Abstract
Impaired glucose tolerance and type 2 diabetes were induced in guinea pigs to model the emerging comorbidity of Mycobacterium tuberculosis infection in diabetic patients. Type 2 diabetes mellitus was induced by low-dose streptozotocin in guinea pigs rendered glucose intolerant by first feeding a high-fat, high-carbohydrate diet before M. tuberculosis exposure. M. tuberculosis infection of diabetic guinea pigs resulted in severe and rapidly progressive tuberculosis (TB) with a shortened survival interval, more severe pulmonary and extrapulmonary pathology, and a higher bacterial burden compared with glucose-intolerant and nondiabetic controls. Compared with nondiabetics, diabetic guinea pigs with TB had an exacerbated proinflammatory response with more severe granulocytic inflammation and higher gene expression for the cytokines/chemokines interferon-γ, IL-17A, IL-8, and IL-10 in the lung and for interferon-γ, tumor necrosis factor-α, IL-8, and monocyte chemoattractant protein-1 in the spleen. TB disease progression in guinea pigs with impaired glucose tolerance was similar to that of nondiabetic controls in the early stages of infection but was more severe by day 90. The guinea pig model of type 2 diabetes–TB comorbidity mimics important features of the naturally occurring disease in humans. This model will be beneficial in understanding the complex pathogenesis of TB in diabetic patients and to test new strategies to improve TB and diabetes control when the two diseases occur together.
Host susceptibility to Mycobacterium tuberculosis is influenced by a variety of chronic communicable and noncommunicable diseases that increase the risk of infection and the development of active tuberculosis (TB) disease. Moreover, M. tuberculosis–infected patients with concurrent diseases often have more severe TB, which further complicates treatment responses to conventional antimicrobial drugs. The risk factors most frequently linked to M. tuberculosis susceptibility are HIV infection, malnutrition, tobacco use, air pollution, alcoholism, extremes in age, chronic kidney disease, and diabetes. The highest relative risk for TB is associated with profound immunosuppression associated with HIV infection. However, recent epidemiologic evidence suggests that in the face of a growing diabetes epidemic, the population-attributable risk of diabetes may be equivalent to or exceed that of HIV/AIDS. In countries with the highest TB and diabetes incidences, type 2 diabetes mellitus in particular may account for up to 20% of active TB cases, whereas <5% may be attributable to HIV.1,2
Type 2 diabetes accounts for approximately 95% of diabetes cases and is associated with obesity, poor diet, and a sedentary lifestyle, all of which are often linked to urbanization. An estimated 371 million people were diagnosed as having type 2 diabetes in 2012, with most residing in low- and middle-income countries. Moreover, an additional 280 million people are prediabetic, many of whom have undiagnosed insulin resistance with nondiabetic hyperglycemia and impaired glucose tolerance (IGT).3 Furthermore, the global incidence of type 2 diabetes is projected to rise to approximately 552 million by 2030.4 Evidence in human studies suggests that glucose control, more than any other feature of altered metabolism in diabetic patients, influences the susceptibility to M. tuberculosis infection, highlighting the importance of uncontrolled hyperglycemia in TB risk.5 The increased difficulty in controlling blood glucose levels in diabetic patients with TB, combined with poor responses to antimicrobial drug treatment, has the potential to further hamper current TB control efforts worldwide.6 An animal model that more closely mimics the pathogenesis of this comorbidity in humans is essential for identifying more effective strategies for antimicrobial drug treatment and for control of blood glucose levels in diabetic patients with TB. In addition, the influence that IGT and insulin resistance have on TB susceptibility and pathogenesis has not been adequately investigated.
Currently, the animal models most often used to study the pathogenesis of TB concurrent with diabetes are inbred strains of mice treated with high doses of the cytotoxic drug streptozotocin (STZ).7,8 These models mimic chronic hyperglycemia resulting from total insulin deficiency, as in human type 1 diabetes mellitus. Although this strategy has provided valuable information on how absolute insulin deficiency and persistent hyperglycemia affect active TB disease, it fails to take into account a variety of other metabolic defects associated with IGT and type 2 diabetes through dietary manipulation. Unlike murine models, lipid metabolism in the guinea pig more closely resembles that of humans, making it ideal for studying cardiovascular disease risks and other consequences of altered glucose and lipid metabolism associated with type 2 diabetes.9 Moreover, most mouse strains used to model TB, including those previously used in diabetes-TB comorbidity studies, fail to respond to M. tuberculosis infection with the development of pulmonary and extrapulmonary granulomata with caseous necrosis, as is typical in humans, as well as guinea pigs, nonhuman primates, and rabbits.10 The value of the guinea pig TB model has been further validated recently in studies of natural transmission of M. tuberculosis from human patients with TB to guinea pigs. A subpopulation of guinea pigs exposed to aerosols from patients with TB developed active TB disease and an array of clinical and pathologic responses, which more accurately reflects the clinical variation of naturally occurring M. tuberculosis infection in humans.11
Clinical studies have shown that the increased susceptibility of diabetic patients to M. tuberculosis is accompanied by an altered host response to infection. Diabetic patients with active TB have higher bacterial burdens based on sputum culture and are refractory to first-line antimicrobial combination therapy, with longer time to sputum conversion and higher mortality rates during therapy.12–14 Diabetic patients often have atypical radiographic findings, with more frequent involvement of lower lung lobes, and some studies indicate a higher rate of cavitary disease, consistent with more severe pulmonary pathology.15,16 However, the impact that diabetes has on the development of extrapulmonary TB in humans is conflicting with some studies, indicating a relative risk similar to or less than that of nondiabetic patients, whereas others show a predisposition for extrapulmonary and even miliary disease patterns in diabetic patients.5,17,18 In patients with TB and poorly controlled type 2 diabetes, an exaggerated innate and type 1 cytokine response has been demonstrated clinically.19 However, the impact that insulin resistance alone has on the response to M. tuberculosis infection is unknown. The goal of this study was to develop an animal model that more closely mimics the clinical and immunologic manifestations of diabetes-TB comorbidity in humans.
Materials and Methods
Induction and Confirmation of Glucose Intolerance and Type 2 Diabetes
Sixty outbred Dunkin-Hartley guinea pigs, weighing 300 to 400 g, were obtained from Charles River Laboratories (Wilmington, MA) and were maintained in individual housing. Guinea pigs were divided into three groups: nondiabetic, IGT, and type 2 diabetic (n = 20 per group). Eleven weeks before infection with M. tuberculosis, guinea pigs were fed a custom-formulated high-fat, high-carbohydrate (HFHC) diet (Dyets Inc., Bethlehem, PA) ad libitum to induce IGT. The diet consisted of 18% protein, 30% fat, and 52% carbohydrate, with the carbohydrate portion consisting of 45% sucrose and 55% fructose. The dietary fat composition consisted of an equivalent kilocalorie per kilogram of beef tallow and Primex vegetable shortening (Stratas Foods, Memphis, TN), creating a fatty acid composition of 42% saturated, 50% monounsaturated, and 8% polyunsaturated fatty acids. After 8 weeks of the HFHC diet, type 2 diabetes was induced in half of the guinea pigs with IGT using a single s.c. injection of STZ to induce subtotal β-cell cytotoxicity.20 STZ treatment consisted of an optimized single dose of 200 mg/kg in citrate buffer (pH 4.5) after anomer equilibration at 4°C for 2 hours21 and 20 minutes after pretreatment with an intramuscular injection of 0.5 mg/kg of the α2-adrenergic receptor antagonist yohimbine.22 A standardized oral glucose challenge [oral glucose tolerance test (OGTT)] was used to assess the severity of glucose intolerance by administering a 2-g/kg dose of d-glucose (0.5 g/mL) after a 12-hour fasting period and measuring glucose levels 0, 60, 90, 120, and 150 minutes after administration using the FreeStyle Lite glucometer (Abbott Diabetes Care, Alameda, CA), which has been validated for accuracy in the guinea pig against the glucose oxidase method, as previously described.23
Infection with M. tuberculosis and Euthanasia
Low-dose aerosol exposure of guinea pigs to M. tuberculosis was performed using the Madison chamber aerosol generation device (College of Engineering Shops, University of Wisconsin, Madison, WI) calibrated to deliver approximately 20 bacilli of the H37Rv strain of M. tuberculosis (TMC 102; Trudeau Institute, Saranac Lake, NY) isolated during log phase growth in Proskauer-Beck media. The course of infection was evaluated in nondiabetic, glucose-intolerant, and diabetic guinea pigs at the predetermined end points of TB disease progression or days 30, 60, or 90 of infection or to humane end points, and then the animals were euthanized (n = 5 per group per time point). On day 30 of infection, 7 of 20 diabetic guinea pigs remained and were euthanized owing to declining clinical condition. Euthanasia was performed by anesthetic induction with 40 mg of ketamine and 1 mg of diazepam before i.p. injection of an overdose of 1.5 mL/kg of sodium pentobarbital.
