-immuno-precipitation of human resistin and CAP1 in THP-1 (Figure 3A). Also, we performed a “far Western analysis”(Wu et al., 2007) to confirm the interaction between resistin multimer and CAP1 in vitro. Briefly, purified mFc-hResistin was transferred to a nitrocellulose membrane, as in standard Western blotting but in non-reducing condition. The membrane then was blocked and incubated with recombinant human CAP1 (rhCAP1). This approach identified CAP1 on spots in the membrane corresponding to the multimeric assembly of mFc-hResistin (Figure 3B), demonstrating that resistin multimer and CAP1 interact directly. Further, we performed a bimolecular fluorescence complementation (BiFC) assay, which is based on complementation between two non-fluorescent fragments of the fluorescent protein when they are brought together by interaction between proteins fused to each fragment(Hu et al., 2002). Using this non-immunoglobulin binding approach, we could visualize the interaction between human resistin and CAP1 in living cells (Figure 3C).
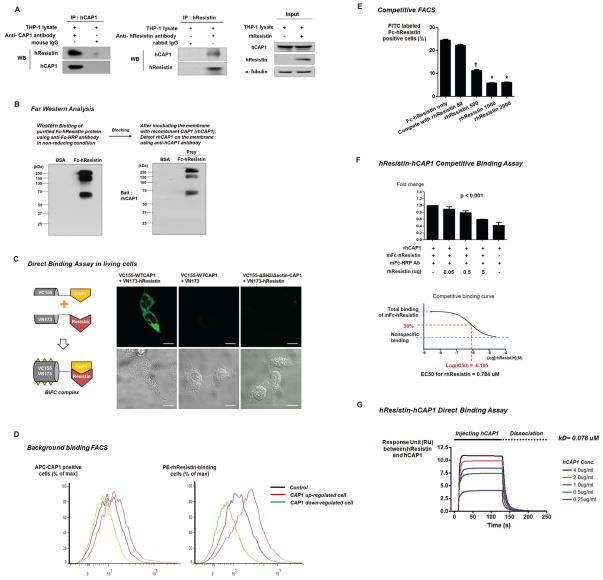
Figure 3. Human resistin binds directly to CAP1.
(A) Representative co-immuno-precipitation of human resistin and CAP1. (B) Representative far-Western blot of multimeric mFc-hResistin and recombinant human CAP1 (rhCAP1). (C) Representative bimolecular fluorescence complementation (BiFC) assay visualizing the human resistin-CAP1 interaction in living cells. The highest fluorescence intensity was exhibited when both CAP1 and resistin fused to each fragment (pVC155, pVN173) was expressed (left panel). However, when human CAP1 was expressed alone, detectable fluorescence was not exhibited (middle panel). When SH3- and actin- binding domain deletion mutant was cloned into pVC155 instead of wild-type CAP1 and co-expressed with pVN173-hResistin fusion protein, detectable fluorescence was not exhibited (right panel). (D) Representative classic background flow cytometry analysis (FACS)-based binding assay. PE-hResistin-binding cells were more increased in CAP1 up-regulated cell group than in control or CAP1 down-regulated cell groups. (E) Competitive FACS binding assay. FITC-Fc-hResistin was displaced by the non-labeled rhResistin in a dose-dependent manner (n=3, *p< 0.001, †p< 0.01). The error bars represent standard error of the mean (SEM). (F) Competitive ELISA binding assay. Recombinant human resistin (rhResistin) bound to rhCAP1 and was competitively inhibited by mFc-hResistin (n=3, *p< 0.001). The error bars represent SEM. (G) Direct binding assay between human resistin and CAP1.
In addition, we performed a classical flow cytometry analysis (FACS)-based binding assay. Firstly, the purified multimeric human resistin was fluorescently labeled and then we analyzed the binding of the labeled resistin to THP-1 cells, either overexpressed or lack of CAP1. Resistin-binding cells were more increased in CAP1 up-regulated cell group than in control or CAP1 down-regulated cell group (Figure 3D). Moreover, a competitive FACS assay further validated the direct interaction demonstrating that the binding can be effectively competed with non-labeled resistin (Figure 3E). As another receptor-binding competition assay, rhCAP1 was dispensed into ELISA plates, and was incubated with mFc-hResistin in which binding had been quantitated by fluorescence before and after incubation with excessive un-conjugated rhResistin. As shown in Figure 3F, mFc-hResistin was displaced by the recombinant protein in a dose-responsive manner and the competitive binding curve showed that recombinant resistin competed with mFc-hResistin for CAP1 binding (EC50 = 0.784 μM; calculated EC50 likely is related to the Kd). To calculate EC50, we assumed a one-site receptor-ligand binding model and applied the Cheng/Prusoff-equation(Cheng and Prusoff, 1973) using GraphPad Prism.
Finally, we underwent a direct binding assay using rhResistin and rhCAP1 (Figure 3G). The binding curves showed increased response unit (RU) between hResistin and hCAP1 in a dose dependent manner. And the calculated dissociation equilibrium constant (KD) from the dissociation curves was 0.078 uM. These findings provide the strong evidences of the direct binding and interaction between hCAP1 and hResistin.
