Abstract
Primary liver cancers, including hepatocellular carcinoma and intrahepatic cholangiocarcinoma, are leading causes of cancer-related death worldwide. Recent large-scale genomic approaches have identified a wide number of genes whose deregulation is associated with hepatocellular carcinoma and intrahepatic cholangiocarcinoma development. Murine models are critical tools to determine the oncogenic potential of these genes. Conventionally, transgenic or knockout mouse models are used for this purpose. However, several limitations apply to the latter models. Herein, we review a novel approach for stable gene expression in mouse hepatocytes by hydrodynamic injection in combination with Sleeping Beauty–mediated somatic integration. This method represents a flexible, reliable, and cost-effective tool to generate preclinical murine models for liver cancer research. Furthermore, it can be used as an in vivo transfection method to study biochemical cross talks among multiple pathways along hepatocarcinogenesis and to test the therapeutic potential of drugs against liver cancer.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Primary liver cancer represents a major health problem worldwide. According to the World Health Organization, liver cancer represents the third leading cause of cancer-related death worldwide, accounting for approximately 695,000 deaths in 2008.1
Hepatocellular carcinoma (HCC) accounts for approximately 80% of all primary liver cancers. Epidemiological and molecular studies have demonstrated that the development of HCC spans several decades. Patients with hepatitis B (HBV) or hepatitis C (HCV) chronic infection, especially when accompanied by liver cirrhosis, are at a much higher risk of developing HCC than noninfected people.1,2 Other risk factors for HCC include alcohol abuse, diabetes, obesity, and related metabolic syndrome. Development of HCC is a multistep process.3 Although HBV or HCV infection has been clearly linked to HCC pathogenesis, the molecular events underlying this association remain poorly understood. Because HCC often arises in the context of liver cirrhosis, it has been hypothesized that HCC development might be triggered by the repeated rounds of hepatocyte death and proliferation occurring in the cirrhotic liver. This incessant hepatocyte loss and compensatory replication might generate a permissive environment for the occurrence of genetic and/or epigenetic changes, which result in activation of oncogenes and/or loss of function of tumor-suppressor genes, eventually leading to HCC formation.4,5 Genetic alterations observed in HCCs include mutations of p53 and β-catenin genes, activation of c-Met and insulin-like growth factor receptor tyrosine kinases, and aberrant CpG island hypermethylation of tumor-suppressor genes, such as APC, RASSF1A, and E-cadherin.6–10 HCC is a deadly disease with limited treatment options. Indeed, tumor resection and liver transplantation can only be applied to a few patients, and sorafenib, the only drug available for the treatment of unresectable HCC, prolongs the survival of patients with HCC for only 2 to 3 months.11,12
Intrahepatic cholangiocarcinoma (ICC) accounts for approximately 10% of primary liver cancer.13–15 In the past decades, the incidence of ICC has been increasing in the United States and the Western world.16,17 Liver fluke infection is the major risk factor in countries, such as Thailand, where ICC is prevalent. The etiology of ICC in Western countries is less well defined, but a recent study of meta-analysis of all published ICC epidemiological data suggests that HBV or HCV infection, alcohol abuse, diabetes, and obesity are major risk factors for ICC.18 This body of evidence indicates that different primary tumor types of the liver, including HCC and ICC, might share some etiological agents. ICC is a deadly malignancy with few treatment options. In fact, to our knowledge, there is no U.S. Food and Drug Administration–approved targeted therapy for ICC. Because of its orphan status, few clinical trials for the treatment of ICC have been conducted.
During the past decades, genetic studies have uncovered major signaling pathways involved in hepatocarcinogenesis. Recently, high-throughput oncogenomic studies, including microarrays, array-based comparative genomic hybridization, and deep sequencing, in combination with bioinformatics and other computational biological approaches, have identified many genes that are deregulated along HCC and ICC development. However, most of these candidate genes are likely to be passenger genes with limited implication in hepatocarcinogenesis. On the basis of these considerations, an important question arises: how can we identify the driver oncogenes and tumor-suppressor genes required for liver tumor initiation and progression? The use of liver cancer cell lines and in vitro studies has significant limitations because these cell lines are already of tumor origin. Mouse models can instead be critical to validate the oncogenic potential of a genetic event or an aberrantly altered signaling pathway. Also, because of the growing understanding of liver cancer molecular pathogenesis, mouse models represent an essential tool for in vivo screening of innovative therapeutic approaches against this deadly malignancy.
Mouse Models of Liver Cancer
Commonly used mouse models for liver cancer research have been previously and thoroughly described (Table 1).19–22 Genetically engineered mouse models, including knockout or transgenic mice, are required to demonstrate the oncogenic or tumor-suppressor potential of the target genes and to illustrate how these genes contribute to tumor initiation and progression. For instance, by using liver-specific Pten knockout mice, it has been demonstrated that ablation of Pten leads to hepatic steatosis, nonalcoholic steatohepatitis, and, eventually, liver cancer formation at approximately 1 year of age, providing strong evidence that Pten functions as a tumor suppressor for liver cancer development.40 However, these genetically modified murine models have several limitations. Indeed, the generation of germ-line knockout or transgenic mice is costly, is time consuming, and requires a high level of expertise. Also, often, oncogenes or tumor-suppressor genes are critical for embryonic or fetal development. Thus, overexpression or deletion/inactivation of these genes in the germ-line frequently results in early lethality or developmental defects. To overcome these limitations, tissue-specific, inducible-knockout, or transgenic mouse models are needed. Furthermore, multiple genetic alternations are generally necessary to transform a normal cell (hepatocyte) into an invasive tumor cell. In accordance with the latter assumption, overexpression or deletion of one gene is often not sufficient to promote liver tumor formation in vivo. In this scenario, one would need to cross multiple mouse strains to determine how several genes and related deregulation act synergistically to promote liver tumor formation. Finally, the mouse genetic background has a critical role in tumor development. Because mouse models are often generated in different genetic backgrounds, researchers, in many cases, need to backcross the mice into a pure genetic background before the mice can be used to study cancer development or to be crossed with other mouse strains. All these crosses can be labor intensive, time consuming, and expensive.
Table 1.
Mouse Liver Cancer Models Generated Using Hydrodynamic Transfection
| Genes | Tumor type | Mouse strain | Latency | Reference |
|---|---|---|---|---|
| Nras-FAH and shP53 | HCC | Fah−/− | ∼10 weeks | Wangensteen et al, 200823 |
| HBx-FAH and shP53 | HCC | Fah−/− | ∼10 weeks | Keng et al, 201124 |
| Rtl1 and FAH | HCC | Fah−/− | ∼9 months | Riordan et al, 201325 |
| NRasV12 | Mixed HCC and ICC | Ink4A/Arf−/− | ∼7 weeks | Carlson et al, 200526 |
| c-Met and ΔN90-β-catenin | HCC | WT FVB/N | ∼3 months | Tward et al, 200727 |
| NRasV12 and ΔN90-β-catenin | HCC | WT FVB/N | ∼3 months | Lee et al, 200828 |
| Spry2Y55F and ΔN90-β-catenin | HCC | WT FVB/N | ∼6 months | Lee et al, 200828 |
| Cyclin D1 and c-Met | HCC | WT FVB/N | ∼6 months | Patil et al, 200929 |
| Bmi1 and NRasV12 | HCC | WT FVB/N | ∼6 months | Xu et al, 200930 |
| c-Met and Spry2Y55F | HCC | WT FVB/N | ∼6 months | Lee et al, 201031 |
| myr-AKT | HCC | WT FVB/N | ∼6 months | Calvisi et al, 201132 |
| myr-AKT and NRasV12 | Mixed HCC and ICC | WTFVB/N | 3–4 weeks | Ho et al, 201233 |
| c-Myc | HB | WT FVB/N | ∼6 weeks | Chow et al, 201234 |
| myr-AKT and ΔN90-β-catenin | HCC | WT FVB/N and C57BL/6 | ∼4 weeks | Stauffer et al, 201135 |
| myr-AKT and Spry2Y55F | HCC with emperipolesis | WT FVB/N | ∼3 to 4 months | Wang et al, 201236 |
| HRasV12 and shP53 | Undifferentiated liver tumors | WT C57BL/6 | ∼1 week | Ju et al, 201337 |
| NICD1 | ICC | WT FVB/N | ∼5 months | Fan et al, 201238 Evert et al, 201339 |
| myr-AKT and NICD | ICC | WT FVB/N | ∼3 weeks | Fan et al, 201238 |
An alternative approach to the traditional genetic/knockout murine models is to transform embryonic hepatoblasts ex vivo, a technique that has been developed by Zender et al.41 By using this innovative approach, Zender et al41 validated the oncogenic potential of Yap and cIAP1 genes in hepatocarcinogenesis because these genes are found to be amplified in human HCC. This approach significantly reduces the time of experiment and number of mice required for a given study. However, it is technically challenging. Hepatoblast cells, in fact, need to be isolated from embryonic mouse livers and purified using E-cadherin–based fluorescence activated cell sorting. These cells are then cultured on feeder layers and infected with retroviral vectors. The modified hepatoblasts can be finally introduced into mice using the intrasplenic surgical injection procedure. However, not all laboratories are equipped to perform such complicated cell biological and animal studies. Furthermore, HCCs develop from hepatoblasts in this setting, in contrast with the widely accepted hypothesis that HCC originates from mature hepatocytes. Therefore, HCC induced from hepatoblasts may not fully recapitulate the biological process of hepatocarcinogenesis in humans.