Histopathologic Evaluation and Lesion Burden Determination
To determine the impact that IGT and diabetes had on TB disease progression, histopathology was evaluated and lesion burden quantified on days 30, 60, and 90 of M. tuberculosis infection. Tissues were fixed in 4% buffered paraformaldehyde. Standardized sampling of the lung was performed by midsagittal sectioning of the left caudal lung lobe from each guinea pig at a predetermined anatomical location, irrespective of visible lung lesions. The head of the spleen from each animal was also uniformly sectioned for histopathologic evaluation. The tissues were paraffin embedded, and 5-μm sections were stained with H&E for histopathologic evaluation using routine methods. Morphometric analysis was performed using an Eclipse 80i microscope (Nikon Instruments, Melville, NY) and Stereo Investigator software version 10.02 (MBF Bioscience, Williston, VT), with tissue area and lesion area estimated using the area fraction fractionator method and expressed as a percentage ratio of lesion to total tissue area, as previously described.24
Quantification of Tissue Bacterial Burden
Lung, spleen, and liver were homogenized in 1 mL of PBS, plated in serial dilutions on nutrient 7H11 agar, and incubated at 37°C for 3 to 6 weeks. Colony-forming units were log transformed and are expressed as colony-forming units per gram of tissue. In addition, the intrathoracic trachea was removed at necropsy, flushed with 1 mL of PBS, and plated on 7H11 agar undiluted. Data are expressed as either positive or negative for M. tuberculosis growth.
Quantification of Serum Lipids
Total serum triglycerides were quantified by sequential enzymatic conversion with lipoprotein lipase, glycerol kinase, and glycerol phosphate oxidase, followed by peroxidase-mediated colorimetric change (Cayman Chemical Co., Ann Arbor, MI) measured spectrophotometrically at an absorbance of 450 nm. Total serum free fatty acids (FFAs) were quantified by sequential enzymatic conversion with ascorbate oxidase and acyl CoA oxidase, followed by peroxidase-mediated generation of fluorescence (Cayman Chemical Co.) measured spectrophotometrically at excitation 530 nm and emission 585 nm, as previously described.23
Quantification of Tissue and Serum Advanced Glycation End Products
Lung tissue was mechanically homogenized in 1 mL of PBS and then was centrifuged to remove insoluble material, and the supernatant was used for advanced glycation end product (AGE) detection. Serum and cell lysate from lung homogenate were assayed for total protein by the bicinchoninic acid method and were diluted to 10 μg/mL for AGE detection by enzyme-linked immunosorbent assay (ELISA) (Cell Biolabs Inc., San Diego, CA). Quantification was performed by interpolation from a standard curve derived from AGE-modified bovine serum albumin, and data are expressed as microgram of AGE-modified protein per milliliter. Antibodies used in this assay detect glycolaldehyde-derived AGEs, including two of the most biologically prevalent: carboxymethyl-lysine and pentosidine.
RNA Isolation from Lung Tissue
The pulmonary circulation was perfused with 40 U/mL of RNasin RNase inhibitor (Promega Corp., Madison, WI) in HBSS at the time of necropsy. Perfused lung was collected in incomplete Dulbecco’s modified Eagle’s medium and was maintained at 4°C unless otherwise indicated. Tissues were digested with 0.7 mg/mL of collagenase D and 100 U of DNaseI at 37°C for 30 minutes and then were pushed through a 70-μm cell strainer. Viable cells were separated from necrotic cell debris using Ficoll-Paque PREMIUM medium (GE Life Sciences, Pittsburg, PA) and were washed twice in HBSS, and 1 mL of TRIzol reagent (Life Technologies, Grand Island, NY) was added to the recovered viable cells. RNA isolation with TRIzol reagent was performed as instructed by the manufacturer, followed by treatment with 10 U of DNaseI and recovery with an additional phenol/chloroform separation and sodium acetate–ethanol precipitation. RNA integrity was assessed using the RNA 6000 nano chip (Agilent Technologies Inc., Santa Clara, CA), and samples with an RNA integrity number greater than 5 were used for relative gene expression.
Relative Gene Expression by Quantitative RT-PCR
The influence of IGT and diabetes on the immune response to M. tuberculosis infection was measured by relative gene expression of selected key cytokines. After cDNA synthesis, quantitative RT-PCR was performed using the SYBR Green detection kit (Bio-Rad Laboratories, Hercules, CA) using the Bio-Rad CFX-96 real-time thermal cycler per manufacturer instructions, with each reaction containing 0.2 mmol/L of each primer and 50 ng of cDNA template. Under these reaction conditions, primer design was optimized for equivalent amplification efficiencies. Gene expression was normalized to two reference genes—hypoxanthine-guanine phosphoribosyltransferase (HPRT) and TATA-box binding protein (TBP)— both of which were validated for consistent expression under these experimental conditions. The HPRT, IL-12p40, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-1β, transforming growth factor-β, and FoxP3 primer sequences used in this study are previously published.25–27 TBP, IL-17A, IL-23, monocyte chemoattractant protein-1 (MCP-1), and IL-10 primers were designed using transcripts from the EMBL Ensembl annotated genome browser28 for the guinea pig with the aid of Primer3 primer design software version 2.2.3,29 and primers targeting IL-4 were designed from the recently published sequence.30 The primer sequences (forward and reverse) are as follows: TBP (ENSCPOT00000001200), 5′-CCAAGCGGTTTGCTGCTGTA-3′ and 5′-GGCTCCTGTGCACACCATCTT-3′; IL-10 (ENSCPOT00000009023), 5′-GCCTTTGGCAGGGTGAAGAC-3′ and 5′-GGCTTGGCASACCCAGGTAAC-3′; IL-17A (ENSCPOT00000010600), 5′-AATGCCGTTACTCGGGCTGT-3′ and 5′-AGCGGGCAGTTCTGAGGTTC-3′; MCP-1 (ENSCPOT00000013601), 5′-AGCAGCAGGTGTCCCCAAGA-3′ and 5′-TCTCTGGTCCAGTTTGGCAATG-3′; IL-23 (ENSCPOT00000015072), 5′-GCAACCACCACACCTTGCAAGAAA-3′ and 5′-ATCAGCAAAGACGTCCGTGACCAGC-3′; and IL-4 (NCBI NM_001257263), 5′-GCAACCACCACACCTTGCAAGAAA-3′ and 5′-ATCAGCAAAGACGTCCGTGACCAGC-3′. RNA isolates without reverse transcription were amplified for each animal to ensure a lack of contaminating genomic DNA, and all assays performed included no-template controls. Data are expressed as normalized fold expression on a log2 scale.
Quantification of Leukocyte Phenotype in Tissue by Flow Cytometry
Single-cell suspensions from lung, tracheobronchial lymph node, and spleen were prepared as previously described.31 Cell suspensions were labeled with anti–guinea pig CD4-RPE, CD8-FITC, pan T-cell–APC, CD45-FITC, MIL4 (granulocytes), MR1 (macrophages), and MHC-II antibodies (AbD Serotec, Raleigh, NC) as previously described.32 Unconjugated antibodies were detected with a secondary anti-mouse IgG antibody conjugated with RPE fluorochrome for CD45 detection on CD8 T cells, MHC-II, and MIL4 antibodies and with FITC fluorochrome for the MR1 antibody. Data acquisition was performed using the LSR II flow cytometer (BD Biosciences, San Jose, CA), and data were analyzed using FACSDiva software version 7.0 (BD Biosciences) using a minimum of 100,000 events. Compensation for spectral overlap was performed as previously described.32
Data Analysis
Analysis was performed using SAS software version 9.3 (SAS Institute, Inc., Cary, NC). A one-factor analysis of variance was used for glucose tolerance testing, morphometric microscopy, tissue bacterial culture, and cytokine data with log transformation to correct for unequal variance, and the Tukey honestly significant difference was used for pairwise comparisons. A two-factor analysis was used on the log-transformed flow cytometry data, and the Tukey honestly significant difference was used for pairwise comparisons between groups at each time point. To determine differences between survival curves, a log-rank test with Wilcoxon adjustment for multiple comparisons was used. Significance was set at P ≤ 0.05.
