Abstract
Yes-associated protein (YAP) is a transcriptional co-activator of hippo signaling pathway, which plays an important role in organ size control and tumorigenesis. Here we report that YAP and its downstream transcriptional targets CCN1 and CCN2 are markedly elevated in keratinocytes in human skin basal cell carcinoma tumor islands. In human keratinocytes, knockdown of YAP significantly reduced expression of CCN1 and CCN2, and repressed proliferation and survival. This inhibition of proliferation and survival was rescued by restoration of CCN1 expression, but not by CCN2 expression. In basal cell carcinoma stroma, CCN2-regulated genes type I collagen, fibronectin, and α-smooth muscle actin were highly expressed. Furthermore, atomic force microscopy revealed increased tissue stiffness in basal cell carcinoma stroma compared to normal dermis. These data provide evidence that up-regulation of YAP in basal cell carcinoma impacts both aberrant keratinocyte proliferation, via CCN1, and tumor stroma cell activation and stroma remodeling, via CCN2. Targeting YAP and/or CCN1 and CCN2 may provide clinical benefit in basal cell carcinoma.
Yes-associated protein (YAP) is a major downstream effector of the hippo signaling pathway, which was originally recognized as a vital regulator of organ size in animals.1,2 YAP activity is primarily regulated by subcellular localization, which is responsive to cell-cell interactions through the hippo pathway3 or mechanical forces through unknown mechanisms.4 Phosphorylation of YAP sequesters it in the cytoplasm and consequently inhibits YAP nuclear translocation and function as a transcriptional co-activator. Recently, YAP was identified as an oncogene in various human cancers.3,5,6 Enhanced expression of YAP has been reported in solid tumors, such as colon adenocarcinoma, lung adenocarcinoma, ovarian serous cystadenocarcinoma, and medulloblastoma.6,7 However, the expression of YAP in human skin cancer has not been studied.
Cysteine-rich protein 61, connective tissue growth factor, nephroblastoma overexpressed (CCN) family members cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) have emerged as direct transcriptional targets of YAP.7–9 Members of the CCN family of proteins exhibit diverse cellular functions, such as regulation of cell proliferation, chemotaxis, apoptosis, adhesion, motility, ion transport, and extracellular matrix (ECM) production.10–12 CCN proteins are secreted and associate with the ECM in connective tissue.13,14 YAP up-regulation of CCN1 has been implicated in proliferation and survival of mouse keratinocytes.8 However, the role of CCN1 and CCN2 in the oncogenic actions of YAP is poorly understood.
Recently, increased nuclear localization of YAP has been shown to be linked to cutaneous squamous cell carcinoma-like tumors in transgenic mice.15 Nuclear localization of YAP is regulated in part by α-catenin, which acts as a tumor suppressor by retaining YAP in the cytoplasm, thereby limiting proliferation of epidermal stem/progenitor cells.16 Cutaneous basal cell carcinoma (BCC) is the most common form of human cancer, with incidence estimated to be four times greater than cutaneous SCC. Little is known regarding the functional significance of YAP and its target genes CCN1 and CCN2 in BCC. We demonstrate that YAP and both CCN1 and CCN2 are markedly elevated in BCC tumor cells. CCN1 and CCN2 appear to serve a distinct function as YAP effectors; CCN1 regulates keratinocytes growth and survival, whereas CCN2 likely mediates alterations in stromal ECM microenvironment.
Materials and Methods
Human Skin Samples
Normal human skin samples were obtained by punch biopsy (4 mm) from sun-protected hips and buttocks of healthy adults (age, 40 to 50 years), as previously described.17,18 Completely de-identified BCC samples were obtained from the University of Michigan Cutaneous Oncology Unit, Ann Arbor, MI. Research involving human subjects was approved by the University of Michigan Institutional Review Board, and participating subjects provided written informed consent.