Signaling pathway of resistin via CAP1
Because CAP1 is known to play a role in adenylyl cyclase activation, the finding that resistin binds to CAP1 suggests that resistin might activate adenylyl cyclase. As expected, the enzyme-linked immunosorbent assay (ELISA) showed resistin significantly increased cAMP levels in THP-1 cells (Figure 4A). Consistent with this, resistin increased the activities of both PKA and NF-κB in THP-1 cells (Figure 4B) and subsequently up-regulated the mRNA and protein levels of inflammatory cytokines such as IL-6, TNFα, and IL-1β (Figure 4C).
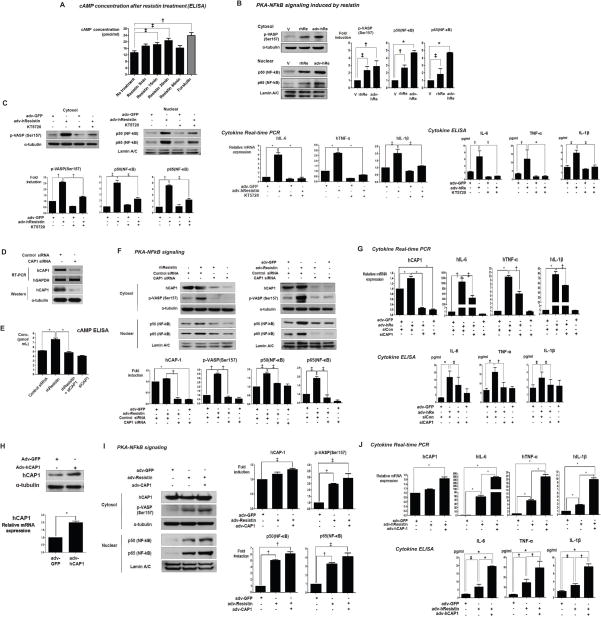
Figure 4. Resistin signaling pathway via CAP1.
(A) cAMP concentration was increased with the treatment of a physiologic dose (50ng/ml) of recombinant human resistin in THP-1 cells. (n=3, †p< 0.01, ‡p< 0.05). (B) Both recombinant protein resistin (rhRe, 50ng/ml) and gene-transfected resistin (adv-hRe) increased the activities of PKA and NF-κB significantly (n=3, *p< 0.001, †p< 0.01, ‡p< 0.05), (C) and subsequently induced inflammatory cytokines at the mRNA and protein levels. The PKA inhibitor, KT5720, abolished resistin-induced NF-κB activation and inflammatory cytokine expression (n=3, *p< 0.001, †p< 0.01, ‡p< 0.05). (D–G) siRNA targeting CAP1 abolished resistin-induced increases in cAMP concentration, PKA and NF-κB activities, and cytokine production (n=3, *p< 0.001, †p< 0.01, ‡p< 0.05). (H–J) Adenoviral-mediated over-expression of CAP1 enhanced the intracellular signals and biological effects of resistin. All the expression data was quantified using real-time qPCR, and the mRNA expression of cytokines was validated by ELISA (n=3, *p< 0.001, †p< 0.01, ‡p< 0.05). The error bars represent standard error of the mean (SEM).
See also Figure S2.
The cellular responses to resistin were greater when this molecule was introduced via adenoviral gene transfection than when cells were directly exposed to recombinant resistin protein (Figure 4B), perhaps reflecting the many cysteine residues in resistin leading to formation of disulfide-dependent multimeric structure(Aruna et al., 2003; Patel et al., 2004; Raghu et al., 2004) that is active and easily formed by adenoviral transfection while formed in difficulty by treatment with recombinant protein form. Non-reducing SDS gel analysis of cell lysates transfected with adenovirus-delivered resistin identified the resistin protein in fractions corresponding to molecular masses of approximately 50 kDa and 20 kDa (Figure S2). These likely represented the resistin trimer and dimer forms(Aruna et al., 2008; Gerber et al., 2005).
Since resistin induces cytokine expression via the activation of NF-κB(Silswal et al., 2005), we hypothesized that this activation may be downstream of adenylyl cyclase activation. Though unusual, this order would be consistent with reports of crosstalk between cAMP/PKA signaling and pro-inflammatory NF-κB pathways in macrophages(Peters-Golden, 2009; Wall et al., 2009). Indeed, treatment of THP-1 cells with PKA inhibitors blocked resistin-induced NF-κB activation and cytokine expression (Figure 4C).
To determine whether CAP1 is a functional signaling receptor for human resistin, we manipulated CAP1 expression and evaluated its impact on intracellular signals and pro-inflammatory actions of resistin in monocytes in vitro. CAP1 levels were reduced using specific small-interfering RNAs (siRNAs) in THP-1 cells (Figure 4D). CAP1 knockdown inhibited the ability of resistin to increase cAMP concentration (Figure 4E), PKA, NF-κB activity (Figure 4F), and cytokines production (Figure 4G). Conversely, over-expression of CAP1 by adenoviral transfection (Figure 4H) enhanced the intracellular signals and biological responses of THP-1 cells to resistin, including increased PKA, NF-κB activity (Figure 4I), and cytokine expression (Figure 4J).