Results
HFHC Diet Impairs Glucose Tolerance in Guinea Pigs
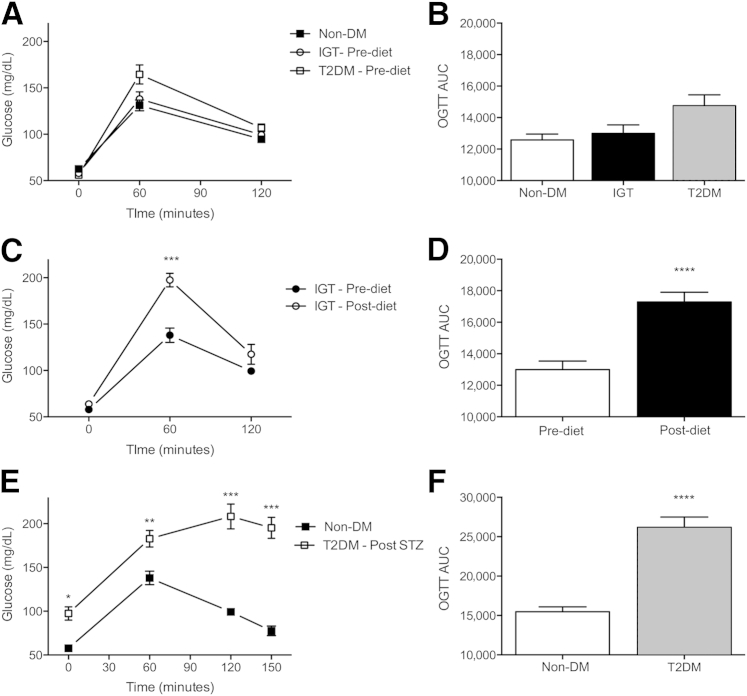
Guinea pigs fed an HFHC diet for 8 weeks consistently developed IGT as determined by OGTT. Delayed glucose use resulted in abnormally elevated blood glucose levels as early as 60 minutes after glucose challenge as expressed as a significant increase in total area under the curve (Figure 1, C and D) compared with OGTT before initiating the diet (Figure 1, A and B).
Figure 1.
Glucose tolerance is impaired in guinea pigs with prediabetic IGT and type 2 diabetes (T2DM). Normal glucose tolerance in nondiabetic (non-DM) guinea pigs before initiating an HFHC diet by OGTT (A) and corresponding area under the curve (AUC) (B). Glucose tolerance was impaired in guinea pigs fed the HFHC diet alone, with reduced glucose disposal at 60 minutes of OGTT (C) and an overall increase in AUC (D) compared with the same guinea pigs before initiating the diet. A diabetic level of IGT in T2DM guinea pigs 11 weeks after combined HFHC/STZ diabetogenic treatment (E) with a marked increase in AUC (F). Data are given as means ± SD. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, and ∗∗∗∗P ≤ 0.0001 compared with non-DM levels.
HFHC Diet Combined with STZ Induces Type 2 Diabetes in Guinea Pigs
OGTT performed 2 weeks after STZ treatment of guinea pigs fed the HFHC diet showed fasting hyperglycemia and further exacerbation of IGT, with glucose levels remaining high 150 minutes after glucose challenge (Figure 1, E and F), criteria consistent with human type 2 diabetes.33 The impact of diabetogenic treatments on serum biochemical parameters after a 12-hour fasting period is summarized in Table 1. Guinea pigs with IGT fed the HFHC diet only for 11 weeks and guinea pigs with 3 weeks of uncontrolled diabetes had elevated fasting triglyceride levels before M. tuberculosis infection. FFA levels were significantly elevated only in the diabetic guinea pigs before M. tuberculosis infection.
Table 1.
Metabolic Parameters from Non-DM, Glucose-Intolerant, and T2DM Guinea Pigs before Infection with M. tuberculosis (Day 0) and 30, 60, and 90 Days after Infection
| Parameter | Model | Day 0 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|
| TG (mg/dL) | Non-DM | 34.7 ± 9.4 | 58.4 ± 16.3 | 40.59 ± 19.3 | ND |
| IGT | 93.1 ± 63.7∗ | 66.9 ± 44.1 | 77.1 ± 42.1 | ND | |
| T2DM | 101 ± 47∗ | 52.2 ± 13.9 | ND | ND | |
| FFA (mmol/L) | Non-DM | 132.3 ± 24.8 | 380 ± 46.2††† | 260.6 ± 8.6† | 252.7 ± 25.4† |
| IGT | 146.6 ± 47.5 | 406.1 ± 84††† | 256.9 ± 19.2† | 240.3 ± 16.6† | |
| T2DM | 172.6 ± 47.4∗ | 454.2 ± 90.7††† | ND | ND | |
| AGE (μg/mL) | Non-DM | 1.079 ± 0.181 | 1.266 ± 0.188 | 1.125 ± 0.179 | 0.956 ± 0.122 |
| IGT | 0.973 ± 0.011 | 1.186 ± 0.273 | 1.007 ± 0.094 | 0.987 ± 0.14 | |
| T2DM | 1.163 ± 0.168 | 2.522 ± 0.532†† | ND | ND |
Data are given as means ± SD.
ND, not determined; non-DM, nondiabetic; T2DM, diabetic; TG, triglyceride.
P ≤ 0.05 compared with non-DM levels.
P ≤ 0.05, ††P ≤ 0.001, and †††P ≤ 0.0001 compared with preinfection levels.
Infection with M. tuberculosis Impairs Glucose Tolerance in Nondiabetic, HFHC-Fed Only, and Diabetic Guinea Pigs
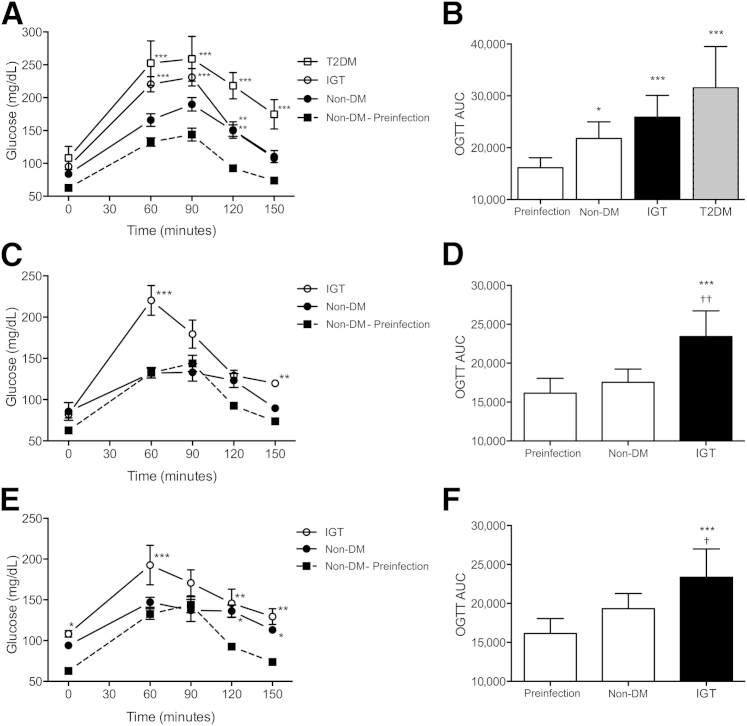
At day 30 of infection, TB induced IGT in nondiabetic guinea pigs, with significantly higher levels 120 minutes after glucose administration and an overall increase in area under the curve compared with preinfection glucose tolerance (Figure 2, A and B). IGT was more severe in HFHC-fed animals and diabetic guinea pigs after M. tuberculosis infection, with total area under the curve exceeding preinfection, postdiabetogenic treatment levels (Figure 2, A and B). Although IGT persisted in HFHC-fed alone guinea pigs through days 60 and 90 of infection, total area under the curve was not significantly different from preinfection glucose tolerance in nondiabetic guinea pigs on days 60 and 90 of infection (Figure 2, C–F). However, despite minimal difference in total area under the curve, IGT was evident at the end point of OGTT in nondiabetic guinea pigs (Figure 2, E and F). Infection with M. tuberculosis also markedly increased serum FFA levels in all the groups. Serum FFA levels were highest on day 30 of infection and subsided as chronic infection was established (Table 1).
Figure 2.
Infection with M. tuberculosis further impairs glucose tolerance in nondiabetic (non-DM), HFHC-fed only, and diabetic (T2DM) guinea pigs. OGTT on day 30 of infection (A) showed amplification of IGT in non-DM, HFHC-fed only, and T2DM guinea pigs due to M. tuberculosis infection with an increased area under the curve (AUC) (B) compared with preinfection values. On day 60 of infection (C), glucose tolerance was comparable with preinfection levels in non-DM guinea pigs, whereas IGT and an increased AUC (D) remained in guinea pigs with preexisting IGT. Similarly, IGT persisted on day 90 of infection (E) in guinea pigs with preexisting IGT as indicated by an elevated AUC (F), whereas overall glucose tolerance was comparable with preinfection levels in non-DM controls, although blood glucose levels remained elevated in this group 2 hours after administration. Data are given as means ± SD. ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001 compared with non-DM preinfection levels; †P ≤ 0.05, ††P ≤ 0.01 compared to non-DM, infected levels.