Laser Capture Microdissection
For laser capture microdissection (LCM), 15 μm skin samples embedded in optimal cutting temperature were sectioned and stained with H&E, and normal epidermis and cancer cell islands were captured by LCM (Leica ASLMD system; Leica Microsystems, Wetzlar, Germany), as previously described.19 Total RNA was prepared from LCM-captured tissues using a commercial kit (RNeasy Micro kit; Qiagen, Chatsworth, CA). The quality and quantity of total RNA were determined by using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Immunohistology
Immunohistology was performed as previously described.20 Briefly, skin samples embedded in optimum cutting temperature were sectioned (7 μm), fixed in 2% paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS, blocked with rabbit serum (5% in PBS), and incubated for 1 hour at room temperature with YAP, CCN1, or CCN2 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with secondary antibody for 1 hour at room temperature. After staining, the slides were examined using a digital imaging microscope (Zeiss, Oberkochen, Germany). Specificity of staining was determined by substituting isotype-control immunoglobulin (mouse IgG2a) for the primary antibodies. No detectable staining was observed with isotype controls (Supplemental Figure S1).
Cell Culture
Human primary keratinocytes were obtained from Cascade Biologics Inc. (Portland, OR). Adult human primary dermal fibroblasts were prepared from punch biopsies of normal adult buttock skin (age, 21 to 55 years), as previously described.21 Keratinocytes were maintained in EpiLife medium (Life Technologies, Grand Island, NY) supplemented with keratinocyte growth supplement. Dermal fibroblasts were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). EpiLife medium and Dulbecco’s modified Eagle’s medium were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were cultured in a humidified incubator with 5% CO2 at 37°C. Cells were plated at 70% to 80% confluence, and used 1 day after plating. Human keratinocytes were transfected with indicated siRNAs and CCN1 or CCN2 expression vectors. Six days after transfection, cultures were imaged (phase-contract microscopy), and cell numbers determined by hemocytometer.
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was extracted using TRizol reagent (Invitrogen) or RNeasy micro kit (Qiagen, Gaithersburg, MD). The cDNA for PCR templates was prepared by reverse transcription of 100 ng total RNA using the Taqman Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Real-time PCR was performed on a 7300 Sequence Detector (Applied Biosystems) using Taqman Universal PCR Master Mix Reagents (Applied Biosystems). YAP real-time PCR primers were designed and purchased from Sigma (St. Louis, MO): sense primer 5′-AGCAGGATGGTGGGACTCAAAAT-3′; antisense primer 5′-AGGTGCCACTGTTAAGGAAAGGAT-3′. CCN1, CCN2, and 36B4 primer sequences have been previously described.13 Target gene mRNA expression levels were normalized to the housekeeping gene 36B4 as an internal control for quantification.
Adeno-X Expression Vector Construction and Infection
CCN2 cDNA was amplified by PCR and gel purified PCR product was subcloned into a TA Cloning vector (pCR2.1; Invitrogen). Purified pCR2.1 DNA was digested with restriction enzymes, and the excised inserts were gel purified and ligated into pShuttle. CCN2 pShuttle plasmids were used to generate the Adeno-X expression vector using the Adeno-X Expression System (Clontech Laboratories, Inc., Temecula, CA) according to the manufacturer's protocol. All constructs were subjected to restriction enzyme digestion analysis and nucleotide sequencing to verify correct sequence and orientation. Adenoviruses were amplified by infection of HEK-293FT cells and purified by sucrose gradient centrifugation. Cells were infected with control adenovirus or CCN2 adenovirus at a multiplicity of infection of 100 plaque-forming units per cell.
Transfection
CCN1 cDNA was amplified by PCR and gel purified PCR product was cloned into expression vector (pCR3.1; Invitrogen).22 YAP siRNA (5′-GACAUCUUCUGGUCAGAGA-3′), was designed and purchased from Sigma. CCN1 and CCN2 siRNAs have been previously described.13,23 CCN1 expression vector and siRNAs were transiently transfected into human skin keratinocytes by electroporation (Amaxa Nucleofector, Koeln, Germany), and analysis was performed after two days transfection.