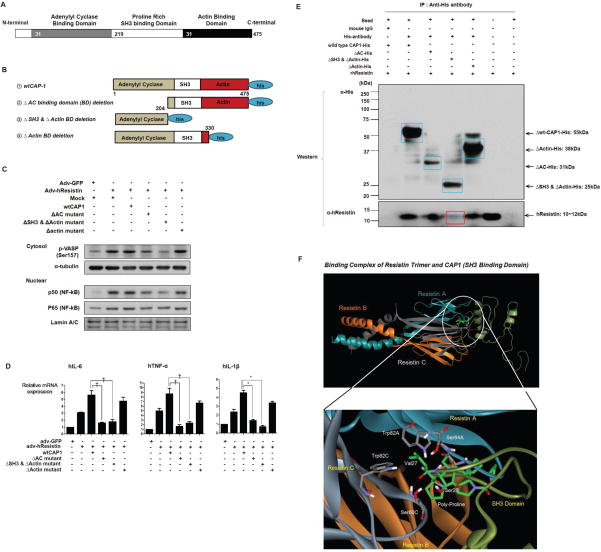
Resistin binding domain of CAP1
CAP1 is comprised of three major structurally and functionally distinct domains (Figure 5A). The highly conserved carboxyl-terminal domain of CAP1 binds to monomeric actin and is necessary for normal cellular morphology. The centrally-located proline-rich domain interacts with Src homology 3 (SH3) domains of specific proteins, including the yeast actin-associated protein Abp1p(Freeman et al., 1996; Lila and Drubin, 1997). Finally, the amino-terminal domain of CAP1 interacts with adenylyl cyclase in yeast. In higher eukaryotes, however, the function of the CAP1 N-terminus is unknown. The amino-termini of CAP1 molecules can interact with each other or with the CAP1 carboxyl terminus, suggesting the possibility of either parallel or anti-parallel dimers(Hubberstey and Mottillo, 2002). Notably, either dimer topology could allow the poly-proline SH3 interacting domain to bind target proteins.
Figure 5. Resistin binding domain of CAP1.
(A,B) Schematics of three major domains and three deletion mutants of human CAP1. (C,D) Resistin-induced PKA/NF-κB signaling and inflammatory cytokine production following overexpression of each of three CAP1 mutants in THP-1 cells (n=3 for each mutant, *p< 0.001, †p< 0.01). The error bars represent standard error of the mean (SEM). (E) In vitro binding assays between each CAP1 mutant and rhResistin. Binding of resistin and CAP1 (bottom row) was decreased significantly when samples were incubated with mutants deleted for the SH3 domain. (F) Predicted secondary structures of the resistin trimer-CAP1 SH3 binding domain complex. The resistin trimer are shown in Grey, cyan and orange schematic ribbon. CAP1-SH3 binding domain is shown in dark green ribbon. The detail interactions were illustrated in the magnified white circle. We targeted Val27 and Ser28 in CAP1-SH3 binding domain to induce point mutations and substitute glycine for Val27 and Ser28. See also Figure S3.
We cloned three deletion mutants of human CAP1 using lentiviral vectors (Figure 5B): an adenylyl cyclase (AC) binding domain deletion mutant (ΔAC binding domain [BD] deletion), a mutant in which both the SH3 binding domain and the actin binding domain were deleted (ΔSH3Δactin BD deletion), and an actin binding domain deletion mutant (Δactin BD deletion). Following resistin stimulation, PKA/NF-κB signaling (Figure 5C) and inflammatory cytokine production (Figure 5D) were not affected in the Δactin BD deletion mutant overexpressed cells, whereas they were suppressed in the ΔAC BD deletion and in the ΔSH3Δactin BD deletion mutant overexpressed cells. These data indicate that the actin binding domain of CAP1 is not necessary for the resistin ligand binding and/or receptor signaling, whereas the other two domains are crucial. Further, to identify the exact resistin binding sites on CAP1, we performed in vitro binding assays between each CAP1 mutant and rhResistin. After overexpressing His-tagged CAP1 mutants in 293A cells and treating rhResistin, we immune-precipitated the whole-cell extracts with anti-His antibodies. Then samples were Western blotted using both anti-resistin and anti-His antibodies. The band at approximately 12 kDa corresponding to rhResistin was not detectable in the ΔSH3Δactin BD deletion mutant, whereas detected in the ΔAC BD deletion mutant and in the Δactin BD deletion (Figure 5E). These observations confirm that human resistin binds to CAP1 via the proline-rich SH3 BD and that the AC domain likely plays a key role in receptor signaling.
Neither X-ray crystallography nor nuclear magnetic resonance spectroscopy data are available to deduce the molecular structure of CAP1. Thus, we predicted the structure of resistin-binding domain using homology modeling(Fernandez-Fuentes et al., 2007; Sternberg et al., 1999) with the known structure of cytidylyltransferase from Thermus thermophilus HB8 (2PX7), which has 20% sequence identity and 32% sequence homology with the region of CAP1 (Figure S3A). Using Discovery Studio 2.5 (Accelrys Inc.), we were able to visualize the predicted structure of resistin-binding domain and the known structures of resistin trimer(Patel et al., 2004). And then, to predict the structure of the resistin-CAP1 complex, we virtually analyzed the surface geometry of the complex using a protein-protein docking simulation and the score function analysis. Using the Pairwise Shape Complementarity function-based docking algorithm, ZDOCK(Chen et al., 2003; Chen and Weng, 2003), we inferred several three-dimensional binding structures between the resistin trimer and the resistin-binding domain of CAP1. Then, we evaluated the binding free energy of each complex using the Poisson-Boltzmann surface area method(Zoete et al., 2010). Figure 5F depicts the structure of the resistin trimer-CAP1 binding complex with the lowest binding free energy, which could be the conformation observed in nature. Further, we performed Molecular dynamics (MD) simulations for predicting the binding energy between resistin and mutated CAP1. And we found that the point mutations in CAP1-SH3 BD abolish interaction between Resistin and CAP1-SH3 BD. As shown in Figure 5F (magnified circle), hydrophobic interactions increase the binding affinity between the side chain of Val27 in CAP1-SH3 BD and Trp82A, Trp82C in resistin trimer. Also, the hydrogen bonds between Ser28 in CAP1-SH3 binding BD and Ser64A, Ser60C in resistin trimer increase the binding affinity. So, we targeted Val27 and Ser28 in CAP1-SH3 BD to induce point mutations and substitute glycine for Val27 and Ser28. After two nanoseconds of MD simulations, we calculated the difference of binding free energy between wild-type complex and mutant complex. The wild-type CAP1 is predicted to bind more favorable than its mutant with −9.0 kcal/mol. It suggests that substitution glycine for Val27 and Ser28 in CAP1-SH3 may abolish interaction between Resistin and CAP1-SH3 BD.