Several virus-based methods can be used for long-term gene expression in the liver and can, therefore, in theory, be used to generate liver tumor models. For example, adeno-associated virus can efficiently and stably deliver genes into hepatocytes, and has been tested clinically for correcting genetic disorders.42–44 However, the adeno-associated virus vector has a limited genome size (in general, <5 kb) and can be technically challenging to generate.45 HIV-based lentiviral vectors are known to efficiently transduce both dividing and nondividing cells. However, lentiviral gene delivery into hepatocytes tends to be poor. Studies have shown that efficient lentiviral transduction of the liver requires hepatocyte cycling in vivo, which can be achieved by partial hepatectomy.46,47 To the best of our knowledge, no study has been performed using these viral-based vectors to induce liver tumor in mice.
Generation of Novel Mouse Models for Liver Cancer Research
Herein, we review a new method that combines hydrodynamic gene delivery and Sleeping Beauty–mediated somatic integration for long-term gene expression in mouse hepatocytes, and how this technology has been used in developing novel murine models for liver cancer research. In this review, we will use the term hydrodynamic transfection to describe this technology.
To understand the rationale of this technology, some anatomical issues have to be introduced. The main reason why parenchymal cells are targeted by hydrodynamic transfection is the fact that capillary endothelium and parenchyma cells are closely associated. This anatomical feature allows the immediate access of DNA in parenchyma cells once the endothelial barrier is breached. Hydrodynamic transfection uses a hydrodynamic force produced by the pressurized injection of a large volume of DNA solution into the blood vessel, which permeabilizes the capillary endothelium and generates pores in the plasma membrane of the surrounding parenchyma cells. DNA has access to the intracellular compartment through these pores. Subsequently, the pores of the plasma membrane close, trapping the DNA inside the parenchymal cells. The most successful application of the hydrodynamic technique is gene delivery to hepatocytes in mice, which has been developed by Liu et al48 at the University of Pittsburgh (Pittsburgh, PA). The standard procedure consists of a rapid (5 to 9 seconds) tail vein injection of physiological solution, equivalent to 10% of body weight, in which the plasmid DNA is diluted. The injection of such a large volume of DNA solution entering directly into the inferior vena cava stretches myocardial fibers over the optimal length for contraction, induces cardiac congestion, and drives the injected solution into the liver in retrograde. As a consequence, liver is the organ with the major uptake of plasmid DNA in the body, and approximately 10% to 40% of hepatocytes can be transfected after hydrodynamic tail vein injection. Transfection efficiency in all other organs, including kidney, spleen, lung, and heart, is <0.1% of that of the liver. Therefore, this transfection technology appears to be rather specific for the liver. Subsequent studies demonstrated that the transfection of the gene of interest affects predominantly the hepatocytes at the pericentral region (zone 3 of the liver acinus).49 Hydrodynamic injection does lead to liver injury. However, the injury is transient and the liver heals in approximately 1 week.49
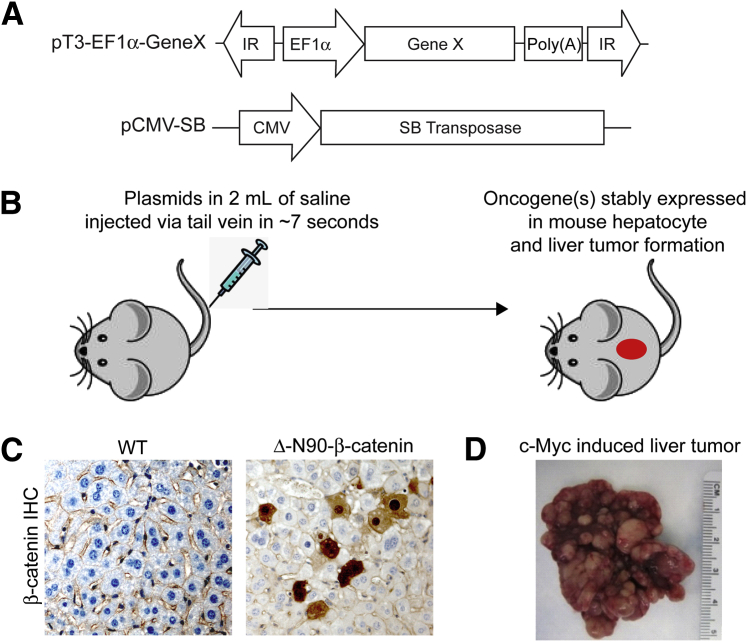
One of the major limitations for the application of hydrodynamic transfection to liver cancer research resides in the fact that transfected genes are rapidly degraded in hepatocytes. Indeed, the expression levels of the gene of interest peak approximately 8 to 24 hours after hydrodynamic injection, and decrease >1000-fold over 7 days.48 However, genes need to be continuously expressed in hepatocytes to induce liver cancer formation. To overcome this major technical challenge, Sleeping Beauty (SB) transposase50–mediated somatic integration is applied in combination with hydrodynamic transfection for stable and long-term target gene expression in the liver.51,52 In brief, SB transposase recognizes and binds to specific inverted repeats flanking a DNA sequence. It excises the DNA sequence and inserts the latter at a new location within a TA dinucleotide. The process can be viewed as cut and paste of DNA sequences. To achieve the goal of long-term gene expression in hepatocytes, two plasmids are needed: one encoding the SB transposase, and the second encoding the gene of interest under a mammalian promoter and flanked by inverted repeats (pT; Figure 1A). Because cytomegalovirus promoter is known to be silenced in hepatocytes, EF1α, PGK, or CAGGS promoters are commonly used. The two plasmids are then mixed together (ratio of SB/gene of interest is generally between 1:10 and 1:25), diluted into saline (volume of saline equals 10% of mouse body weight), and injected into the lateral vein of the mouse tail via hydrodynamic injection (Figure 1B). The long-term integration and gene expression efficiency is generally approximately 2% to 10% of hepatocytes (Figure 1C). Finally, once the oncogene is stably expressed in mouse hepatocytes, this can eventually lead to tumor formation (Figure 1D).
Figure 1.
Principles of hydrodynamic transfection for inducing liver cancer formation in mice. A: Structures of constructs used for the study. B: Overall diagram of study design. C: IHC staining of β-catenin in uninjected WT mouse liver and an activated form of β-catenin (Δ-N90-β-catenin) injected mouse liver. There is sporadic nuclear/cytoplasm localization of Δ-N90-β-catenin in hepatocytes transfected with the construct, whereas β-catenin is localized at the plasma membrane in WT livers. D: Gross image of a mouse liver tumor induced by hydrodynamically injecting the c-Myc proto-oncogene into the mouse. Original magnification, ×200 (C).