Western Blot Analysis
Proteins were prepared from skin tissue or cells and equal amounts of protein (approximately 50 μg per lane) were analyzed by resolving on 10% SDS-PAGE. The SDS gels were transferred to the polyvinylidene difluoride membrane, and the membranes were blocked with 0.1% Tween 20 in PBS (PBST) containing 5% nonfat milk for 1 hour at room temperature. Primary antibodies [YAP, SC-101199; CCN1, SC-13100; CCN2, SC-14939; COL-1, SC-8782; FN, SC-18827; αSMA, SC-53015 (Santa Cruz Biotechnology) and β-actin, A5441 (Sigma)] were incubated with the polyvinylidene difluoride membrane for 1 hour at room temperature, after which membranes were washed three times with PBST solution and incubated with appropriate secondary antibodies for 1 hour at room temperature. After washing the membranes three times with PBST, they were developed with Vistra ECF Western blotting system, (GE Healthcare, Piscataway, NJ) following the manufacturer's protocol. The membranes were scanned with a STORM Molecular Imager (Molecular Dynamics, Sunnyvale, CA), the fluorescence intensities of each band were quantified by ImageQuant (GE Healthcare), and were normalized using β-actin as a marker for equal protein loading.
AFM Imaging
The mechanical properties of normal dermis and BCC stroma were measured by atomic force microscopy (AFM) using previously established techniques in our laboratory with minor modifications.24 Briefly, 20 μm cryosections of BCC tissues were mounted on microscope glass slides (25 × 75 × 1.0 mm; Fisher Scientific, Pittsburgh, PA), and allowed to air dry for at least 48 hours before AFM analysis. Tissue stiffness was measured by Dimension Icon AFM system (Bruker-AXS, Santa Barbara, CA) using PeakForce Quantitative NanoMechanics mode using a silicon AFM probe (NSC15/AIBS, MikroMasch, San Jose, CA). AFM was conducted at the Electron Microbeam Analysis Laboratory, University of Michigan College of Engineering, Ann Arbor, MI, and was analyzed using Nanoscope Analysis software version 120R1sr3 (Bruker-AXS).
Statistical Analysis
Data are expressed as means ± SEM. Student's t-test was used to evaluate the statistical differences among the groups. All P values are two-tailed, and values <0.05 were considered statistically significant.
Results
YAP Protein Expression and Localization in Normal Human Skin and BCC
First we investigated the expression of YAP protein in normal adult human skin. YAP protein expression was detectable in the basal layer of the interfollicular epidermis (Figure 1A) and outer root sheath of the hair follicle (Figure 1B). The majority of YAP protein was localized within the cytoplasm of epidermal keratinocytes (Figure 1A). YAP was primarily localized in the nucleus in keratinocytes in the outer root sheath (Figure 1B). YAP staining in the dermis was not readily apparent (Figure 1, A and B). Interestingly, YAP was strongly expressed in tumor cell islands in BCC (Figure 1C). YAP was present in both nuclei and cytoplasm of cells throughout BCC islands, suggesting aberrant regulation of YAP expression and activity. YAP expression in epidermis adjacent to BCC was limited to the basal layer, as observed in normal adult skin (Figure 1D and Supplemental Figure S2A). Based on Western blot analysis, YAP protein levels were approximately 10-fold greater in BCC tumor islands compared to epidermis (Supplemental Figure S2B). BCC specimens from 12 patients were examined and all displayed substantial tumor island YAP staining. BCC stroma was mostly negative for YAP staining.
Figure 1.