AGEs Accumulate When Diabetes and TB Are Combined
Serum AGEs were elevated above preinfection levels by approximately threefold on day 30 of infection only in diabetic guinea pigs, whereas no difference was evident between nondiabetic guinea pigs and those with IGT on days 30, 60, or 90 of M. tuberculosis infection (Table 1). Increased pulmonary tissue AGEs were present in M. tuberculosis–infected nondiabetic, glucose-intolerant, and diabetic guinea pigs to a similar degree (Table 1) at approximately threefold over uninfected nondiabetic lung (data not shown).
Diabetic Guinea Pigs Develop Rapidly Progressive TB Disease
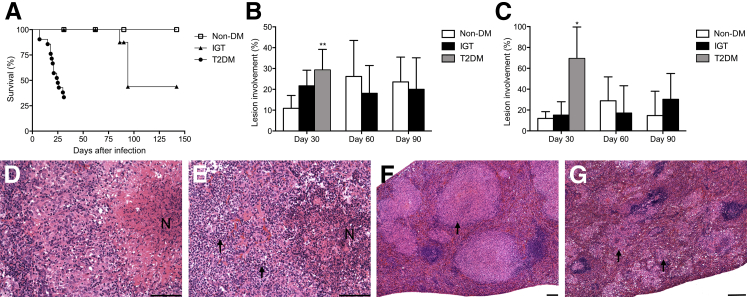
Progression of TB was rapid in diabetic guinea pigs, with 65% mortality before day 30 of infection and median survival of 25 days (Figure 3A). Guinea pigs with IGT showed intermediate, but not statistically significant, mortality rates compared with nondiabetic controls. Mortality in guinea pigs with IGT was 50% before termination of the study on day 145, with median survival of 95 days. Guinea pigs with IGT and infected with M. tuberculosis were euthanized owing to respiratory distress and had developed marked cranial mediastinal and tracheobronchial lymph node enlargement with compression of primary bronchi. No mortality occurred in nondiabetic guinea pigs before the terminal end point on day 145 of infection.
Figure 3.
TB is more severe in guinea pigs with type 2 diabetes (T2DM). A: Markedly reduced survival in T2DM guinea pigs and intermediate, but nonsignificant, susceptibility seen in guinea pigs with IGT. B: An approximately threefold increase in lung disease severity in T2DM guinea pigs on day 30 of infection was measured by lesion burden, whereas no significant differences were present between glucose-intolerant and nondiabetic (non-DM) controls. C: Spleen lesion burden was markedly increased in T2DM guinea pigs on day 30 of infection, whereas no significant differences were present between glucose-intolerant and non-DM controls. Compared with normal granuloma morphology in the lung of non-DM guinea pigs (D), there is much higher granulocyte infiltration (arrows) and disruption of granuloma architecture in T2DM guinea pigs (E). Compared with typical large and discrete granulomas (arrow) in the spleen of non-DM guinea pigs (F), T2DM guinea pigs have a widespread miliary pattern of lesion dissemination in the spleen consisting of small and coalescing granulomas (arrows) (G). Data are given as means ± SD (A–C). ∗P ≤ 0.05, ∗∗P ≤ 0.01 compared with non-DM levels. Scale bars: 100 μm (D–G). H&E (D–G). N, necrosis.
M. tuberculosis Infection in Guinea Pigs with Diabetes Accelerates Bacilli Dissemination and Exacerbates TB Disease Severity
Pulmonary lesion burdens were markedly increased in diabetic guinea pigs, with a 2.7-fold increase in mean percentage involvement over nondiabetic controls. Although pulmonary lesions were higher in guinea pigs with IGT on day 30, this difference was not statistically significant, and no significant differences were present on days 60 and 90 of infection between glucose-intolerant and nondiabetic guinea pigs (Figure 3B). In contrast to typical well-structured granulomas of nondiabetic guinea pigs and those with IGT (Figure 3D), diabetic guinea pigs had marked granulocytic inflammation that disrupted lung granuloma architecture, often resulting in airway erosions (Figure 3E). Splenic lesion burden, as a measure of extrapulmonary TB disease, was increased in diabetic guinea pigs on day 30 of infection, reaching as high as 97% involvement (Figure 3C). In addition to extensive involvement, guinea pigs with diabetes had altered lesion morphology characterized by widely disseminated yet smaller and often coalescing aggregates of macrophages (Figure 3G) compared with nondiabetic controls (Figure 3F). Progressive lesions developed in the lymph nodes draining the lungs in all guinea pigs regardless of their diabetic state.
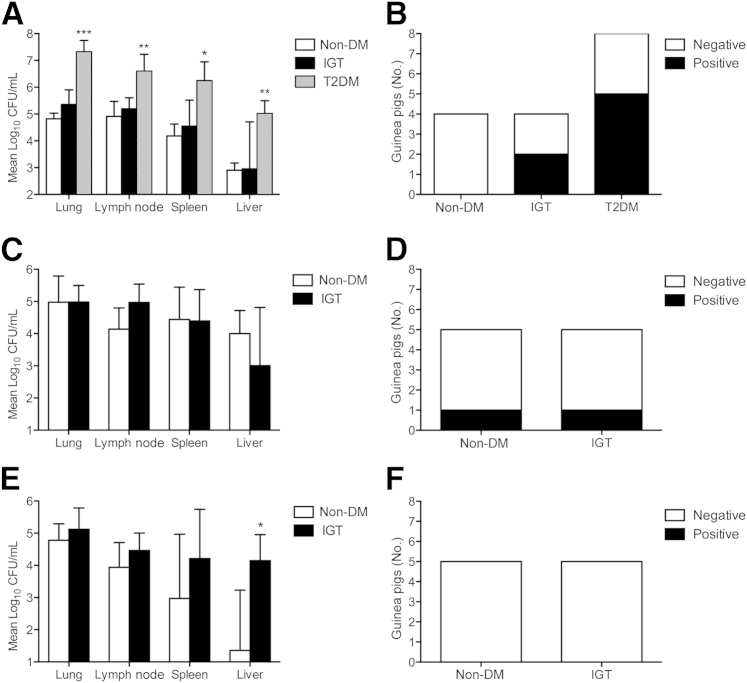
Diabetic Guinea Pigs Have a Higher Bacterial Burden
On day 30 of infection, diabetic guinea pigs had approximately 2 log10 higher bacterial burdens in all tissues evaluated compared with nondiabetic controls and guinea pigs with IGT (Figure 4A). Although no significant differences were present on day 60 of infection (Figure 4C), the bacterial burden was lower in spleen and liver of nondiabetics on day 90 compared with guinea pigs with IGT, whose extrapulmonary burden remained elevated (Figure 4E). The higher lung bacterial burden in diabetic guinea pigs was accompanied by increased shedding of bacilli into the upper airways. Although M. tuberculosis could not be isolated from the tracheal wash fluid of any nondiabetic controls (n = 4) on day 30 of infection, M. tuberculosis was cultured from two of four guinea pigs with IGT and five of eight diabetic guinea pigs (Figure 4B). As chronic infection was established, the frequency of viable bacilli cultured from the tracheal wash of guinea pigs with IGT was reduced. Tracheal washes were culture positive from one nondiabetic and glucose-intolerant guinea pig on day 60 of infection (Figure 4D), but all were negative by day 90 (Figure 4F).
Figure 4.
Guinea pigs with diabetes (T2DM) have a higher bacterial burden. A: T2DM guinea pigs on day 30 of infection had a much higher bacterial burden in pulmonary and extrapulmonary organs. B: There was higher frequency of M. tuberculosis growth from tracheal wash fluid of T2DM guinea pigs, exceeding positive samples from guinea pigs with IGT compared with no growth in nondiabetic (non-DM) controls. There were no significant differences in tissue bacterial burden on day 60 of infection between glucose-intolerant and non-DM guinea pigs (C) and similar tracheal wash culture results, which declined in guinea pigs with IGT (D). E: By day 90 of infection, extrapulmonary burden declined in non-DM guinea pigs, whereas it was persistently elevated in guinea pigs with IGT. F: All tracheal wash cultures from non-DM and glucose-intolerant guinea pigs were negative on day 90 of infection. Data are given as means ± SD (A, C, and E). ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001 compared with non-DM levels. CFU, colony-forming unit.