There are several obvious advantages of this technology in stably expressing genes in the liver and for the establishment of novel murine models for liver cancer in comparison to the traditional transgenic or knockout mouse models. First, in the traditional transgenic or knockout mouse models, virtually all hepatocytes overexpress or delete a specific gene. In contrast, hydrodynamic transfection delivers target genes in a relatively low percentage of hepatocytes (approximately 2% to 10% of hepatocytes) (Figure 1), so that target genes will be expressed by relatively few liver cells surrounded by normal/non-transfected hepatocytes. Thus, the sporadic expression of the target gene better resembles that in human liver cancer in comparison to the traditional transgenic or knockout mouse models. Second, the injection is performed in 6- to 8-week-old mice, thus not inducing any effect on mouse embryonic development. Third, this technology avoids the generation of costly transgenic or knockout mice and subsequent breeding, and significantly reduces the number of mice needed in the experiments. In addition, hydrodynamic transfection can be applied to mice from different genetic backgrounds. Thus, the hydrodynamic transfection method significantly accelerates the speed of the experiments while reducing the cost of the study. Finally, for effective liver tumor development, multiple genes may need to be co-expressed. This can be achieved by simply adding a second or third expressing vector flanked by inverted repeats into the plasmid mix, and injecting the mix into the mice. Overall, hydrodynamic transfection is a reliable, flexible, and cost-effective method to generate novel mouse models of liver cancer. It can also be used to study the biochemical cross talk between signaling pathways and test novel therapeutic agents for the treatment of this deadly disease.
HCC Mouse Models of Liver Cancer Using Hydrodynamic Transfection
We provide a review of the applications of hydrodynamic transfection for the study of liver tumor development.
Hydrodynamic Transfection in Fah-Null Mice
One way to generate murine liver cancer models is to combine hydrodynamic transfection with Fah-null mice. Loss of the Fah (fumaryl-acetoacetate hydrolase) gene leads to neonatal death due to liver failure.53 The lethality can be rescued by feeding the mice with nitisinone (NTBC), a drug that inhibits the upstream enzyme, 4-hydrozyphenylpyruvate diogenease, in the tyrosine metabolism pathway. Transplant of Fah-expressing hepatocytes results in the correction of Fah deficiency because Fah+ hepatocytes repopulate the mouse liver and mice can survive without NTBC.54,55 To use this model, one needs to clone the candidate oncogene together with Fah in the pT expression vector. The constructs are then co-injected with SB plasmid into Fah-null mice. The Fah-null mice are normally maintained on NTBC drinking water, and need to be replaced with normal drinking water immediately after hydrodynamic injection to allow the expansion of hepatocytes that receive the injected plasmids and express the Fah gene. The candidate oncogene is expressed in the repopulated hepatocytes and may induce liver cancer formation. One of the advantages of using Fah-null mice is that the selective repopulation of cells carrying the transfected genes and the proliferating hepatocytes provides additional stimuli that may favor oncogenesis. By using this technology, Keng et al24 characterized the oncogenic potential of HBx, a major oncogenic component of HBV. Specifically, the HBx gene was cloned under the PGK promoter and Fah was cloned under the CAG promoter. Both genes were inserted into one pT2 vector flanked by the inverted repeats.24 The HBx-Fah vector was injected into Fah-null mice, alone or with NRasV12, shRNAmir-based silencing of p53 (shP53), or NRasV12 plus shP53. A few HBx-injected mice developed hyperplastic nodules at approximately 20 weeks after injection. shP53, but not NRasV12, cooperated with HBx to induce liver cancer formation in this selective repopulation model, supporting the critical role of TP53 tumor-suppressor gene in HBV-induced HCC formation.24
Hydrodynamic Transfection of a Single Oncogene
A more popular way to generate liver cancer models is to directly hydrodynamically transfect genes into wild-type (WT) mice without relying on hepatocyte repopulation. c-Myc is a well-characterized oncogene, frequently amplified and/or overexpressed in human HCC and hepatoblastoma (HB) tissues.56,57 Hydrodynamic transfection of the Myc (alias c-Myc) gene into the mouse liver resulted in liver tumor formation and lethality at 5 to 8 weeks after injection.34 Histological evaluation revealed that the transfected hepatocytes were composed of small and highly proliferative tumor cells, which are highly similar to human HB. Similar HB-like tumor can also be induced via conditional induction of c-Myc expression in the mouse liver using the genetic mouse approach [ie, by crossing LAP-tTA mice (tetracycline-controlled transactivator under the control of liver-specific rat LAP promoter58) and TRE-c-Myc mice (human c-Myc under tetracycline-regulated element promoter59) to obtain LAP-tTA;TRE-c-Myc double-transgenic mice].59 For a second example, the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (mTOR) signaling pathway is the central regulator of multiple cellular processes, including cell metabolism, growth, proliferation, and survival.60 Activation of the phosphatidylinositol 3-kinase/AKT/mTOR cascade has been reported in human HCCs, with poor outcome.61 Hydrodynamic transfection of constitutively activated AKT (myr-AKT) leads to hepatocyte proliferation and increased de novo lipogenesis, resulting in hepatic steatosis and, eventually, liver cancer development by 6 months after injection (Figure 2).32 The process mimics what has been observed using liver-specific Pten-knockout mice (AlbCre;Ptenfl/fl mice; ie, ablation of Pten in the mouse liver also leads to hepatic steatosis and eventually liver cancer formation).40 All these studies demonstrate that the hydrodynamic transfection is a reliable, yet cost-efficient, method to generate liver tumor models, and the results recapitulate what is observed using traditional transgenic or knockout mouse approaches.
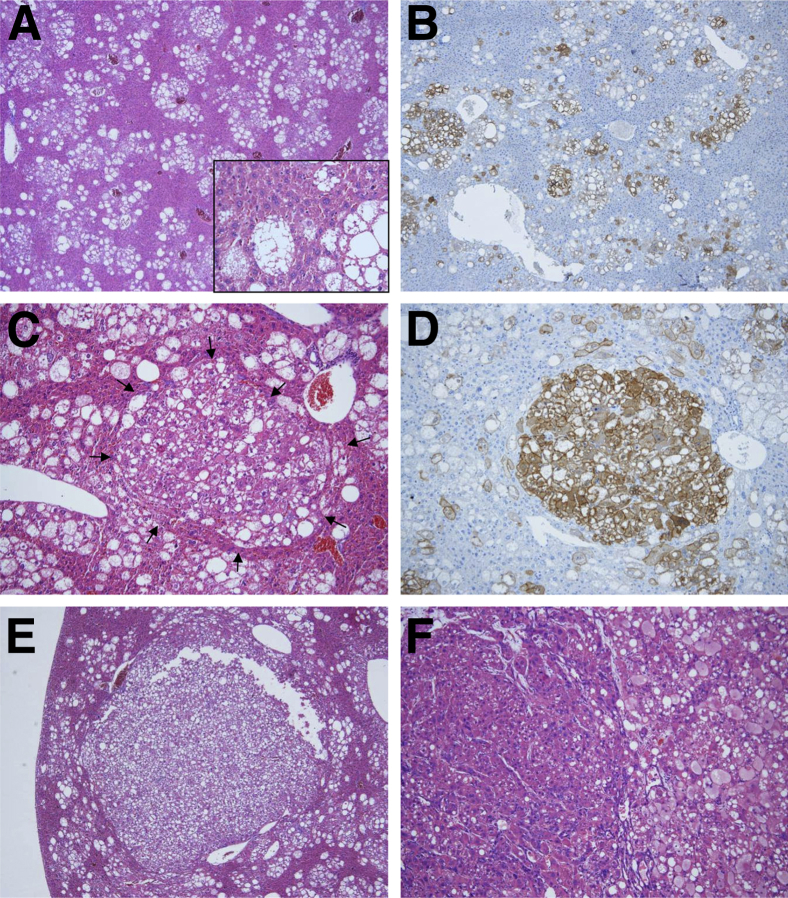
Figure 2.
Liver tumor development after hydrodynamic transfection of myristylated (activated) AKT (myr-AKT) into the mouse liver. A: Histological features of myr-AKT– overexpressing livers 12 weeks after hydrodynamic injection. At this time point, altered hepatocytes occupy approximately 40% to 50% of the liver parenchyma and form focal lesions. The cytoplasm of altered cells is clear and enlarged owing to the high content in lipids (inset). B: Subsequent staining for the HA tag shows that the altered hepatocytes are, in fact, expressing the exogenous myr-AKT gene. C and D: At the same time point, small hepatocellular adenomas (HCAs; arrows, C) also start to emerge in the liver parenchyma of myr-AKT–transfected mice and are positive for HA-tag staining. E and F: At 28 weeks after hydrodynamic transfection, large HCAs (E) and carcinomas (HCCs; F) develop. Although HCAs retain the clear-cell phenotype characteristic of focal, nontumorous lesions, HCCs most often display a macrotrabecular growth, less intracytoplasmic lipid content, and increased cytoplasmic basophilia. Nevertheless, areas with macrotrabecular (F; left part of the tumor) and solid/clear-cell (F; right part of the tumor) features often co-exist in HCCs developed in myr-AKT–transfected mice. Original magnification: ×40 (A, B, and E); ×400 (inset, A); ×100 (C and D); ×200 (F).