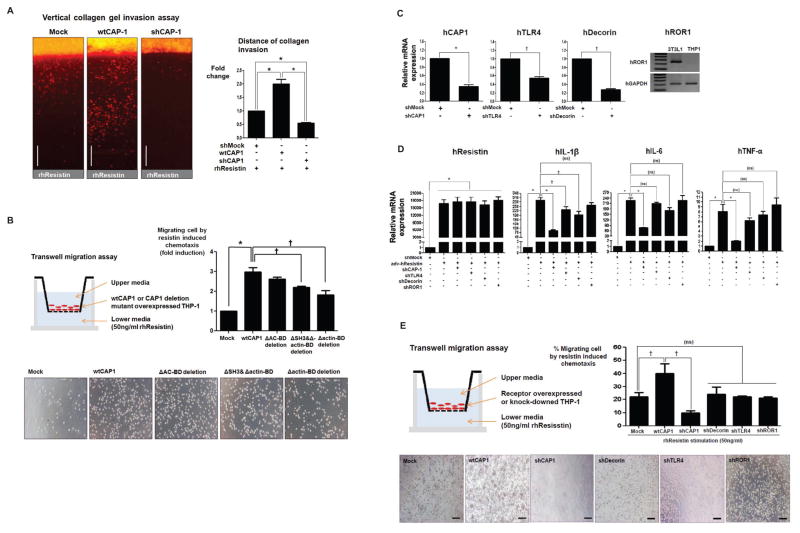
CAP1 and migration activity of monocytes
We previously demonstrated that resistin has chemoattractive effects on monocytes(Cho et al., 2011). Using the same assay of vertical collagen gel invasion(Cho et al., 2011; Hur et al., 2007; Yoon et al., 2005), in the present study, we showed that adenovirus-mediated CAP1 over-expression significantly enhanced the invasion of human THP-1 cells toward resistin (Figure 6A). In contrast, suppression of CAP1 expression by short hairpin RNAs (shRNAs) abrogated the resistin-mediated infiltration of monocytes (Figure 6A). These data indicate that the chemotaxis of macrophages or THP-1 cells to resistin is dependent upon CAP1. Further, for evaluating the role of the actin BD on chemotaxis, we performed the transwell migration assay by comparing wild type CAP1 with deletion mutant of CAP1. Interestingly, the migration ability of CAP1 was not changed significantly in ΔAC BD deletion mutant group whereas over-expressing ΔSH3Δactin BD deletion or Δactin BD deletion CAP1 mutant inhibited the resistin-mediated infiltration of monocytes (Figure 6B). Thus, the actin binding domain of CAP1 might have a pivotal role on the resistin-related migration activity of human monocytes.
Figure 6. CAP1 and migration activity of monocytes.
(A) Vertical collagen gel invasion assay. Monocytes over-expressing CAP1 migrate toward resistin significantly more frequently than those with CAP1 down-regulated did (n=3, *p<0.001). Scale=500 μm. (B) Transwell migration assay. Over-expressing ΔSH3Δactin BD deletion or Δactin BD deletion CAP1 mutant inhibited the resistin-mediated infiltration of monocytes (n=3, *p<0.001, †p<0.01). (C) Suppression of human decorin, TLR4 and ROR1 expression in human monocytes (D) showed little effects on pro-inflammatory cytokine production that was stimulated by human resistin (n=3, *p<0.001, †p<0.01, ‡p<0.05, ns=not significant). (E) Also, human decorin, TLR4 and ROR1 did not affect the migration of human monocytes toward human resistin (n=3, †p<0.01, ns=not significant). The error bars represent standard error of the mean (SEM).
See also Figure S4.
In addition, to demonstrate that CAP1 is indeed the bona fide receptor of human resistin, we assessed the role of the three previously reported receptors in the inflammatory actions of monocytes induced by human resistin. After suppressing the expression of human decorin, ROR1, and TLR4 using specific shRNAs (Figure 6C), we found that the expression level of human decorin, ROR1 and TLR4 in human monocytes had little effect on the production of pro-inflammatory cytokines that was stimulated by human resistin (Figure 6D). Transwell migration assay also demonstrated that human decorin, ROR1, and TLR4 did not affect the migration of human monocytes toward human resistin (Figure 6E). Only CAP1 had a great effect on the production of pro-inflammatory cytokine and the infiltration property of human monocytes which were induced by human resistin. When compared to CAP1 expression, moreover, both decorin and ROR1 were scarcely expressed in human cells particularly in human PBMC or THP-1 (Figure S4A and S4B). And even if applied resistin exogenously or using viral transfection, the expression level of decorin and ROR1 were not increased (Figure S4C).