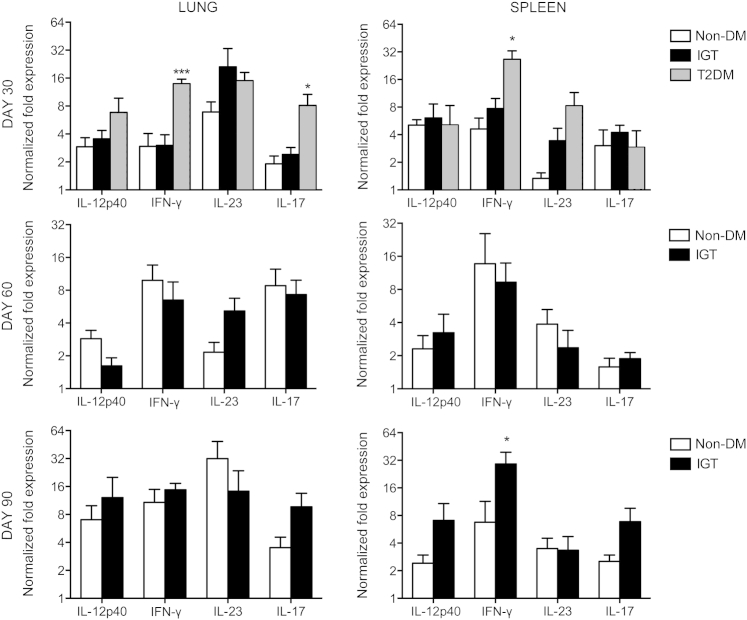
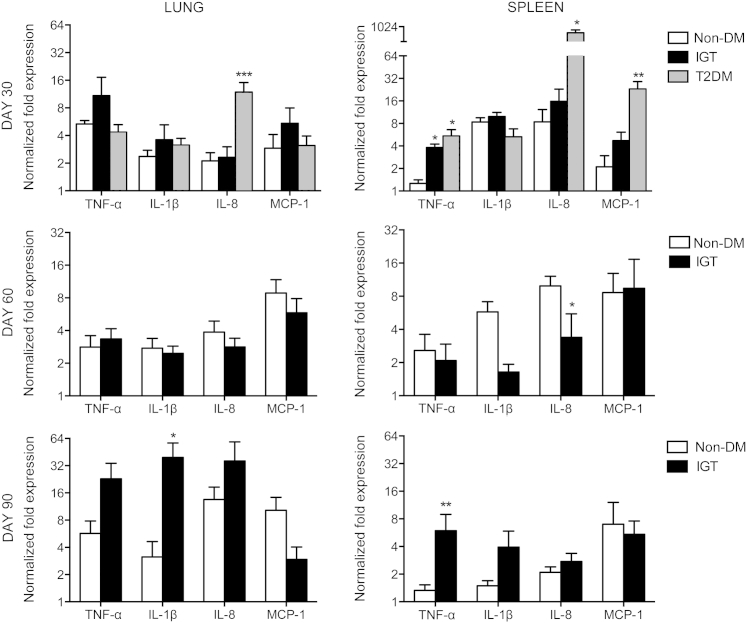
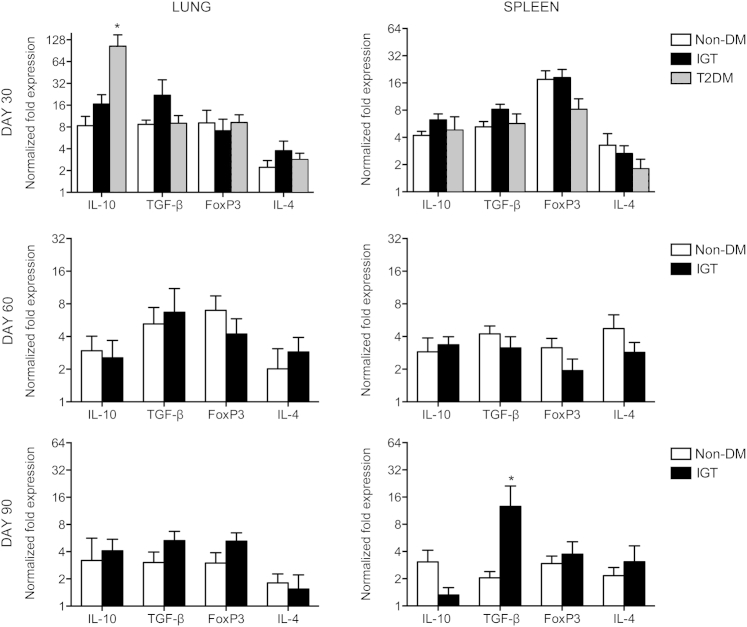
Guinea Pigs with IGT and Diabetes Respond to M. tuberculosis Infection with Altered Proinflammatory and Anti-Inflammatory Cytokine Profiles
On day 30 of infection, elevations in cytokine and chemokine expression in the lungs of diabetic guinea pigs included IL-12p40 (2.3-fold), IFN-γ (4.7-fold), IL-17A (4.3-fold), IL-8 (5.6-fold), and IL-10 (12.6-fold) compared with nondiabetic controls (Figures 5, 6, and 7). In contrast, nondiabetic guinea pigs and those with IGT had similar cytokine profiles early in infection and through day 60 (Figures 5, 6, and 7), except for a 4.0- and 12.6-fold increase in TNF-α (P = 0.1) and IL-1β, respectively, on day 90 of infection in guinea pigs with IGT compared with nondiabetic controls (Figure 6). Diabetic guinea pigs had elevated IFN-γ (5.8-fold), TNF-α (4.3-fold), IL-8 (39.8-fold), and MCP-1 (11.0-fold) levels in the spleen on day 30 of infection, whereas guinea pigs with IGT had elevated TNF-α (3.0-fold) compared with nondiabetic controls (Figures 5 and 6). Differences in cytokine expression in the spleen on day 90 of infection were present in guinea pigs with IGT with elevated IFN-γ (4.4-fold), TNF-α (4.5-fold), and transforming growth factor-β (6.2-fold) levels (Figures 5, 6, and 7). Expression of FoxP3 and IL-4 did not differ between any groups at any point throughout the study (Figure 7).
Figure 5.
Guinea pigs with type 2 diabetes (T2DM) respond to M. tuberculosis infection with a more robust type 1 cytokine response. Relative gene expression of cytokines promoting a Th1-biased T-cell response was measured by quantitative RT-PCR. Lung IFN-γ and IL-17 expression and spleen IFN-γ expression were higher in T2DM guinea pigs infected with M. tuberculosis on day 30 of infection. Elevated IFN-γ expression was not seen in guinea pigs with IGT until day 90 of infection. No significant differences were observed in IL-12p40 or IL-23 expression at any time point evaluated. Data are given as log2 ± SEM. ∗P ≤ 0.05, ∗∗∗P ≤ 0.001 compared with nondiabetic (non-DM) levels.
Figure 6.
Guinea pigs with type 2 diabetes (T2DM) respond with an elevated innate chemokine/cytokine response early in M. tuberculosis infection. Relative gene expression of innate cytokines and chemokines most involved in TB pathogenesis was measured by quantitative RT-PCR. Expression of the neutrophil and macrophage chemokines IL-8 and MCP-1, respectively, as well as splenic TNF-α expression were significantly elevated in guinea pigs with T2DM on day 30 of infection. In contrast, elevated expression of IL-1β and TNF-α occurred only on day 90 of infection in guinea pigs with IGT. Data are given as log2 ± SEM. ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001 compared with nondiabetic (non-DM) levels.
Figure 7.
Increased IL-10 expression does not impair the inflammatory response in type 2 diabetic (T2DM) guinea pigs. Relative gene expression of cytokines and transcription factors that oppose the cell-mediated response during TB were measured by quantitative RT-PCR. A productive type 1 cytokine response persisted in T2DM guinea pigs despite elevated IL-10 expression on day 30 of infection with M tuberculosis. IL-4 expression, as an indicator of Th2 T-cell differentiation, or FoxP3 expression, as an indicator of regulatory T-cell differentiation, did not differ between nondiabetic (non-DM), insulin-resistant, or T2DM states at any time point evaluated. Data are given as log2 ± SEM. ∗P ≤ 0.05 compared with non-DM levels. TGF-β, transforming growth factor-β.