Hydrodynamic Transfection with Multiple Oncogenes
HCC development is a complex process. In general, tumor development requires the activation of multiple signaling pathways and, in many cases, the mutation of one gene is not sufficient to promote HCC formation. One such example is the wingless-type MMTV integration site family/β-catenin pathway. Activating mutations of β-catenin occur in 15% to 30% of human HCC samples.62,63 However, overexpression of activated mutant forms of β-catenin via hydrodynamic transfection fails to induce liver tumor formation, even over a long latency.27 The same results were obtained using transgenic mouse models overexpressing oncogenic forms of β-catenin.64,65 On the other hand, the activated mutant of β-catenin was found to cooperate with activated Ras or AKT pathways to induce liver tumor formation in the mouse.27,28,35 Overexpression of NRasV12 (a persistently active form of N-Ras), c-Met, or dominant negative Spry2 (Spry2Y55F) can all lead to activation of the Ras/mitogen-activated protein kinase (MAPK) signaling. Activating mutations of Ras are rarely found in human HCCs.35 On the other hand, the Ras/MAPK cascade is frequently activated in human HCCs.35 The goal of using NRasV12 is to mimic the activation of the Ras/MAPK pathway in vivo. These studies demonstrated that hydrodynamic cotransfection of ΔN90-β-catenin with NRasV12, c-Met, or Spry2Y55F triggered HCC formation in mice.27,28 In particular, liver tumor cells showed high levels of extracellular signal–regulated kinase protein activation and nuclear β-catenin, thus supporting the hypothesis that activation of both signaling cascades is required for HCC formation in vivo. Similar to that described for β-catenin, overexpression of NRasV12 alone is also unable to promote liver tumor formation in mice. This is presumably because of the fact that strong activation of the Ras/MAPK pathway promotes cellular senescence in hepatocytes.66 Indeed, NRasV12-expressing hepatocytes become senescent soon after hydrodynamic transfection and are subsequently eliminated by immune cells.66 A second signal, such as the activated mutant form of β-catenin,28 overexpression of the stem cell factor, Bmi1,30 or loss of the Ink4A/Arf locus,26 is required to cooperate with NRasV12 to induce HCC formation in mice.
The flexibility of the hydrodynamic transfection method makes it the ideal approach to demonstrate the in vivo oncogenic potential of novel gene(s) or pathway(s). For instance, Spry2 was first identified through genomic analysis to be significantly down-regulated in human HCC samples compared with nontumor normal liver tissues.67 Further analysis showed that Spry2 is located at 13q, which is deleted in approximately 50% of human HCCs.28 Spry2 is also among genes whose promoters are frequently methylated in HCCs.31,68 In addition, previous biochemical studies showed that Spry2 functions as a negative-feedback regulator of the Ras/MAPK pathway and supports the role of Spry2 as a tumor-suppressor gene along hepatocarcinogenesis.69 To validate the tumor-suppressor role for Spry2, the traditional method would require generating Spry2-knockout mice. However, most Spry2-knockout mice have severe gastrointestinal tract defects and survival of <1 month after birth and, therefore, are not suitable for the study.70 To avoid the early lethality, AlbCre mice need to be crossed with Spry2fl/fl mice to generate liver-specific Spry2-knockout mice (AlbCr;Spry2fl/fl). However, because loss of Spry2 alone is not sufficient to induce HCC formation in vivo,28 liver-specific Spry2-knockout mice have to be crossed eventually with additional knockout or transgenic mice to determine whether ablation of Spry2 cooperates with other gene(s) to promote hepatocarcinogenesis. By using hydrodynamic transfection, we recently generated the dominant-negative form of Spry2 (Spry2Y55F),71 which was shown to efficiently block Spry2 activity in cancer cells, and overexpressed it into the mouse liver alone or together with other common genetic events observed in human HCCs, including the activated form of β-catenin28 or AKT36 or overexpression of c-Met.31 The latter studies demonstrated that Spry2Y55F is able to synergize with other genetic events to promote HCC formation in vivo by sustaining high levels of the Ras/MAPK cascade. This experimental evidence suggests a new molecular mechanism for unrestrained activation of the Ras/MAPK cascade in the absence of Ras or Raf mutations along hepatocarcinogenesis.
Molecular Characterization of Murine Models Generated by Hydrodynamic Transfection
Murine models generated via hydrodynamic transfection can be used to analyze biochemical cross talk between signaling pathways; characterize cellular phenotypes, such as cancer stem cells; and evaluate drug responsiveness. For example, AKT/mTOR and Ras/MAPK pathways are frequently and concomitantly activated in human HCC samples. To investigate the functional interaction between the two signaling cascades, we have generated a mouse model by hydrodynamically transfecting activated forms of AKT and NRas proto-oncogenes into the mouse liver.33 As discussed previously, activated Ras alone did not induce liver tumor formation,66 and activated AKT alone led to HCC formation over a long latency period (approximately 30 weeks after injection).32 In contrast, co-expression of activated forms of AKT and NRas (referred to herein as AKT/Ras) in the mouse liver significantly accelerated hepatocarcinogenesis, leading to large tumor formation and mouse lethality by 6 weeks after injection.33 Biochemical analyses demonstrated that increased mTORC1, but not mTORC2, activity occurred in AKT/Ras tumor samples when compared with corresponding lesions from AKT mice. The increased mTORC1 activity in AKT/Ras mice was due to, at least partly, the Ras/MAPK-mediated phosphorylation and inactivation of serine 664 residue of tuberous sclerosis 2 protein, an mTORC1 suppressor.33 Further molecular analyses showed a strong up-regulation of c-Myc and FoxM1 in AKT/Ras tumor cells.33 Intriguingly, in vitro assays demonstrated that the up-regulation of c-Myc and FoxM1 contributed to AKT/Ras-induced hepatocarcinogenesis in an mTORC1-independent manner.33 In a follow-up study, we showed that rapamycin, an allosteric partial mTORC1 inhibitor, was able to restrain AKT/Ras-induced hepatocarcinogenesis.72 However, rapamycin treatment also triggered activation of the MAPK signaling in the residual tumor cells.72 Subsequent in vitro studies using a primary cell line isolated from an AKT/Ras mouse HCC demonstrated that cotargeting of mTORC1 and Ras/MAPK pathways was highly detrimental for the growth of these cells.72 These results provide strong preclinical evidence that concomitant inhibition of mTOR and MAPK cascades may be required for efficient treatment of HCC patients. As a second example, cancer stem cells (CSCs) are characterized as having enhanced tumor-initiating capabilities compared with other tumor cells,73 and have been identified in several solid tumors, including liver cancer.74 By using c-Myc transgenic mice and mice hydrodynamically transfected with c-Myc or AKT/Ras, Chow et al34 identified a subset of tumor cells that excludes Hoechst 33,342 dye in liver tumors induced by c-Myc, but not AKT/Ras. This side population (SP) of cells functions as CSCs, because they exhibited increased tumor-initiating properties compared with non-SP tumor cells using allograft experiments performed in NOD/Scidil2Rγ−/− mice.34 In addition, these SP tumor cells expressed markers of hepatic progenitor cells, such as CD44, Epcam, and Bmi1, and they could readily differentiate into more mature non-SP hepatic cancer cells.34 The latter study demonstrated that different initiating oncogenes can induce distinct CSC population formation.