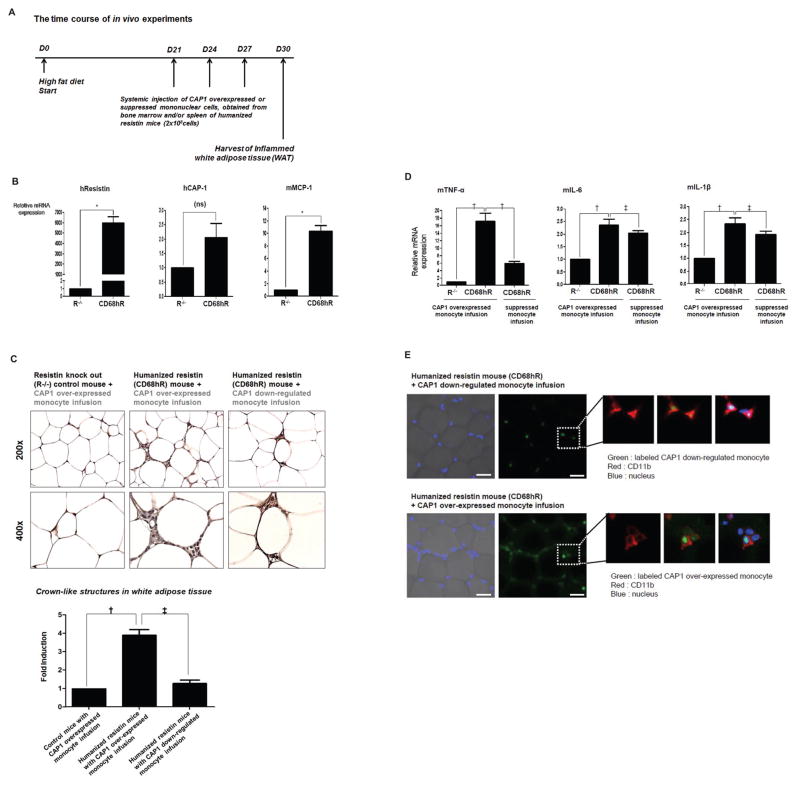
In vivo analysis of resistin-CAP1 interaction
Mice humanized by expressing human resistin in monocytes/macrophage lineage were known to develop an exacerbation of inflammation in the WAT when fed with a high fat diet(Qatanani et al., 2009). Before starting animal experiments using these transgenic mice (“humanized resistin mice”), we addressed an evidence of the interaction between human resistin and murine CAP1 (Figure S5). Murine CAP1 was known to have 97% homology with human CAP1 although their function was rarely known in both species so far(Hubberstey and Mottillo, 2002). Finally, we evaluated whether alteration of CAP1 expression in the monocytes of humanized resistin mice affects resistin-induced WAT inflammation. The time course of the in vivo experiments is illustrated in Figure 7A.
Figure 7. In vivo analysis of resistin-CAP1 interaction.
(A) The time course of in vivo experiments. (B) White adipose tissue of humanized resistin mice (CD68hR) fed with a high-fat diet exhibited higher levels of hResistin, MCP-1, and CAP-1 than resistin knock-out control mice (R−/−) (n=9 for each group, *p< 0.001, ns=not significant). The error bars represent standard error of the mean (SEM). (C) Immunohistochemical detection of the monocyte/macrophage-specific antigen CD11b in adipose tissue from humanized resistin and control mice. When CD68hR mice were systemically infused with monocytes over-expressing CAP1, macrophages heavily infiltrated into the white adipose tissue. When CAP1-suppressed monocytes were infused instead, macrophages infiltrated into the white adipose tissue less heavily (n=9, †p<0.01, ‡p<0.05). Magnification, ×200 (upper panels); ×400 (lower panels). The error bars represent SEM. (D) Consequently, inflammatory cytokines in white adipose tissue were more induced following systemic administration of CAP1-over-expressing monocytes than after administration of CAP1-suppressed monocytes (n=9, †p<0.01, ‡p<0.05). The error bars represent SEM. (E) Representative immune-fluorescence staining images indicate that infused monocytes migrated to inflamed white adipose tissue and that CAP1-over-expressing monocytes infiltrated adipose tissue much more heavily than CAP1-suppressed cells did. Scale=100 μm.
See also Figure S5.
Following a 1-month period of a high-fat diet to induce WAT inflammation, interestingly, CAP1 expression was elevated in WAT of humanized resistin mice along with human resistin and monocyte chemotactic protein-1 (MCP-1) (Figure 7B). Consequently, humanized resistin mice exhibited a greater accumulation of CAP1-overexpressed macrophages in the WAT than control mice (Figure 7C). However, when CAP1-suppressed monocytes were administered into humanized resistin mice, the accumulation of macrophages in WAT decreased significantly (Figure 7C). This effect was concordant with a reduction in several inflammatory markers, including TNF-α (Figure 7D). The identity of the infiltrating macrophages in WAT was confirmed by immune-fluorescence staining, which indicated that the infused monocytes migrated to inflamed WAT and that monocytes over-expressing CAP1 infiltrated WAT to a much greater extent than did CAP1-suppressed monocytes (Figure 7E). These findings strongly suggest that CAP1 serves as a receptor for resistin in vivo while acting as a key physiological regulator of the resistin-induced inflammatory action of monocytes.