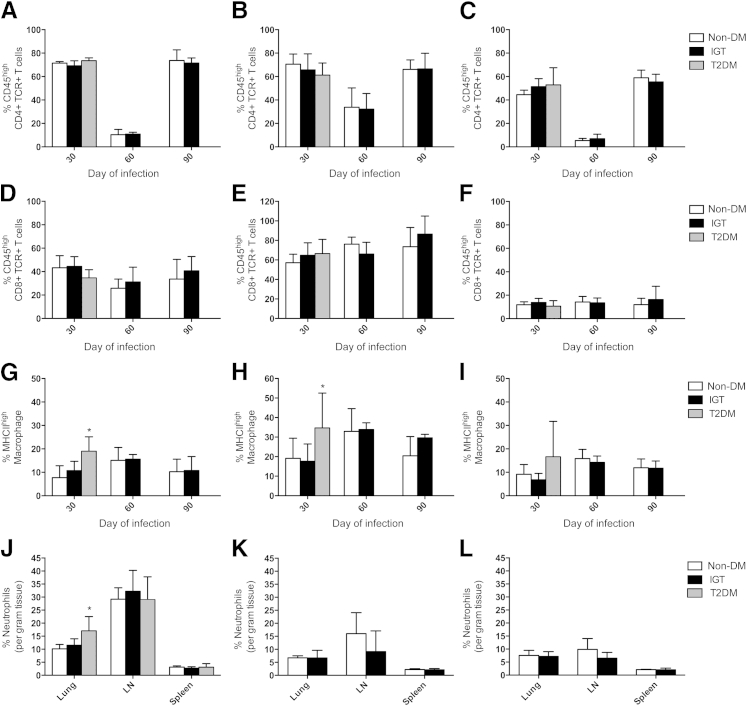
Guinea Pigs with Diabetes Have Higher Levels of Macrophage Activation and Neutrophil Infiltration Early in Disease, Whereas Innate Immune Cell Phenotypes Increase Late in Disease in Guinea Pigs with IGT
Absolute quantification of immune cell phenotypes per gram of lung, lymph node, and spleen is depicted in Table 2. Diabetic guinea pigs responded to M. tuberculosis infection with higher total numbers of activated MHCIIhigh macrophages in lung, lymph node, and spleen (Table 2) and a greater proportion of activated macrophages out of all macrophage cells recovered from lung and lymph node compared with nondiabetic and glucose-intolerant controls (Figure 8, G and H). This correlated with higher total numbers of activated CD45high CD4 T cells from the lymph node of diabetic guinea pigs, although the percentage of activated CD45high T cells out of total CD4 T cells recovered did not differ (Figure 8, A–C). In addition, diabetic guinea pigs had a higher proportion of granuloyctes in the lung on day 30 of infection compared with nondiabetic controls (Figure 8J). In contrast, guinea pigs with IGT had lower cell numbers of all phenotypes recovered from lymph node on day 30 of infection, but higher numbers of activated MHCIIhigh macrophages as well as granulocytes were present on day 90 of infection. An overall decrease in the proportion of activated CD45high CD4 T cells occurred in all organs in nondiabetic guinea pigs and those with IGT between days 30 and 60 of infection but increased again on day 90 of infection (Figure 8, A–C).
Table 2.
Immune Cell Phenotypes in Absolute Number as Measured by Flow Cytometry in Lung, Lymph Node, and Spleen at 30, 60, and 90 Days of Infection
| Cell phenotype | Model | Lung |
Lymph node |
Spleen |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 days | 60 days | 90 days | 30 days | 60 days | 90 days | 30 days | 60 days | 90 days | ||
| CD4 TCR | Non-DM | 3.09 ± 0.58 | 8.13 ± 1.04 | 5.31 ± 1.12 | 16.32 ± 1.53∗∗ | 52.10 ± 9.48 | 21.71 ± 7.07 | 6.86 ± 0.84 | 29.73 ± 8.56 | 10.09 ± 1.74 |
| IGT | 3.24 ± 0.33 | 9.92 ± 1.65 | 8.05 ± 0.83 | 5.75 ± 1.33†† | 46.30 ± 8.92 | 19.86 ± 5.22 | 7.65 ± 1.43 | 28.20 ± 5.01 | 13.59 ± 5.25 | |
| T2DM | 2.00 ± 0.22 | 24.59 ± 4.55 | 6.30 ± 0.67 | |||||||
| CD4 TCR CD45high | Non-DM | 2.20 ± 0.41 | 0.88 ± 0.26 | 4.03 ± 0.94 | 11.38 ± 0.87∗ | 17.96 ± 3.85 | 13.61 ± 3.81 | 3.08 ± 0.44 | 1.85 ± 0.72 | 6.10 ± 1.23 |
| IGT | 2.24 ± 0.22 | 1.08 ± 0.19 | 5.79 ± 0.70 | 3.96 ± 1.18†† | 15.50 ± 4.26 | 13.69 ± 4.21 | 4.07 ± 0.99 | 2.12 ± 0.81 | 8.06 ± 3.31 | |
| T2DM | 1.47 ± 0.16 | 15.31 ± 3.14 | 3.42 ± 0.65 | |||||||
| CD8 TCR | Non-DM | 2.42 ± 0.58 | 2.35 ± 0.22 | 3.06 ± 0.92 | 11.91 ± 1.94∗ | 12.29 ± 3.11 | 8.49 ± 1.95 | 6.95 ± 0.85 | 16.90 ± 4.63 | 18.84 ± 5.03 |
| IGT | 2.53 ± 0.42 | 3.91 ± 0.66 | 5.34 ± 0.74 | 3.92 ± 0.73††† | 16.29 ± 4.00 | 13.60 ± 4.48 | 7.00 ± 1.41 | 17.49 ± 5.53 | 17.78 ± 7.01 | |
| T2DM | 1.78 ± 0.16 | 21.38 ± 3.97 | 5.76 ± 1.68 | |||||||
| CD8 TCR CD45high | Non-DM | 1.07 ± 0.27 | 0.60 ± 0.09∗ | 0.91 ± 0.23 | 6.81 ± 1.18 | 25.06 ± 13.60 | 6.36 ± 1.98 | 0.81 ± 0.09 | 2.66 ± 0.93 | 2.62 ± 1.24 |
| IGT | 1.15 ± 0.24 | 1.11 ± 0.18 | 2.12 ± 0.38 | 2.45 ± 0.42††† | 10.53 ± 2.37 | 11.20 ± 3.92 | 0.96 ± 0.19 | 2.60 ± 1.04 | 2.63 ± 0.84 | |
| T2DM | 0.60 ± 0.06 | 14.01 ± 2.64 | 0.73 ± 0.34 | |||||||
| MHCIIlow MAC | Non-DM | 51.00 ± 5.32 | 47.08 ± 7.02 | 84.16 ± 13.95 | 145.9 ± 16.14 | 157.5 ± 24.26 | 150.1 ± 46.24 | 58.92 ± 7.71 | 106.3 ± 23.43 | 115.9 ± 23.42 |
| IGT | 45.86 ± 3.28 | 49.64 ± 8.02 | 154.20 ± 14.36 | 100.1 ± 10.94† | 113.9 ± 17.38 | 199.8 ± 17.44 | 68.00 ± 7.18 | 109.6 ± 21.43 | 185.7 ± 57.52 | |
| T2DM | 35.96 ± 3.87 | 167.1 ± 12.11 | 93.03 ± 15.16 | |||||||
| MHCIIHigh MAC | Non-DM | 4.62 ± 1.63† | 8.36 ± 1.88 | 9.73 ± 2.79 | 47.87 ± 18.57 | 89.46 ± 32.07 | 26.70 ± 6.62 | 6.42 ± 1.99 | 20.50 ± 5.38 | 15.12 ± 2.35 |
| IGT | 5.32 ± 0.59 | 9.07 ± 1.40 | 19.14 ± 6.24 | 22.42 ± 6.47† | 56.28 ± 4.97 | 86.68 ± 8.30 | 5.28 ± 1.34† | 17.43 ± 3.30 | 22.50 ± 5.05 | |
| T2DM | 8.44 ± 1.44 | 103.6 ± 31.98 | 19.18 ± 6.75 | |||||||
| MIL4 Gran | Non-DM | 11.39 ± 1.20 | 8.08 ± 1.55 | 14.91 ± 2.99∗ | 133.0 ± 25.87 | 92.22 ± 31.12∗∗ | 34.54 ± 10.69 | 3.49 ± 0.47 | 6.24 ± 1.78 | 5.47 ± 0.70 |
| IGT | 12.83 ± 1.26 | 8.70 ± 2.05 | 25.68 ± 4.58 | 92.42 ± 17.41† | 35.22 ± 15.65 | 35.22 ± 15.65 | 3.71 ± 0.79 | 5.94 ± 1.31 | 8.45 ± 3.02 | |
| T2DM | 15.86 ± 2.71 | 178.3 ± 33.21 | 5.98 ± 1.41 | |||||||
| B cell | Non-DM | 41.20 ± 5.20 | 42.94 ± 6.99 | 74.74 ± 11.33∗∗ | 91.46 ± 13.54 | 163.3 ± 39.14 | 123.1 ± 37.71 | 28.10 ± 2.56 | 85.10 ± 19.44 | 94.06 ± 12.57 |
| IGT | 41.92 ± 2.54 | 47.38 ± 7.30 | 138.4 ± 14.74 | 55.70 ± 8.90† | 101.6 ± 7.24 | 188.0 ± 25.59 | 40.70 ± 9.42 | 89.16 ± 20.30 | 149.5 ± 48.68 | |
| T2DM | 27.96 ± 2.29 | 106.3 ± 14.21 | 60.40 ± 9.78 | |||||||
Results are expressed as means ± SD at ×105 cells and normalized to per gram of tissue. Blank cells corresponding to T2DM guinea pigs on days 60 and 90 of infection were not determined.
Gran, granulocyte; MAC, macrophage; non-DM, nondiabetic; T2DM, diabetic.
P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 compared with IGT.
P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001 compared with T2DM.
Figure 8.