Combination of Hydrodynamic Transfection with Traditional Transgenic and Knockout Mouse Models
Another major advantage of the hydrodynamic transfection technology is the possibility to combine it with the use of transgenic or knockout mice to study the genetic interactions between different genes in liver cancer development. For example, Chow et al34 identified an SP of cells functioning as CSCs in the c-Myc liver cancer model. The avidin-biotin complex transporter proteins, multidrug resistance protein 1 (MDR1) and ATP-binding cassette, sub-family G (WHITE), member 2 (BCRP), have both been shown previously to efflux Hoechst 33,342 dye and, therefore, may contribute to SP cell formation.75,76 To functionally determine which transporter is required for SP formation in c-Myc liver cancer, Chow et al34 applied hydrodynamic transfection of c-Myc in either Mdr1a/1b−/− or Bcrp−/− mice. The results showed that SP cells could be readily isolated in c-Myc–injected Bcrp−/− mice, but not in Mdr1a/1b−/− mice. Furthermore, it was shown that MDR1 expression renders CSC cells resistant to chemotherapeutic drugs that are MDR1 substrates, such as doxorubicin.34 In these studies, we found that breeding homozygous Mdr1a/1b−/− or Bcrp−/− mice and injecting them with c-Myc provides a definitive answer to the hypothesis 6 to 8 weeks after injection. In contrast, by using traditional mouse genetic approaches, one has to breed LAP-tTA (tetracycline-controlled transactivator under the control of liver-specific rat LAP promoter58), TRE–c-Myc (human c-Myc under tetracycline-regulated element promoter59), and Mdr1a/1b−/− or Bcrp−/− mice (three different strains of mice) together to obtain LAP-tTA;TRE-c-Myc;Mdr1a/1b−/− mice and LAP-tTA;TRE-c-Myc;Bcrp−/− mice. The breeding is likely to require 1 to 2 years, and only a few resulting mice (between 1 of 16 and 1 of 8, depending on the breeding strategy) have the desired genotypes. The latter experiments are both time consuming and expensive. Hydrodynamic transfection instead significantly reduced the cost and time of the experiments, decreased mouse numbers, and significantly improved experimental efficiency.
A second example is provided by the study on the role of Bmi1 in hepatocarcinogenesis, which we recently performed.77 Bmi1 is a polycomb group transcriptional repressor and regulates self-renewal and proliferation of many types of stem or progenitor cells.78 Bmi1 is found to be overexpressed in human HCC samples, and in vitro studies support the critical role of Bmi1 in liver cancer development.79 However, whether Bmi1 is required for tumor formation in vivo was not previously investigated. We showed that Bmi1 expression is up-regulated in liver tumors induced by activated forms of AKT and Ras.77 Also, we determined whether Bmi1 expression is required for AKT/Ras tumor formation.77 For this purpose, hydrodynamic transfection of AKT/Ras into Bmi1−/− mice and Bmi1+/+ control littermates was performed.77 We found that ablation of Bmi1 significantly delayed hepatocarcinogenesis induced by AKT and Ras co-expression.77 The latter study provides the evidence, for the first time to our knowledge, that Bmi1 expression is required for liver cancer development in vivo, thus representing a promising target for innovative treatments against human HCC.
ICC Mouse Models of Liver Cancer Using Hydrodynamic Transfection
ICC is another major type of liver cancer, but has limited treatment options owing to the poor understanding of the molecular pathogenesis of this deadly disease.14,80 Subcutaneous xenograft models of ICC have been generated for the development of novel therapeutic strategies.81–84 However, preclinical data derived from these xenograft systems correlate poorly with the clinical outcome.85–88 Also, only a few ICC murine models are available, and are difficult to be applied in preclinical studies.89,90 In a recent study, we applied hydrodynamic transfection to overexpress an activated form of Notch1 (NICD) or co-express activated AKT and Notch (AKT/NICD) into the mouse liver.38 We found that NICD1 alone is sufficient to promote ICC development, although after 20 to 25 weeks of latency.39 More important, cholangiocarcinogenesis was tremendously accelerated by co-expression of AKT and NICD, leading to ICC development 3 to 5 weeks after injection.38 As we have discussed previously, hydrodynamic transfection delivers genes into the pericentral region (zone 3 of the liver acinus). Indeed, it was found that oncogene-expressing cells are all hepatocytes located around the central vein. On the other hand, bile duct cells are located at the portal triad (zone 1 of the liver acinus). This raised an intriguing question: where do the ICC cells originate from? Powered by the lineage-tracing experiment in combination with morphological analysis using electron microscopy, Fan et al38 demonstrated that ICCs induced by AKT/NICD derived from mature hepatocytes. The hepatocyte origin of ICC in mice was subsequently confirmed in a chemically induced ICC murine model using the traditional genetic approach,91 as well as a study by the electroporating KRasV12 oncogene into p53-null hepatocytes.92 These novel findings suggest that mature hepatocytes can be the cellular origin of ICCs, and provides a previously overlooked mechanism of human ICC formation. Clearly, whether hepatocytes are the cell origin during human ICC pathogenesis needs to be further investigated. In accordance with these results, a recent meta-analysis of risk factors for human ICCs revealed that HBV or HCV infection, alcohol abuse, diabetes, and obesity, all well-characterized etiological factors for HCC, are also major risk factors for ICC,18 thus supporting the common pathogenesis of HCC and ICC. In addition, the studies by Fan et al38 and Evert et al39 suggested Notch signaling as the driver oncogenic pathway in ICC development. The conclusion was supported by several recent studies using in vitro approaches or traditional transgenic mouse models.93,94 Altogether, the study by Fan et al showed that ICC models can be generated via hydrodynamic transfection, and demonstrated that targeting the Notch signaling cascade might represent a novel and promising therapeutic target against human ICC.
Limitations of Hydrodynamic Transfection
Although we have discussed many advantages of hydrodynamic transfection in generating novel murine liver cancer models, some limitations also apply to this technology. One of the major limitations resides in the fact that hydrodynamic injection delivers genes predominantly into hepatocytes in the pericentral region (zone 3 of the liver acinus). Therefore, the technology cannot be applied to study tumors originating from hepatic stem cells or biliary epithelial cells. To achieve the goal of long-term gene expression in liver cells other than hepatocytes, it would be possible to combine Sleeping Beauty–mediated somatic integration with other delivery methods to stably target genes into hepatic stem cells and biliary epithelial cells. For example, in a recent preliminary report, it has been shown that intrabiliary injection of activated forms of AKT and Yap, together with Sleeping Beauty transposase, followed by bile duct ligation and IL-33 stimulation, resulted in ICC formation in mice (American Association for the Study of Liver Diseases 2013 annual meeting).
Another limitation is the difference between human liver tumors and those generated by hydrodynamic transfection in the mouse. Indeed, only a few (in most cases, one or two) liver tumor nodules developed in a patient. After hydrodynamic transfection, in contrast, at least 1% to 2% of hepatocytes are transfected, and all these cells can potentially produce tumors. This leads to numerous tumor nodules throughout the mouse liver, and in many cases, the tumor nodules are too many to be counted. Future studies are required to develop additional approaches, allowing the expression of target genes into few (ideally, one or two) hepatocytes in mice.
Most human HCCs develop in the context of a fibrotic or cirrhotic liver. Hydrodynamic transfection has been instead used to deliver genes into the normal liver. Clearly, induction of cirrhosis in mice before hydrodynamic transfection of oncogenes would be required to study how oncogenes promote tumor development in a cirrhotic microenvironment. The most common approach to induce inflammation and fibrosis in mouse liver is by hepatotoxins, such as treating the mice with carbon tetrachloride or thioacetamide. In addition, liver fibrosis can also be induced in transgenic mouse models. For example, overexpression of platelet-derived growth factor (PDGF) family members is able to induce fibrosis in mice.95 Indeed, we found that hydrodynamic transfection of PDGF-C is able to promote fibrosis in mice (C. Wang, unpublished data). Thus, it would be possible to express oncogenes via hydrodynamic transfection after inducing liver fibrosis by hepatotoxins or PDGFs. This would eventually allow us to study how the oncogenes may contribute to liver tumor development in the background of fibrotic liver. However, it remains unknown whether hydrodynamic transfection can achieve a high enough efficiency to deliver genes into the fibrotic liver and in the presence of the altered vasculature characteristic of this condition. Furthermore, many of these stimuli, especially hepatotoxins, are known to induce random mutations of DNA in hepatocytes, eventually leading to HCC or ICC formation in mice. This may complicate the understanding of the contribution of specific oncogenes or signaling pathways in liver tumor development. Altogether, whether hepatotoxin- or growth factor–induced fibrotic mouse models are suitable to combine with hydrodynamic transfection to study hepatocarcinogenesis requires further investigation.
Finally, the method is highly useful to study the contribution of oncogenes to tumor initiation, but not tumor progression. To overcome this limitation, hydrodynamic transfection requires to be coupled to other approaches. To investigate the role of specific oncogenes in tumor progression, indeed, hydrodynamic transfection should be performed in environmental (ethanol consumption, high-fat diet, and exposure to hepatocarcinogens) or genetic (injection in mice depleted of tumor-suppressor genes and co-injection with weak oncogenes) cancer-prone conditions.