DISCUSSION
Here we identified CAP1 as a resistin receptor that mediates the pro-inflammatory effects of human resistin in vitro and in vivo. The various competitive and direct bio-chemical binding assays that were lacking in previous receptor studies validated the direct interaction between CAP1 and resistin. Moreover, we have identified the region of CAP1 that binds resistin, and provided structural evidence of the plausibility of the resistin-CAP1 interaction.
Resistin is part of a unique family of cysteine-rich peptides that includes resistin-like molecules (RELMs) α and β, raising the question of whether CAP1 might bind to the RELMs. RELMα is not conserved in humans (Yang et al., 2003), but in preliminary studies we found that human resistin-like molecule β (RELMβ) interacts with the same domain of CAP1 that binds resistin (Figure S3B), and CAP1 had an effect on the production of pro-inflammatory cytokine induced by human RELMβ (Figure S3C). RELMβ is known to contribute to local immune responses in gut and bronchial epithelial cells (He et al., 2003), and a recent report showed that RELMβ is abundantly expressed in activated macrophages and contributes to atherosclerosis development via lipid accumulation and inflammatory facilitation (Kushiyama et al., 2013). That is similar to the role of resistin in aggravating atherosclerosis by stimulating monocytes, which is demonstrated in our previous study (Cho et al., 2011).
In the present study, we further assessed the role of the three previously reported receptors for resistin: isoform of decorin, ROR1 and TLR4. The gain- and loss-of function study demonstrated that all of the three putative receptors had little effect on the pro-inflammatory actions of human resistin in human monocytes. Furthermore, decorin and ROR1 were scarcely expressed in human mononuclear cells and the expression levels of decorin and ROR1 were not increased, as ever, regardless of resistin treatment. Also, the decorin identified as a potential receptor was an extracellular cleavage product of decorin and their model cell line may not perform the necessary proteolytic cleavage. Taken together, our results support that CAP1 is indeed the bona fide resistin receptor in humans.
With regard to CAP1 localization to the cell membrane, our detection of CAP1 in the membrane fraction of human monocytes using surface FACS as well as Western blotting and immunofluorescence staining is consistent with an earlier report which showed that CAP1 is mainly associated with plasma membrane in human monocytes (Wakeel et al., 2009). Moreover, we demonstrated that human resistin elicited a change in CAP1 localization to membrane surface. As CAP1 lacks a transmembrane domain, the cell biological mechanism underlying its membrane location is unclear and will require future investigation.
Resistin and receptor CAP1 up-regulated intracellular cAMP concentration, PKA activity and NF-kB related transcription of many inflammatory cytokines in human monocytes. As a contributor to the CAP1 receptor signaling that precedes inflammation, cAMP/PKA-dependent signaling pathway might be the key mechanism of the action of resistin, modulating monocytes to lead chronic inflammation. Considering that resistin was produced by activated monocytes and that CAP1 was detected in monocytes both in cytosol and near the plasma membrane, the present study suggests that CAP1 may serve as an autocrine/paracrine receptor or an endogenous receptor for resistin particularly during inflammatory conditions so that it can more aggravate the inflammatory actions of monocytes. Therefore, CAP1 could be a target in the treatment of inflammatory cardio-metabolic diseases, such as obesity or atherosclerosis.
EXPERIMENTAL PROCEDURES
See also “Extended Experimental Procedures” in Supplemental information.
SDS-PAGE chromatography
To identify the molecule that binds to mFc-conjugated hResistin in THP-1, which believed to be a receptor for human resistin, we performed immunopreciptation (IP) using mFc-conjugated hResistin protein as a primary antibody with mFc-specific beads. Briefly, THP-1 cells were incubated with human Fc Receptor binding inhibitor (eBioscience, Cat. No.: 16–9161) for 1hr to inhibit the non-specific receptor mediated binding, and then THP1 cells were washed with ice-cold PBS and whole cell lysates were prepared with a lysis buffer (20mM Tris pH7.5, 150mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 1mM EDTA, 1Mm NaF, and 1mM Na3VO4) containing protease inhibitor cocktail (Roche). The cell lysates were incubated with mFc-conjugated hResistin or recombinant mouse IgG1 Fc (Sino Biological Inc. Cat. No. : 10690-MNAH) as negative control at 4°C on rotator for overnight. After incubation, the protein complexes were pulled down using CaptureSelect Multi Species Fc matrix (the Bio Affinity Company) and microcentrifuged to pellet the protein-mFc matrix. After washing with PBS, the immunoprecipitated sample was resuspended with 2X reducing electrophoresis buffer and boiled at 95–100°C for 5 minutes to denature the protein and separate it from the protein-mFc matrix. The proteins were separated by electrophoresis on 8% polyacrylamide SDS gel and the gel was stained with Coomassie Brilliant Blue R250. We found the specific band around the size of 55kDa only in the lane of THP-1 whole lysate with mFc-hResistin fusion protein. To identify this unknown molecule, we excised the band from the gel and performed matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF).