YAP protein expression and localization in normal human skin and BCC. Skin sections were immunostained with mouse anti-YAP. A: YAP protein immunostaining in normal interfollicular skin. B: Normal skin hair. C: Basal cell carcinoma (BCC). D: Epidermis adjacent to BCC. Representative image from 6 (A), 3 (B), 12 (C), and 5 (D) individuals. Isotype control antibody (mouse IgG2a) immunohistochemical staining in normal skin, BCC, and epidermis adjacent to BCC were negative (Supplemental Figure S3). Scale bars: 100 μm (A–D).
YAP Target Genes CCN1 and CCN2 Co-Localize with YAP and Are Up-Regulated in BCC
The products of YAP-regulated genes CCN1 and CCN2 co-localized with YAP protein in tumor cells in BCC islands (Figure 2A). To quantify mRNA levels, BCC islands and normal epidermis were isolated by LCM and analyzed by real-time RT-PCR (Supplemental Figure S3). YAP mRNA expression was elevated approximately 2.7-fold in LCM-captured BCC tumor islands (Figure 2B). Additionally, CCN1 mRNA (Figure 2C) and CCN2 mRNA (Figure 2D) were elevated 28-fold and 56-fold, respectively, in LCM-captured BCC, compared to normal epidermis.
Figure 2.
YAP target genes CCN1 and CCN2 co-localize with YAP and are up-regulated in BCC. A: Co-immunofluorescence staining of YAP and CCN1 (top row), and YAP and CCN2 (bottom row), in BCC tumor islands. DAPI staining reveals cell nuclei. Images are representative of five individuals. BCC tumor islands and adjacent normal epidermis were obtained by laser capture microdissection, and total RNA was extracted from captured tissue (Supplemental Figure S2) (B–D). YAP (B), CCN1 (C), and CCN2 (D) mRNA levels were quantified by real-time RT-PCR. Target gene mRNA levels were normalized to internal control housekeeping gene 36B4 for quantification. Data are means ± SEM. N = 6. ∗P < 0.05. Scale bars: 100 μm (A).
YAP Regulates Keratinocyte Proliferation through CCN1 But Not CCN2
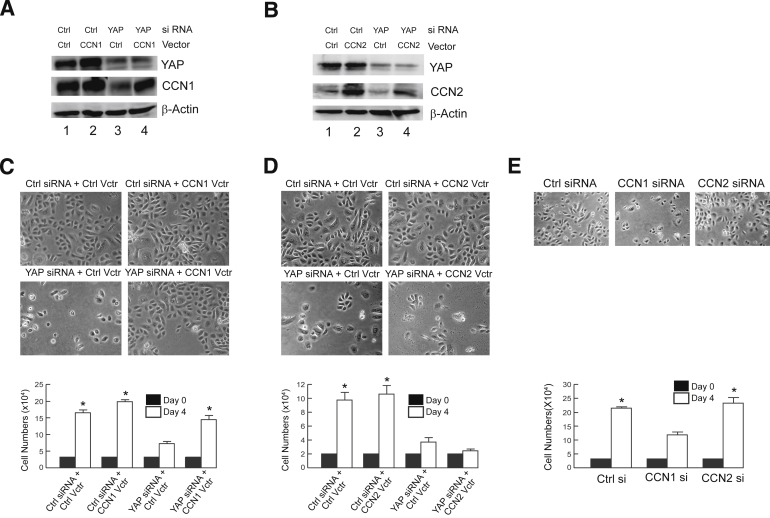
Although both CCN1 and CCN2 are generally recognized as prominent YAP target genes, their roles in mediating YAP function are poorly understood. To gain insight into the functionality of elevated YAP and it target genes CCN1 and CCN2 in BCC, we conducted loss- and gain-of-function studies in primary human epidermal keratinocytes. Knockdown of YAP expression by siRNA significantly reduced expression of CCN1 (Figure 3A) and CCN2 (Figure 3B). YAP knockdown resulted in near complete loss of keratinocyte proliferation, determined by cell number 4 days after knockdown (Figure 3, C and D). Furthermore, at 5 days after YAP knockdown, the cell number decreased, indicating that YAP depletion was detrimental to keratinocyte survival (Supplemental Figure S4). To investigate the role of CCN1 and CCN2 in YAP-mediated keratinocyte growth control, CCN1 (Figure 3A) or CCN2 (Figure 3B) was restored in YAP knockdown cells. Restoration of CCN1 expression (Figure 3C), but not the restoration of CCN2 expression (Figure 3D), markedly rescued keratinocyte proliferation and survival in YAP knockdown cells. These differential effects of YAP effectors on keratinocyte proliferation were confirmed by knockdown studies. Knockdown of CCN1, but not CCN2, significantly reduced keratinocyte proliferation (Figure 3E). These data indicate that CCN1, but not CCN2, plays a key role in mediating YAP regulation of keratinocyte proliferation and survival.