Future Directions
Despite some limitations, hydrodynamic transfection holds great promise to both advance our knowledge on the cellular and molecular mechanisms underlying hepatocarcinogenesis and develop novel murine models for preclinical testing of innovative therapeutic approaches against this deadly disease. Combining hydrodynamic transfection with important etiological factors of HCC is worth exploring. For example, only one study described HCC development induced by HBx and shP53 using the Fah-null mouse model and hydrodynamic transfection. Because both HBV and HCV are critical etiological factors for human hepatocarcinogenesis, it would be important to combine the transfection of various cellular oncogenes into transgenic mice expressing HBV or HCV oncogenes, such as HBx96,97 or HCV Core.98 These models will be highly useful to understand the mechanisms by which viral oncoproteins cooperate with common genetic events observed in HCC to promote tumor development. In addition, alcohol intake and obesity have been implicated in HCC development.99,100 Furthermore, hydrodynamic transfection could be combined with alcohol feeding or high-fat diet feeding to determine whether these environmental/lifestyle factors can accelerate oncogene-induced hepatocarcinogenesis.
Perhaps the most promising aspect of hydrodynamic transfection is to screen for candidate oncogenes and tumor-suppressor genes. Indeed, recent genomic studies identified many genes whose expression is altered in HCCs, genes that are mutated in HCCs, and genes that are amplified and deleted in HCCs.101,102 The flexibility of hydrodynamic transfection renders it the ideal approach to determine the in vivo oncogenic potential of the candidate oncogenes or oncogenic mutations. By using the traditional transgenic approach, the generation of a transgenic mouse line for each of the candidate oncogenes or oncogenic mutants is instead necessary. Overexpression of the candidate oncogene might be insufficient to promote liver cancer development in vivo. Thus, these transgenic mice may need to be crossed with other transgenic or knockout mice to further evaluate their combined oncogenic potential. This approach is presumably unrealistic in the screening of many candidate oncogenes, most of which are likely to be passenger genes or mutants with limited contribution to liver tumor initiation and progression. By using hydrodynamic transfection, one can easily clone the candidate genes into a vector flanked by inverted repeats and injected into mice to determine whether overexpressing one candidate gene alone is sufficient to induce liver cancer formation. Furthermore, these genes can be co-injected with other common genetic events, such as the activated mutant of β-catenin or c-Met, into the liver to investigate whether these genetic events cooperate to promote hepatocarcinogenesis.
Although most of the current studies focus on overexpressing oncogenes into the mouse liver, efforts should be put into inhibiting the expression of genes to study their possible tumor-suppressor activity or investigating whether specific genes or pathways are required for oncogene-induced hepatocarcinogenesis. For this purpose, the direct silencing of candidate genes via hydrodynamic transfection of shRNA constructs might be applied. However, knocking down gene expression in vivo via shRNA could be challenging. Studies from our laboratory suggested that overexpression of the 19-mer stem-loop-stem shRNA, driven by U6 promoter, is highly toxic to hepatocytes, and all hepatocytes that received the shRNA underwent apoptosis (C. Wang, unpublished data). The molecular mechanisms underlying this event are not clear, but it is likely that the exogenous shRNA binds to the endogenous Dicer complex and inhibits the endogenous miRNA process, leading to cell toxicity. This observation was recently confirmed by Wuestefeld et al.103 Interestingly, the latter study suggests that miRNA-based shRNA (shRNAmir) is not toxic to the mouse liver, and the group successfully applied this technology to study liver regeneration.103 To date, the only successful shRNA-based silencing experiment using hydrodynamic transfection is shP53.23,24 The effectiveness of shRNAmir in gene silencing in liver tumor models requires further evaluation. If successful, this approach can provide a powerful method to study the downstream targets of various oncogenes or oncogenic signals. In addition, similar to that described by Wuestefeld et al,103 using the shRNA pools against candidate tumor-suppressor genes identified from human cancer genomic studies, in combination with hydrodynamic transfection, followed by deep sequencing, it would be possible to identify driver tumor suppressors directly in vivo.
Another area requiring further investigation is the study of liver tumor progression, metastasis, and tumor regression in the murine models. Thus far, virtually all studies focus on tumor initiation (ie, to determine which oncogene or what combinations of oncogenes, when overexpressed in mouse hepatocytes, can lead to liver tumor formation). None of these murine models resulted in tumor metastasis, and the experiments addressing molecular events from preneoplastic lesions to malignant tumors are still lacking. Notably, this is a challenge facing the entire liver cancer mouse modeling field, and it is not unique to murine models generated by hydrodynamic transfection. Although multiple genes are clearly implicated in promoting liver tumor metastasis, these genes have not been studied in mouse liver cancer models. Clearly, it is pivotal to further investigate the functional role(s) of these genes in vivo using either traditional genetic models or hydrodynamic transfection models. To study whether tumor cells are addicted to a specific oncogene or oncogenic pathway, it would be ideal if the oncogene can be turned off when tumors are already formed. In traditional transgenic mouse models, this can be achieved by using the doxycycline-inducible system. For example, one can breed LAP-tTA and TRE-c-Myc to generate LAP-tTA; TRE-c-Myc double-transgenic mice. When these mice are fed with regular chow (without doxycycline), c-Myc is expressed in mouse liver, leading to liver tumor formation in these mice. When the tumor-bearing mice are fed with doxycycline-containing chow, doxycycline turns off the expression of c-Myc oncogene, resulting in tumor regression.59 The result suggests that these tumor cells are highly dependent on the activity of the c-Myc oncogene, and targeting c-Myc is likely to be highly efficient, treating liver tumors with high levels of c-Myc expression, such as those tumors harboring c-Myc amplification. It would be of great interest to combine the doxycycline-inducible system with hydrodynamic transfection to allow the control of on-and-off status of the oncogene. These studies would provide critical information on whether the oncogene or oncogenic pathway would serve as an efficient therapeutic target.
In summary, hydrodynamic transfection is a flexible, efficient, and reliable method to generate novel mouse models for liver cancer research. The models developed using this technology have been proved to be highly helpful for the understanding of hepatocarcinogenesis and are receiving increasing attention by scientists from the liver cancer research field. It is likely that hydrodynamic transfection will be soon widely applied by many research groups and will contribute to a better understanding of the molecular pathogenesis of human liver cancer.
Footnotes
Supported by NIH grant R01CA136606 (X.C.).
Disclosures: None declared.
Contributor Information
Xin Chen, Email: xin.chen@ucsf.edu.
Diego F. Calvisi, Email: diego.calvisi@uni-greifswald.de.