Bimolecular Fluorescence Complementation (BiFC) assay
Full length of human CAP1, SH3/Actin domain CAP1 deletion mutant and human resistin were cloned into pVC155 and pVN173, respectively. pVC155 and pVN173 were kindly gifted by Prof. Chang S, from Seoul National University College of Medicine, Seoul, Korea. (See more details about lentiviral vector cloning of full length and three different deletion mutants of human CAP1 in “Extended Experimental Procedures” in Supplemental information.) After verifying sequences, HEK293A cells were transfected with 500 ng of each corresponding BiFC pairs using polyethylenimine (PEI). After 24hrs of transfection, cells were fixed with 2% paraformaldehyde and fluorescence was imaged with confocal microscope (LSM710, Zeiss, Germany).
Direct binding assay: surface plasmon resonance
The protocol of surface plasmon resonance spectroscopy (SPR) in this study of real-time direct binding of hResistin and hCAP1 was performed by using a Biacore X-100 (GE Healthcare, Piscataway, NJ, USA). The Sensor chip CM5 with pre-immobilized hResistin (200 ug/ml) in one flow cell was first saturated with hResistin protein. To analyze the binding kinetics, various concentrations of hCAP1 diluted in HBS-EP buffer (consisting of 0.01M HEPES, pH7.4 / 0.15M NaCl / 3mM EDTA / 0.005% Surfactant P20) were injected onto the sensor chip for 120 sec at 30 ul/min, and the response unit (RU) was then recorded. After injection of the analyte was stopped, HBS-EP buffer was poured over the chip for 130 sec at 30 ul/min to allow the bound analytes to dissociate from the immobilized hResistin, and dissociation curves were obtained. The RU elicited by injecting HBS-EP buffer was used as the vehicle control. Biacore X-100 control software was used to measure the changes in RU and to plot the binding curve. The curves obtained from the SPR experiments were analyzed and the dissociation equilibrium constant (KD) of hCAP1 to immobilized hResistin were calculated using kinetic evaluation software. The dissociation equilibrium constant KD (M) was derived from the equation, KD = kd/ka, where kd and ka are dissociation- and association-rate constants, respectively.
Transwell migration assay
Monocyte chemotaxis was measured using a 24-well Micro Transwell Permeable Supports (Corning). Wild type CAP1 and its three different deletion mutants overexpressed or CAP1, Decorin, TLR4 and ROR1 knock-downed THP1 cells using lentivirus were transferred to the upper chamber of the Micro Chemotaxis chamber. (See more details about RNA interference and cloning of shRNA constructs in “Extended Experimental Procedures” in Supplemental information.) As a chemoattractant, recombinant human Resistin (50ng/ml) was added to the lower chamber. The lower and upper chambers were separated by a polycarbonate membrane (5um pore size). Transmigration was performed for 6hr to 12hr at 37°C in a humidified atmosphere with 5% CO2. All cells migrating through the polycarbonate membrane to lower chamber were counted and taken by light microscopy.
In vivo analysis of resistin-CAP1 interaction
Age-matched (9- to 10-week-old) male humanized resistin mice expressing human resistin in their monocyte/macrophage lineages and not expressing murine resistin (Retn−/− CD68hR) were used in this study. Retn−/− littermates were used as controls (R−/−). All animals were fed a high-fat diet (60% fat, D12492; Uni Faith, Inc.) for 1 month, which induced WAT inflammation. During this period, excess mononuclear cells (2 × 108 cells) that either over-expressed or suppressed for CAP1 were infused systemically. To obtain the mononuclear cells expressing human resistin, we harvested bone marrow and/or spleen from humanized resistin mice and then isolated the mononuclear cells using panning procedures and cell sorting. CAP1 expression was modulated by lentiviral transduction, as described above.
Statistics
All experiments had been repeated at least three times, and all data were calculated as mean ± standard error of the mean (SEM). In each experiment, biological duplicates or triplicates were included. Comparisons between groups were performed by Student’s t-tests, and the results of in vivo experiments were compared using paired t-tests. SPSS v.11.0 (SPSS, Chicago, IL) was used for all analyses, and statistical significance was assigned at p < 0.05. The error bars represent SEM.
Supplementary Material
HIGHLIGHTS.
Human resistin binds directly to CAP1 at proline-rich SH3 binding domain
Resistin stimulates cAMP/PKA dependent signaling pathway via CAP1
CAP1 is the bona fide resistin receptor leading to inflammation in human
Acknowledgments
This study was supported by grants from the Innovative Research Institute for Cell Therapy (A062260), the National Research Foundation, MEST (2010-0020258), the Korean Health Technology R&D Project (A100476), and the National Institutes of Health (grant DK49210). Dr. Hyo-Soo Kim also is Professor of Molecular Medicine & Biopharmaceutical Sciences at Seoul National University; he is sponsored by the World Class University program of the Ministry of Education and Science, Korea
Footnotes
Supplemental information includes Extended Experimental Procedures and five figures.
AUTHOR CONTRIBUTION
First two authors contributed equally to this work. The other authors of this research paper also have directly participated in the planning, execution, or analysis of the study. They have read and approved the final version submitted.