Figure 3.
YAP regulates keratinocyte proliferation through CCN1, but not CCN2. Human keratinocytes were transfected with indicated expression vectors. YAP and CCN1 (A), and YAP and CCN2 (B), protein levels were determined by Western blot analysis (N = 3). C–E: Keratinocyte proliferation was determined 4 days after addition of indicated siRNAs and expression vectors. Results are means ± SEM. ∗P < 0.05. Original magnification, ×100 (C and D).
YAP Target Gene CCN2 Promotes Stroma Fibroblast Activation and ECM Remodeling
CCN2 is known to regulate ECM production in connective tissue.14,25,26 CCN2 is markedly elevated in numerous fibrotic disorders, where it is believed to stimulate excessive deposition of collagen and other ECM proteins, the hallmark of fibrosis.26–28 It is likely, therefore, that elevated production of CCN2 by BCC tumor cells impacts the function and composition of the tumor stromal microenvironment. In support of this possibility, we observed that BCC stromal cells adjacent to BCC tumor cell islands expressed high levels of CCN2 target gene products, including the major structural proteins type I collagen and fibronectin, and the myofibroblast marker, α-smooth muscle actin, compared to normal human skin dermis (Figure 4A). These alterations were associated with increased stiffness of the tumor stroma ECM, as measured by atomic force microscopy (Figure 4B). Furthermore, expression of CCN2 in adult human dermal fibroblasts resulted in increased levels of type I collagen, fibronectin, and α-smooth muscle proteins (Figure 4C). These data suggest that secretion of elevated CCN2, by BCC tumor cells, remodels the stromal ECM microenvironment through paracrine signaling.
Figure 4.
A: YAP target gene CCN2 promotes stroma fibroblast activation and ECM remodeling. Immunostaining of indicated proteins in normal human dermis and BCC stroma. Images are representative of six individuals. Arrows indicate positive staining (red) in stroma surrounding BCC tumor islands. B: Stiffness of normal dermis and BCC stroma was determined by atomic force microscopy. Results are means ± SEM (N = 8). C: Human skin dermal fibroblasts were transfected with control (CTRL) or CCN2 expression vector, and indicated protein levels were determined by Western blot analysis (N = 3). ∗P < 0.05. Scale bars: 50 μm (A).
Discussion
In normal adult human skin, YAP protein expression is detectable in the basal layer of the interfollicular epidermis and outer root sheath of the hair follicle. This restricted localization of YAP in keratinocytes that possess proliferative potential is consistent with YAP function in promoting epidermal proliferation and maintenance of stem cell populations.8,16 YAP has been identified as an oncogene and its overexpression has been linked to tumorigenesis in various human cancers.3,5,6 Our findings indicate that YAP and its downstream transcriptional targets CCN1 and CCN2 are markedly elevated in keratinocytes in human skin BCC tumor islands.
Both CCN1 and CCN2 are well recognized as prominent YAP target genes, however, their roles in mediating YAP function are poorly understood. Our data indicate that up-regulation of YAP in BCC impacts both aberrant keratinocyte proliferation, via CCN1, and tumor stroma cell activation and stroma remodeling, via CCN2.