References
- 1.El-Serag H. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nault J.C., Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Semin Liver Dis. 2011;31:173–187. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- 4.Buendia M.A. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185–200. doi: 10.1006/scbi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 5.Thorgeirsson S.S., Grisham J.W. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 6.Lee S., Lee H.J., Kim J.H., Lee H.S., Jang J.J., Kang G.H. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371–1378. doi: 10.1016/S0002-9440(10)63495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang B., Guo M., Herman J.G., Clark D.P. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003;163:1101–1107. doi: 10.1016/S0002-9440(10)63469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki I., Wada N., Nakano M., Saito A., Takasaki K., Doi M., Kameyama K., Otani Y., Kubochi K., Niioka M., Watanabe T., Maruyama K. Difference in gene expression for matrix metalloproteinase-1 between early and advanced hepatocellular carcinomas. Hepatology. 1997;25:580–584. doi: 10.1002/hep.510250315. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki I., Mizuta T., Zhao G., Yotsumoto H., Hara T., Kajihara S., Hisatomi A., Sakai T., Yamamoto K. Involvement of the Ets-1 gene in overexpression of matrilysin in human hepatocellular carcinoma. Cancer Res. 2000;60:6519–6525. [PubMed] [Google Scholar]
- 10.Chen Q., Seol D.W., Carr B., Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997;26:59–66. doi: 10.1002/hep.510260108. [DOI] [PubMed] [Google Scholar]
- 11.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T.F., Galle P.R., Seitz J.F., Borbath I., Haussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Kane R.C., Farrell A.T., Madabushi R., Booth B., Chattopadhyay S., Sridhara R., Justice R., Pazdur R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95–100. doi: 10.1634/theoncologist.2008-0185. [DOI] [PubMed] [Google Scholar]
- 13.Fava G., Marzioni M., Benedetti A., Glaser S., DeMorrow S., Francis H., Alpini G. Molecular pathology of biliary tract cancers. Cancer Lett. 2007;250:155–167. doi: 10.1016/j.canlet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Blechacz B., Gores G.J. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel T. Cholangiocarcinoma: controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyson G.L., El-Serag H.B. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charbel H., Al-Kawas F.H. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep. 2011;13:182–187. doi: 10.1007/s11894-011-0178-8. [DOI] [PubMed] [Google Scholar]
- 18.Palmer W.C., Patel T. Are common factors involved in the pathogenesis of primary liver cancers? a meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zender L., Villanueva A., Tovar V., Sia D., Chiang D.Y., Llovet J.M. Cancer gene discovery in hepatocellular carcinoma. J Hepatol. 2010;52:921–929. doi: 10.1016/j.jhep.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heindryckx F., Colle I., Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fausto N., Campbell J.S. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 22.Bakiri L., Wagner E.F. Mouse models for liver cancer. Mol Oncol. 2013;7:206–223. doi: 10.1016/j.molonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wangensteen K.J., Wilber A., Keng V.W., He Z., Matise I., Wangensteen L., Carson C.M., Chen Y., Steer C.J., McIvor R.S., Largaespada D.A., Wang X., Ekker S.C. A facile method for somatic, lifelong manipulation of multiple genes in the mouse liver. Hepatology. 2008;47:1714–1724. doi: 10.1002/hep.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keng V.W., Tschida B.R., Bell J.B., Largaespada D.A. Modeling hepatitis B virus X-induced hepatocellular carcinoma in mice with the Sleeping Beauty transposon system. Hepatology. 2011;53:781–790. doi: 10.1002/hep.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riordan J.D., Keng V.W., Tschida B.R., Scheetz T.E., Bell J.B., Podetz-Pedersen K.M., Moser C.D., Copeland N.G., Jenkins N.A., Roberts L.R., Largaespada D.A., Dupuy A.J. Identification of rtl1, a retrotransposon-derived imprinted gene, as a novel driver of hepatocarcinogenesis. PLoS Genet. 2013;9:e1003441. doi: 10.1371/journal.pgen.1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson C.M., Frandsen J.L., Kirchhof N., McIvor R.S., Largaespada D.A. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc Natl Acad Sci U S A. 2005;102:17059–17064. doi: 10.1073/pnas.0502974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tward A.D., Jones K.D., Yant S., Cheung S.T., Fan S.T., Chen X., Kay M.A., Wang R., Bishop J.M. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.A., Ho C., Roy R., Kosinski C., Patil M.A., Tward A.D., Fridlyand J., Chen X. Integration of genomic analysis and in vivo transfection to identify sprouty 2 as a candidate tumor suppressor in liver cancer. Hepatology. 2008;47:1200–1210. doi: 10.1002/hep.22169. [DOI] [PubMed] [Google Scholar]
- 29.Patil M.A., Lee S.A., Macias E., Lam E.T., Xu C., Jones K.D., Ho C., Rodriguez-Puebla M., Chen X. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69:253–261. doi: 10.1158/0008-5472.CAN-08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C.R., Lee S., Ho C., Bommi P., Huang S.A., Cheung S.T., Dimri G.P., Chen X. Bmi1 functions as an oncogene independent of Ink4A/Arf repression in hepatic carcinogenesis. Mol Cancer Res. 2009;7:1937–1945. doi: 10.1158/1541-7786.MCR-09-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.A., Ladu S., Evert M., Dombrowski F., De Murtas V., Chen X., Calvisi D.F. Synergistic role of Sprouty2 inactivation and c-Met up-regulation in mouse and human hepatocarcinogenesis. Hepatology. 2010;52:506–517. doi: 10.1002/hep.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvisi D.F., Wang C., Ho C., Ladu S., Lee S.A., Mattu S., Destefanis G., Delogu S., Zimmermann A., Ericsson J., Brozzetti S., Staniscia T., Chen X., Dombrowski F., Evert M. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho C., Wang C., Mattu S., Destefanis G., Ladu S., Delogu S., Armbruster J., Fan L., Lee S.A., Jiang L., Dombrowski F., Evert M., Chen X., Calvisi D.F. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55:833–845. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow E.K., Fan L.L., Chen X., Bishop J.M. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56:1331–1341. doi: 10.1002/hep.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauffer J.K., Scarzello A.J., Andersen J.B., De Kluyver R.L., Back T.C., Weiss J.M., Thorgeirsson S.S., Wiltrout R.H. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C., Delogu S., Ho C., Lee S.A., Gui B., Jiang L., Ladu S., Cigliano A., Dombrowski F., Evert M., Calvisi D.F., Chen X. Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis via activation of MAPK and PKM2 pathways. J Hepatol. 2012;57:577–583. doi: 10.1016/j.jhep.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju H.L., Ahn S.H., Kim do Y., Baek S., Chung S.I., Seong J., Han K.H., Ro S.W. Investigation of oncogenic cooperation in simple liver-specific transgenic mouse models using noninvasive in vivo imaging. PLoS One. 2013;8:e59869. doi: 10.1371/journal.pone.0059869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan B., Malato Y., Calvisi D.F., Naqvi S., Razumilava N., Ribback S., Gores G.J., Dombrowski F., Evert M., Chen X., Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evert M., Dombrowski F., Fan B., Ribback S., Chen X., Calvisi D.F. On the role of notch1 and adult hepatocytes in murine intrahepatic cholangiocarcinoma development. Hepatology. 2013;58:1857–1859. doi: 10.1002/hep.26411. [DOI] [PubMed] [Google Scholar]
- 40.Horie Y., Suzuki A., Kataoka E., Sasaki T., Hamada K., Sasaki J., Mizuno K., Hasegawa G., Kishimoto H., Iizuka M., Naito M., Enomoto K., Watanabe S., Mak T.W., Nakano T. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zender L., Spector M.S., Xue W., Flemming P., Cordon-Cardo C., Silke J., Fan S.T., Luk J.M., Wigler M., Hannon G.J., Mu D., Lucito R., Powers S., Lowe S.W. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarty D.M. Self-complementary AAV vectors:; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 43.Asokan A., Schaffer D.V., Samulski R.J. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Laan L.J., Wang Y., Tilanus H.W., Janssen H.L., Pan Q. AAV-mediated gene therapy for liver diseases: the prime candidate for clinical application? Expert Opin Biol Ther. 2011;11:315–327. doi: 10.1517/14712598.2011.548799. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park F., Ohashi K., Chiu W., Naldini L., Kay M.A. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet. 2000;24:49–52. doi: 10.1038/71673. [DOI] [PubMed] [Google Scholar]
- 47.Pichard V., Boni S., Baron W., Nguyen T.H., Ferry N. Priming of hepatocytes enhances in vivo liver transduction with lentiviral vectors in adult mice. Hum Gene Ther Methods. 2012;23:8–17. doi: 10.1089/hgtb.2011.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F., Song Y., Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 49.Zhang G., Gao X., Song Y.K., Vollmer R., Stolz D.B., Gasiorowski J.Z., Dean D.A., Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivics Z., Hackett P.B., Plasterk R.H., Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 51.Mikkelsen J.G., Yant S.R., Meuse L., Huang Z., Xu H., Kay M.A. Helper-independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol Ther. 2003;8:654–665. doi: 10.1016/s1525-0016(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 52.Yant S.R., Meuse L., Chiu W., Ivics Z., Izsvak Z., Kay M.A. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet. 2000;25:35–41. doi: 10.1038/75568. [DOI] [PubMed] [Google Scholar]