DISCLOSURES
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aruna B, Ghosh S, Singh AK, Mande SC, Srinivas V, Chauhan R, Ehtesham NZ. Human recombinant resistin protein displays a tendency to aggregate by forming intermolecular disulfide linkages. Biochemistry. 2003;42:10554–10559. doi: 10.1021/bi034782v. [DOI] [PubMed] [Google Scholar]
- Aruna B, Islam A, Ghosh S, Singh AK, Vijayalakshmi M, Ahmad F, Ehtesham NZ. Biophysical analyses of human resistin: oligomer formation suggests novel biological function. Biochemistry. 2008;47:12457–12466. doi: 10.1021/bi801266k. [DOI] [PubMed] [Google Scholar]
- Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Chen R, Li L, Weng Z. ZDOCK: an initial-stage protein-docking algorithm. Proteins. 2003;52:80–87. doi: 10.1002/prot.10389. [DOI] [PubMed] [Google Scholar]
- Chen R, Weng Z. A novel shape complementarity scoring function for protein-protein docking. Proteins. 2003;51:397–408. doi: 10.1002/prot.10334. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, Lee J, Lee HJ, Lee TK, Park J, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9:74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fuentes N, Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. Comparative protein structure modeling by combining multiple templates and optimizing sequence-to-structure alignments. Bioinformatics. 2007;23:2558–2565. doi: 10.1093/bioinformatics/btm377. [DOI] [PubMed] [Google Scholar]
- Field J, Vojtek A, Ballester R, Bolger G, Colicelli J, Ferguson K, Gerst J, Kataoka T, Michaeli T, Powers S, et al. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990;61:319–327. doi: 10.1016/0092-8674(90)90812-s. [DOI] [PubMed] [Google Scholar]
- Freeman NL, Lila T, Mintzer KA, Chen Z, Pahk AJ, Ren R, Drubin DG, Field J. A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol. 1996;16:548–556. doi: 10.1128/mcb.16.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M, Boettner A, Seidel B, Lammert A, Bar J, Schuster E, Thiery J, Kiess W, Kratzsch J. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–4509. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thumheer MC, Cebra JJ, Lazar MA, Wu GD. Bacterial colonization leads to the colonic secretion of RELMβ/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- Hubberstey AV, Mottillo EP. Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 2002;16:487–499. doi: 10.1096/fj.01-0659rev. [DOI] [PubMed] [Google Scholar]
- Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Kushiyama A, Sakoda H, Oue N, Okubo M, Nakatsu Y, Ono H, Fukushima T, Kamata H, Nishimura F, Kikuchi T, et al. Resistin-like molecule β is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2013;33:1986–1993. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- Li FP, He J, Li ZZ, Luo ZF, Yan L, Li Y. Effects of resistin expression on glucose metabolism and hepatic insulin resistance. Endocrine. 2009;35:243–251. doi: 10.1007/s12020-009-9148-4. [DOI] [PubMed] [Google Scholar]
- Lila T, Drubin DG. Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matviw H, Yu G, Young D. Identification of a human cDNA encoding a protein that is structurally and functionally related to the yeast adenylyl cyclase-associated CAP proteins. Mol Cell Biol. 1992;12:5033–5040. doi: 10.1128/mcb.12.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- Nakata M, Okada T, Ozawa K, Yada T. Resistin induces insulin resistance in pancreatic islets to impair glucose-induced insulin release. Biochem Biophys Res Commun. 2007;353:1046–1051. doi: 10.1016/j.bbrc.2006.12.134. [DOI] [PubMed] [Google Scholar]
- Noegel AA, Rivero F, Albrecht R, Janssen KP, Kohler J, Parent CA, Schleicher M. Assessing the role of the ASP56/CAP homologue of Dictyostelium discoideum and the requirements for subcellular localization. J Cell Sci. 1999;112(Pt 19):3195–3203. doi: 10.1242/jcs.112.19.3195. [DOI] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M. Putting on the brakes: cyclic AMP as a multipronged controller of macrophage function. Sci Signal. 2009;2:pe37. doi: 10.1126/scisignal.275pe37. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Ghosh S, Soundarya K, Haseeb A, Aruna B, Ehtesham NZ. Dimerization of human recombinant resistin involves covalent and noncovalent interactions. Biochem Biophys Res Commun. 2004;313:642–646. doi: 10.1016/j.bbrc.2003.11.156. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- Sanchez-Solana B, Laborda J, Baladron V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol Endocrinol. 2012;26:110–127. doi: 10.1210/me.2011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O’Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Sternberg MJ, Bates PA, Kelley LA, MacCallum RM. Progress in protein structure prediction: assessment of CASP3. Curr Opin Struct Biol. 1999;9:368–373. doi: 10.1016/S0959-440X(99)80050-5. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider KM, Carr DB, Tong J, Wallace TM, Hull RL, Zraika S, Xiao Q, Mistry JS, Retzlaff BM, Knopp RH, et al. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330–2333. doi: 10.1007/s00125-005-1932-y. [DOI] [PubMed] [Google Scholar]
- Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–1745. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, et al. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li Q, Chen XZ. Detecting protein-protein interactions by Far western blotting. Nat Protoc. 2007;2:3278–3284. doi: 10.1038/nprot.2007.459. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW, et al. Comparative studies of resistin expression and phylogeomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- Zoete V, Irving MB, Michielin O. MM-GBSA binding free energy decomposition and T cell receptor engineering. J Mol Recognit. 2010;23:142–152. doi: 10.1002/jmr.1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.