Emerging data demonstrate that tumor stroma is an active and critical participant in tumor formation and maintenance.9,29 For example, elevated production of ECM components, such as collagen and fibronectin, results in increased stromal ECM stiffness, which is directly implicated in promoting tumor cell proliferation and cancer progression.30,31 Recent studies demonstrate that YAP activity is positively regulated by increased ECM stiffness and cytoskeletal tension.4,32,33 Interestingly, our work suggests that YAP promotes ECM stiffness in BCC through up-regulation of its target gene CCN2. Therefore, up-regulation of CCN2 may participate in a positive feedback loop in which YAP-induced CCN2 promotes ECM stiffness, which further activated YAP. These data provide important new insight into the role of YAP in tumorigenesis by regulating tumor microenvironment.
Taken together, our data demonstrate that in normal skin, YAP is detectable in keratinocytes that reside in proliferative regions of the epidermis and hair follicle, supporting a role of YAP in promoting epidermal cell growth. In BCC, YAP and its downstream targets CCN1 and CCN2 are markedly elevated in tumor islands. We propose that YAP contributes to BCC formation through up-regulation of CCN1 and CCN2, which promote keratinocyte growth and survival, and stromal cell activation and ECM remodeling, respectively. Targeting YAP and/or CCN1 and CCN2 may provide clinical benefit in BCC and other cancers in which YAP is elevated.
Acknowledgment
We thank Diane Fiolek for administrative assistance.
Footnotes
Supported by NIH grants RO1 ES014697 (T.Q.), 3R01ES014697-03S1 (T.Q.), and RO1AG031452 ( G.J.F.).
Disclosures: None declared.
Supplemental Data
YAP isotype control antibody (mouse IgG2a) IHC staining in normal human skin (A), hair follicle (B), basal cell carcinoma (BCC; C), and epidermis adjacent to BCC (D). Results are representative of five individuals. Scale bars: 100 μm.
A: YAP protein immunostaining in BCC and adjacent epidermis. The black line indicates the boundary between the epidermis (top) and dermis (bottom). Representative images are from five individuals. B: YAP protein Western blot analysis in BCC and epidermis. Results are means ± SEM. N = 3. ∗P < 0.05. Scale bar = 100 μm (A).
Laser capture microdissection (LCM) coupled real-time RT-PCR. Optimum cutting temperature–embedded skin cryosections (15 μm) were H&E stained, and BCC tumor islands were captured using LCM (Leica ASLMD System, Leica Microsystems). Numbers indicate the locations where BCC islands were captured by LCM. Total mRNA was extracted from captured cells, and mRNA levels were quantified by real-time RT-PCR.
YAP regulates keratinocyte proliferation and survival. Human keratinocytes were transfected with control siRNA or YAP siRNA. Keratinocyte numbers were determined at indicated days after transfection. Results are means ± SEM. N = 3. ∗P < 0.05.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.12.017.