- 53.Grompe M., al-Dhalimy M., Finegold M., Ou C.N., Burlingame T., Kennaway N.G., Soriano P. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 54.Montini E., Held P.K., Noll M., Morcinek N., Al-Dhalimy M., Finegold M., Yant S.R., Kay M.A., Grompe M. In vivo correction of murine tyrosinemia type I by DNA-mediated transposition. Mol Ther. 2002;6:759–769. doi: 10.1006/mthe.2002.0812. [DOI] [PubMed] [Google Scholar]
- 55.Held P.K., Olivares E.C., Aguilar C.P., Finegold M., Calos M.P., Grompe M. In vivo correction of murine hereditary tyrosinemia type I by phiC31 integrase-mediated gene delivery. Mol Ther. 2005;11:399–408. doi: 10.1016/j.ymthe.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Abou-Elella A., Gramlich T., Fritsch C., Gansler T. C-myc amplification in hepatocellular carcinoma predicts unfavorable prognosis. Mod Pathol. 1996;9:95–98. [PubMed] [Google Scholar]
- 57.Cairo S., Armengol C., De Reynies A., Wei Y., Thomas E., Renard C.A. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Kistner A., Gossen M., Zimmermann F., Jerecic J., Ullmer C., Lubbert H., Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shachaf C.M., Kopelman A.M., Arvanitis C., Karlsson A., Beer S., Mandl S., Bachmann M.H., Borowsky A.D., Ruebner B., Cardiff R.D., Yang Q., Bishop J.M., Contag C.H., Felsher D.W. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 60.Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2010;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q., Lui V.W., Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167. doi: 10.2217/fon.11.95. [DOI] [PubMed] [Google Scholar]
- 62.Thompson M.D., Monga S.P. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 63.de La Coste A., Romagnolo B., Billuart P., Renard C.A., Buendia M.A., Soubrane O., Fabre M., Chelly J., Beldjord C., Kahn A., Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cadoret A., Ovejero C., Saadi-Kheddouci S., Souil E., Fabre M., Romagnolo B., Kahn A., Perret C. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 65.Harada N., Oshima H., Katoh M., Tamai Y., Oshima M., Taketo M.M. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 66.Kang T.W., Yevsa T., Woller N., Hoenicke L., Wuestefeld T., Dauch D., Hohmeyer A., Gereke M., Rudalska R., Potapova A., Iken M., Vucur M., Weiss S., Heikenwalder M., Khan S., Gil J., Bruder D., Manns M., Schirmacher P., Tacke F., Ott M., Luedde T., Longerich T., Kubicka S., Zender L. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 67.Fong C.W., Chua M.S., McKie A.B., Ling S.H., Mason V., Li R., Yusoff P., Lo T.L., Leung H.Y., So S.K., Guy G.R. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048–2058. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- 68.Calvisi D.F., Ladu S., Gorden A., Farina M., Lee J.S., Conner E.A., Schroeder I., Factor V.M., Thorgeirsson S.S. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713–2722. doi: 10.1172/JCI31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mason J.M., Morrison D.J., Basson M.A., Licht J.D. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Shim K., Minowada G., Coling D.E., Martin G.R. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Lo T.L., Yusoff P., Fong C.W., Guo K., McCaw B.J., Phillips W.A., Yang H., Wong E.S., Leong H.F., Zeng Q., Putti T.C., Guy G.R. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004;64:6127–6136. doi: 10.1158/0008-5472.CAN-04-1207. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Cigliano A., Delogu S., Armbruster J., Dombrowski F., Evert M., Chen X., Calvisi D.F. Functional crosstalk between AKT/mTOR and Ras/MAPK pathways in hepatocarcinogenesis: implications for the treatment of human liver cancer. Cell Cycle. 2013;12:1999–2010. doi: 10.4161/cc.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalerba P., Cho R.W., Clarke M.F. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 74.Chiba T., Kita K., Zheng Y.W., Yokosuka O., Saisho H., Iwama A., Nakauchi H., Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 75.Bunting K.D., Zhou S., Lu T., Sorrentino B.P. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000;96:902–909. [PubMed] [Google Scholar]
- 76.Scharenberg C.W., Harkey M.A., Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 77.Fan L., Xu C., Wang C., Tao J., Ho C., Jiang L., Gui B., Huang S., Evert M., Calvisi D.F., Chen X. Bmi1 is required for hepatic progenitor cell expansion and liver tumor development. PLoS One. 2012;7:e46472. doi: 10.1371/journal.pone.0046472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddique H.R., Saleem M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells. 2012;30:372–378. doi: 10.1002/stem.1035. [DOI] [PubMed] [Google Scholar]
- 79.Chiba T., Miyagi S., Saraya A., Aoki R., Seki A., Morita Y., Yonemitsu Y., Yokosuka O., Taniguchi H., Nakauchi H., Iwama A. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742–7749. doi: 10.1158/0008-5472.CAN-07-5882. [DOI] [PubMed] [Google Scholar]
- 80.Sia D., Tovar V., Moeini A., Llovet J.M. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fava G., Marucci L., Glaser S., Francis H., De Morrow S., Benedetti A., Alvaro D., Venter J., Meininger C., Patel T., Taffetani S., Marzioni M., Summers R., Reichenbach R., Alpini G. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–11446. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 82.Braconi C., Swenson E., Kogure T., Huang N., Patel T. Targeting the IL-6 dependent phenotype can identify novel therapies for cholangiocarcinoma. PLoS One. 2010;5:e15195. doi: 10.1371/journal.pone.0015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshikawa D., Ojima H., Kokubu A., Ochiya T., Kasai S., Hirohashi S., Shibata T. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br J Cancer. 2009;100:1257–1266. doi: 10.1038/sj.bjc.6604988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng F., Henson R., Lang M., Wehbe H., Maheshwari S., Mendell J.T., Jiang J., Schmittgen T.D., Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 85.Johnson J.I., Decker S., Zaharevitz D., Rubinstein L.V., Venditti J.M., Schepartz S., Kalyandrug S., Christian M., Arbuck S., Hollingshead M., Sausville E.A. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voskoglou-Nomikos T., Pater J.L., Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 87.Sausville E.A., Burger A.M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. discussion 3354. [DOI] [PubMed] [Google Scholar]
- 88.Bibby M.C. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004;40:852–857. doi: 10.1016/j.ejca.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 89.Farazi P.A., Zeisberg M., Glickman J., Zhang Y., Kalluri R., DePinho R.A. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–6627. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 90.Xu X., Kobayashi S., Qiao W., Li C., Xiao C., Radaeva S., Stiles B., Wang R.H., Ohara N., Yoshino T., LeRoith D., Torbenson M.S., Gores G.J., Wu H., Gao B., Deng C.X. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sekiya S., Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gurlevik E., Fleischmann-Mundt B., Armbrecht N., Longerich T., Woller N., Kloos A., Hoffmann D., Schambach A., Wirth T.C., Manns M.P., Zender L., Kubicka S., Kuhnel F. Adjuvant gemcitabine therapy improves survival in a locally induced, R0-resectable model of metastatic intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1031–1041. doi: 10.1002/hep.26468. [DOI] [PubMed] [Google Scholar]
- 93.Jeliazkova P., Jors S., Lee M., Zimber-Strobl U., Ferrer J., Schmid R.M., Siveke J.T., Geisler F. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 94.Zender S., Nickeleit I., Wuestefeld T., Sorensen I., Dauch D., Bozko P., El-Khatib M., Geffers R., Bektas H., Manns M.P., Gossler A., Wilkens L., Plentz R., Zender L., Malek N.P. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784–795. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 95.Campbell J.S., Hughes S.D., Gilbertson D.G., Palmer T.E., Holdren M.S., Haran A.C., Odell M.M., Bauer R.L., Ren H.P., Haugen H.S., Yeh M.M., Fausto N. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim C.M., Koike K., Saito I., Miyamura T., Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 97.Koike K., Moriya K., Iino S., Yotsuyanagi H., Endo Y., Miyamura T., Kurokawa K. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994;19:810–819. [PubMed] [Google Scholar]
- 98.Moriya K., Fujie H., Shintani Y., Yotsuyanagi H., Tsutsumi T., Ishibashi K., Matsuura Y., Kimura S., Miyamura T., Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 99.McKillop I.H., Schrum L.W. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 100.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 101.Woo H.G., Park E.S., Thorgeirsson S.S., Kim Y.J. Exploring genomic profiles of hepatocellular carcinoma. Mol Carcinog. 2011;50:235–243. doi: 10.1002/mc.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Breuhahn K., Gores G., Schirmacher P. Strategies for hepatocellular carcinoma therapy and diagnostics: lessons learned from high throughput and profiling approaches. Hepatology. 2012;53:2112–2121. doi: 10.1002/hep.24313. [DOI] [PubMed] [Google Scholar]
- 103.Wuestefeld T., Pesic M., Rudalska R., Dauch D., Longerich T., Kang T.W., Yevsa T., Heinzmann F., Hoenicke L., Hohmeyer A., Potapova A., Rittelmeier I., Jarek M., Geffers R., Scharfe M., Klawonn F., Schirmacher P., Malek N.P., Ott M., Nordheim A., Vogel A., Manns M.P., Zender L. A direct in vivo RNAi screen identifies MKK4 as a key regulator of liver regeneration. Cell. 2013;153:389–401. doi: 10.1016/j.cell.2013.03.026. [DOI] [PubMed] [Google Scholar]