Activated macrophages and granulocytes are increased in diabetic (T2DM) guinea pigs on day 30 of M tuberculosis infection. Total CD4+ or CD8+ TCR+ T cells and MHCII low- or high-expressing macrophages were quantified by flow cytometry and normalized to per gram of tissue in lung, lymph node (LN), and spleen and then expressed as percentage activated based on proportions of CD45high CD4+ and CD8+ lymphocytes and MHCIIhigh macrophages. A–C: The proportion of activated CD4 T cells decreased in nondiabetic (non-DM) and glucose-intolerant guinea pigs on day 60 of infection but was equally elevated on day 90 of infection in all organs in non-DM and glucose-intolerant guinea pigs. D–F: There were no differences in CD8 T-cell activation at any time point among non-DM, glucose-intolerant, and T2DM guinea pigs. G–I: Higher proportions of activated macrophages were present in T2DM guinea pigs in lung and LN on day 30 of infection. J–L: Granulocytes as a proportion of total leukocytes counted per gram of tissue were increased in the lung of T2DM guinea pigs. Data are given as means ± SD. ∗P ≤ 0.05 compared with non-DM levels.
Discussion
This study represents the first model of type 2 diabetes in the guinea pig and the first description of M. tuberculosis infection in an animal model with diet-induced IGT or type 2 diabetes. The guinea pig model was chosen to better mimic the comorbidity of diabetes and TB in humans based on the guinea pig response to M. tuberculosis infection and similarities to human glucose and lipid metabolism compared with mice or rats. The most common indicator of systemic insulin resistance in human patients is delayed use of glucose, referred to as IGT, as measured by OGTT. In this study, guinea pigs fed an HFHC diet developed IGT evidenced by an elevated 1-hour postload blood glucose level during OGTT, a measurement recently shown to predict early insulin resistance and β-cell dysfunction in patients.34 In addition, guinea pigs developed serum lipid alterations, including elevated fasting triglyceride and FFA levels, consistent with insulin resistance similar to that seen in humans.35 In humans, the progression from prediabetic, IGT, and insulin resistance to type 2 diabetes is gradual and can take years.36 Consequently, low-dose treatment with the cytotoxic drug STZ in these studies was included to accelerate the rate of β-cell loss in guinea pigs with IGT. The combination of the HFHC diet and low-dose STZ use consistently induces fasting hyperglycemia and blood glucose levels exceeding 200 mg/dL in 2-hour OGTT as early as 14 days after STZ treatment, data consistent with the diagnostic standards established for patients with type 2 diabetes.33
Severe systemic inflammation in nondiabetic patients is often associated with hyperglycemia and IGT mediated, in part, by proinflammatory cytokines, especially TNF-α. The pathogenesis of cytokine-mediated hyperglycemia and IGT is directly linked to the release of FFAs, which are potent mediators of insulin resistance.37–39 In this study, IGT was induced by M. tuberculosis infection alone even in nondiabetic guinea pigs, as previously described.23 M. tuberculosis infection in diabetic and nondiabetic guinea pigs fed the HFHC diet only further exacerbated IGT. This finding paralleled the marked elevations in serum FFA levels in infected guinea pigs, which exceeded the modest elevations associated with diabetes induction before infection. In addition, elevations in FFA levels reflected increased cytokine expression during the early stages of infection, which subsided on day 60 of infection, when innate cytokine expression was low. The relationship between cytokine expression and FFA concentrations was also reflected on day 90 of M. tuberculosis infection in guinea pigs with IGT, which also correlated with increased TNF-α and IL-1β expression. IGT and insulin resistance with hyperglycemia has been previously reported in patients with active TB, which may be explained by elevated proinflammatory cytokines consistent with these data. The clinical significance of IGT and hyperglycemia induced by M. tuberculosis infection is that it further complicates the diagnosis and treatment of diabetes in patients with TB.40–43
Overt diabetes had a profound effect on TB progression in this study. An overall increase in disease severity, characterized by a significantly higher bacterial load and more severe and rapidly progressive pulmonary and extrapulmonary disease, contributed to decreased survival in diabetic guinea pigs with TB. These findings are consistent with reports of more severe pulmonary TB in diabetic patients.15,16 Despite the conflicting evidence in patients,5,10,17,18 more severe and rapidly progressing extrapulmonary disease in diabetic guinea pigs was a consistent finding. The altered pattern of lesion dissemination in the spleen of diabetic guinea pigs resembles miliary TB, a pattern of disease that is associated with increased mortality rates, especially in severely immunocompromised patients with TB.44 M. tuberculosis infection in diabetic guinea pigs also resulted in increased shedding of bacilli into the tracheal lumen at the predetermined end points. M. tuberculosis was cultured from the tracheal washes of diabetic guinea pigs with greater frequency than nondiabetic guinea pigs and those with IGT. The low frequency of animal-to-animal transmission of M. tuberculosis in normal guinea pigs is likely related to the failure to routinely develop open cavitary lesions, which typically harbor large numbers of bacilli.10,45 The present data indicate that diabetes induction increases the frequency of airway shedding of M. tuberculosis, even in the absence of cavitation, which may be related to a higher pulmonary bacterial load and/or an alteration in the diabetic airway microenvironment.46,47 With the increasing interest in natural M. tuberculosis transmission from humans to animals or between animals,11 the diabetic guinea pig may represent a viable animal-to-animal transmission model, which warrants further investigation.
In contrast, IGT in guinea pigs seemed to have little effect on TB disease progression in the early stages of disease. Although the initial pulmonary burden was higher in guinea pigs with IGT, the differences were not statistically significant, and the number of activated CD45high lymphocytes in the lung as well as cytokine expression were comparable with nondiabetic controls. Differences in response to M. tuberculosis infection in guinea pigs with IGT were not seen until the chronic stages of infection. On day 90, TNF-α and IL-1β expression and the extrapulmonary bacterial burden in guinea pigs with IGT were higher than in nondiabetic controls. Recently, an increased prevalence of IGT and insulin resistance has also been recognized in patients with TB; however, it is unknown whether these metabolic alterations are a consequence of systemic insulin resistance mediated by M. tuberculosis infection.48 These results provide the first experimental evidence that IGT similar to a prediabetic state also increases TB disease severity, a finding that warrants further investigation in animal models and humans.49 Although guinea pigs with IGT did not develop TB disease that progressed as rapidly as in diabetic guinea pigs, active inflammation persisted, which accounted for more severe clinical disease in the chronic stages of infection. In this study, the more severe TB disease in guinea pigs with hyperglycemia as a result of IGT are similar to previous studies showing that TB disease severity is increased even in nondiabetic guinea pigs with repeated postprandial hyperglycemia.23 Moreover, the present results are similar to those described in diabetic humans and STZ-treated mice with chronic hyperglycemia, which suggest that poor glycemic control is an important determinant of TB disease risk.7,19
Aside from the increased severity of disease, the pathologic features of M. tuberculosis infection in diabetic guinea pigs differed from those in nondiabetic controls. These changes may be related to the altered expression of particular cytokines and subsequent cellular response to M. tuberculosis infection. Increased IL-17 levels, also described in humans with type 2 diabetes and TB,50 as well as elevated IL-8 expression, may be linked to more granulocytic infiltration and pathology seen in diabetic guinea pigs infected with M. tuberculosis. A persistent IL-17 response during TB contributes to excessive inflammation and is generally limited by IFN-γ production, although this was not evident in the diabetic guinea pigs in this study.51,52 We have shown that the accumulation of AGEs occurs as a result of M. tuberculosis–mediated inflammation but is further increased when combined with diabetes. This response was reflected by the marked increase in serum AGE levels only in diabetic guinea pigs with TB. These by-products of chronic hyperglycemia, combined with oxidative stress, induce a proinflammatory response and may have contributed to the more severe inflammation and TB disease in guinea pigs with type 2 diabetes.53–55 A significant increase in MCP-1 expression in diabetic guinea pigs with TB correlated with the pattern of coalescing foci of tissue macrophages without granuloma formation in the spleen. Increased MCP-1 expression in patients with TB is a feature of pulmonary TB; however, the increased expression of this cytokine in diabetic guinea pigs may explain the more severe, miliary pattern of extrapulmonary spread to the spleen, which is prevalent in this species.56
It is generally accepted that a Th1-biased cytokine response is critical for protection during M. tuberculosis infection. Guinea pigs with type 2 diabetes displayed higher macrophage activation, an appropriate response to the increased production of IFN-γ. Despite this seemingly favorable response to M. tuberculosis infection, diabetic guinea pigs failed to limit bacterial growth. A balance between the ability to limit bacterial growth and the development of damaging inflammation during active TB may explain the increase in anti-inflammatory cytokine levels. The increased anti-inflammatory cytokine expression is likely a reciprocal response intended to prevent host injury from the exacerbated innate response to M. tuberculosis infection seen in diabetic guinea pigs. In this study, the increase in IL-10 occurred despite the counterregulatory effects of high IFN-γ expression. These findings suggest possible IFN-γ–mediated induction of IL-10, as has been previously described in association with a high M. tuberculosis burden.57,58 Previously, delayed onset of IFN-γ production in response to M. tuberculosis infection was shown in STZ-treated mice with hyperglycemia, which also fail to control bacterial growth.8 The contribution of rapid disease progression before onset of adaptive immunity to M. tuberculosis in diabetic patients needs to be determined in future studies focusing on the very early stages of diabetes-TB comorbidity in guinea pigs. Previous studies have shown that the accumulation of regulatory T cells expressing FoxP3 during M. tuberculosis infection parallels the activation of a Th1-biased adaptive immune response and, along with the Th2 cytokine IL-4, is thought to impair a productive Th1 lymphocyte response.59 However, in this study, no differences were noted in FoxP3 or IL-4 expression, suggesting that the overexpression of immunosuppressive T-cell subsets is not a major determinant in the increased severity of TB in guinea pigs with type 2 diabetes.