References
- 1.Zhao B., Tumaneng K., Guan K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Justice R.W., Zilian O., Woods D.F., Noll M., Bryant P.J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B., Li L., Lei Q., Guan K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 5.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinhardt A.A., Gayyed M.F., Klein A.P., Dong J., Maitra A., Pan D., Montgomery E.A., Anders R.A. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urtasun R., Latasa M.U., Demartis M.I., Balzani S., Goni S., Garcia-Irigoyen O., Elizalde M., Azcona M., Pascale R.M., Feo F., Bioulac-Sage P., Balabaud C., Muntane J., Prieto J., Berasain C., Avila M.A. Connective tissue growth factor autocrine in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology. 2011;54:2149–2158. doi: 10.1002/hep.24587. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Pasolli H.A., Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai D., Ho K.C., Hao Y., Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 10.Brigstock D.R. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 11.Perbal B., Brigstock D.R., Lau L.F. Report on the second international workshop on the CCN family of genes. Mol Pathol. 2003;56:80–85. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C.C., Lau L.F. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun J.I., Lau L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 15.Silvis M.R., Kreger B.T., Lien W.H., Klezovitch O., Rudakova G.M., Camargo F.D., Lantz D.M., Seykora J.T., Vasioukhin V. Alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J.R., Zhou D., Kreger B.T., Vasioukhin V., Avruch J., Brummelkamp T.R., Camargo F.D. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan T., He T., Kang S., Voorhees J.J., Fisher G.J. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- 18.Quan T., He T., Kang S., Voorhees J.J., Fisher G.J. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- 19.Qin Z., Fisher G.J., Quan T. CCN1 Domain-specific Stimulation of Matrix Metalloproteinase-1 Expression Through alphaVbeta3 Integrin in Human Skin Fibroblasts. J Biol Chem. 2013;288:12386–12394. doi: 10.1074/jbc.M112.424358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher G.J., Wang Z.Q., Datta S.C., Varani J., Kang S., Voorhees J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 21.Fisher G.J., Esmann J., Griffiths C.E., Talwar H.S., Duell E.A., Hammerberg C., Elder J.T., Finkel L.J., Karabin G.D., Nickoloff B.J., Cooper K.D., Voorhees J.J. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- 22.Quan T., He T., Shao Y., Lin L., Kang S., Voorhees J.J., Fisher G.J. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan T., Qin Z., Xu Y., He T., Kang S., Voorhees J.J., Fisher G.J. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J Invest Dermatol. 2010;130:1697–1706. doi: 10.1038/jid.2010.29. [DOI] [PubMed] [Google Scholar]
- 24.Quan T., Wang F., Shao Y., Rittie L., Xia W., Orringer J.S., Voorhees J.J., Fisher G.J. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2012;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan T., Shao Y., He T., Voorhees J.J., Fisher G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130:415–424. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikawa Y., Ng P.S., Endo K., Kondo M., Chujo S., Ishida W., Shirasaki F., Fujimoto M., Takehara K. Neutralizing monoclonal antibody to human connective tissue growth factor ameliorates transforming growth factor-beta-induced mouse fibrosis. J Cell Physiol. 2008;216:680–687. doi: 10.1002/jcp.21449. [DOI] [PubMed] [Google Scholar]
- 27.Grotendorst G.R. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 28.Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 29.Boudreau N., Bissell M.J. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol. 1998;10:640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assoian R.K., Klein E.A. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D., Hammer D.A., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhao B., Li L., Wang L., Wang C.Y., Yu J., Guan K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
YAP isotype control antibody (mouse IgG2a) IHC staining in normal human skin (A), hair follicle (B), basal cell carcinoma (BCC; C), and epidermis adjacent to BCC (D). Results are representative of five individuals. Scale bars: 100 μm.
A: YAP protein immunostaining in BCC and adjacent epidermis. The black line indicates the boundary between the epidermis (top) and dermis (bottom). Representative images are from five individuals. B: YAP protein Western blot analysis in BCC and epidermis. Results are means ± SEM. N = 3. ∗P < 0.05. Scale bar = 100 μm (A).
Laser capture microdissection (LCM) coupled real-time RT-PCR. Optimum cutting temperature–embedded skin cryosections (15 μm) were H&E stained, and BCC tumor islands were captured using LCM (Leica ASLMD System, Leica Microsystems). Numbers indicate the locations where BCC islands were captured by LCM. Total mRNA was extracted from captured cells, and mRNA levels were quantified by real-time RT-PCR.
YAP regulates keratinocyte proliferation and survival. Human keratinocytes were transfected with control siRNA or YAP siRNA. Keratinocyte numbers were determined at indicated days after transfection. Results are means ± SEM. N = 3. ∗P < 0.05.