From this and other studies investigating diabetes-TB comorbidity, it is evident that the diabetic state impairs M. tuberculosis host defenses, resulting in uncontrolled bacterial growth. In addition, altered cytokine expression and more severe pulmonary pathologic abnormalities indicative of a proinflammatory response to M. tuberculosis infection are consistent with features described in diabetes-TB comorbidity of humans. Moreover, these studies demonstrate that M. tuberculosis infection alone is associated with altered host lipid and glucose metabolism, which reveals the possibility of using antiglycemic drugs as an adjunct with antimicrobial drugs to treat TB. Because of the shared features of M. tuberculosis infection in diabetic patients and guinea pigs, this model will be valuable in testing new TB treatments and diabetes control strategies when both diseases occur together.
Footnotes
Supported by American Diabetes Association grant 1-11-BS-08 (R.J.B.) and NIH grants 1R21AI094123-01 (R.J.B.), 5T32RR007072-10 (B.K.P.), and 1DP2OD006450 (D.J.O.).
Disclosures: None declared.
References
- 1.Maurice J. WHO framework targets tuberculosis-diabetes link. Lancet. 2011;378:1209–1210. doi: 10.1016/s0140-6736(11)61527-4. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson C.R., Critchley J.A., Forouhi N.G., Roglic G., Williams B.G., Dye C., Unwin N.C. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. 2007;3:228–245. doi: 10.1177/1742395307081502. [DOI] [PubMed] [Google Scholar]
- 3.Guariguata L. By the numbers: new estimates from the IDF Diabetes Atlas Update for 2012. Diabetes Res Clin Pract. 2012;98:524–525. doi: 10.1016/j.diabres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Leung C.C., Lam T.H., Chan W.M., Yew W.W., Ho K.S., Leung G.M., Law W.S., Tam C.M., Chan C.K., Chang K.C. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–1494. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 6.Jeon C.Y., Murray M.B., Baker M.A. Managing tuberculosis in patients with diabetes mellitus: why we care and what we know. Expert Rev Anti Infect Ther. 2012;10:863–868. doi: 10.1586/eri.12.75. [DOI] [PubMed] [Google Scholar]
- 7.Martens G.W., Arikan M.C., Lee J., Ren F., Greiner D., Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallerskog T., Martens G.W., Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez M.L., Volek J.S. Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab (Lond) 2006;3:17. doi: 10.1186/1743-7075-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padilla-Carlin D.J., McMurray D.N., Hickey A.J. The guinea pig as a model of infectious diseases. Comp Med. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmadhikari A.S., Basaraba R.J., Van Der Walt M.L., Weyer K., Mphahlele M., Venter K., Jensen P.A., First M.W., Parsons S., McMurray D.N., Orme I.M., Nardell E.A. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011;91:329–338. doi: 10.1016/j.tube.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dooley K.E., Tang T., Golub J.E., Dorman S.E., Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–639. [PMC free article] [PubMed] [Google Scholar]
- 13.Restrepo B.I., Fisher-Hoch S.P., Smith B., Jeon S., Rahbar M.H., McCormick J.B. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79:541–544. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C.S., Yang C.J., Chen H.C., Chuang S.H., Chong I.W., Hwang J.J., Huang M.S. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–210. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Guzman C., Torres-Cruz A., Villarreal-Velarde H., Salazar-Lezama M.A., Vargas M.H. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis. 2001;5:455–461. [PubMed] [Google Scholar]
- 16.Wang J.Y., Lee L.N., Hsueh P.R. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–783. [PubMed] [Google Scholar]
- 17.Long R., O'Connor R., Palayew M., Hershfield E., Manfreda J. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinical-pathologic-radiologic correlation. Int J Tuberc Lung Dis. 1997;1:52–58. [PubMed] [Google Scholar]
- 18.Young F., Wotton C.J., Critchley J.A., Unwin N.C., Goldacre M.J. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66:519–523. doi: 10.1136/jech.2010.114595. [DOI] [PubMed] [Google Scholar]
- 19.Restrepo B.I., Fisher-Hoch S.P., Pino P.A., Salinas A., Rahbar M.H., Mora F., Cortes-Penfield N., McCormick J.B. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed M.J., Meszaros K., Entes L.J., Claypool M.D., Pinkett J.G., Gadbois T.M., Reaven G.M. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 21.de la Garza-Rodea A.S., Knaan-Shanzer S., den Hartigh J.D., Verhaegen A.P., van Bekkum D.W. Anomer-equilibrated streptozotocin solution for the induction of experimental diabetes in mice (Mus musculus) J Am Assoc Lab Anim Sci. 2010;49:40–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Nakadate T., Nakaki T., Muraki T., Kato R. Adrenergic receptors and the onset of streptozotocin-induced diabetes in mice. Eur J Pharmacol. 1981;75:45–51. doi: 10.1016/0014-2999(81)90343-5. [DOI] [PubMed] [Google Scholar]
- 23.Podell B.K., Ackart D.F., Kirk N.M., Eck S.P., Bell C., Basaraba R.J. Non-diabetic hyperglycemia exacerbates disease severity in Mycobacterium tuberculosis infected guinea pigs. PLoS One. 2012;7:e46824. doi: 10.1371/journal.pone.0046824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ordway D.J., Shanley C.A., Caraway M.L., Orme E.A., Bucy D.S., Hascall-Dove L., Henao-Tamayo M., Harton M.R., Shang S., Ackart D., Kraft S.L., Lenaerts A.J., Basaraba R.J., Orme I.M. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob Agents Chemother. 2010;54:1820–1833. doi: 10.1128/AAC.01521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen S.S., McMurray D.N. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71:4271–4277. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H., McMurray D.N. Recombinant guinea pig TNF-α enhances antigen-specific type 1 T lymphocyte activation in guinea pig splenocytes. Tuberculosis (Edinb) 2007;87:87–93. doi: 10.1016/j.tube.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Shang S., Harton M., Tamayo M.H., Shanley C., Palanisamy G.S., Caraway M., Chan E.D., Basaraba R.J., Orme I.M., Ordway D.J. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 2011;91:378–385. doi: 10.1016/j.tube.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flicek P., Amode M.R., Barrell D., Beal K., Brent S., Carvalho-Silva D. Ensembl 2012. Nucleic Acids Res. 2012;40:D84–D90. doi: 10.1093/nar/gkr991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 30.Jeevan A., Yoshimura T., Ly L.H., Dirisala V.R., McMurray D.N. Cloning of guinea pig IL-4: reduced IL-4 mRNA after vaccination or Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2011;91:47–56. doi: 10.1016/j.tube.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Takizawa M., Chiba J., Haga S., Asano T., Yamazaki T., Yamamoto N., Honda M. Novel two-parameter flow cytometry (MIL4/SSC followed by MIL4/CT7) allows for identification of five fractions of guinea pig leukocytes in peripheral blood and lymphoid organs. J Immunol Methods. 2006;311:47–56. doi: 10.1016/j.jim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Ordway D., Palanisamy G., Henao-Tamayo M., Smith E.E., Shanley C., Orme I.M., Basaraba R.J. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J Immunol. 2007;179:2532–2541. doi: 10.4049/jimmunol.179.4.2532. [DOI] [PubMed] [Google Scholar]
- 33.Standards of medical care in diabetes: 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi C., Miccoli R., Trombetta M., Giorgino F., Frontoni S., Faloia E., Marchesini G., Dolci M.A., Cavalot F., Cavallo G., Leonetti F., Bonadonna R.C., Del Prato S., GENFIEV Investigators Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired β-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. J Clin Endocrinol Metab. 2013;98:2100–2105. doi: 10.1210/jc.2012-3971. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin T., Abbasi F., Cheal K., Chu J., Lamendola C., Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 36.Tabak A.G., Herder C., Rathmann W., Brunner E.J., Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borst S.E. The role of TNF-α in insulin resistance. Endocrine. 2004;23:177–182. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- 38.Dhar A., Castillo L. Insulin resistance in critical illness. Curr Opin Pediatr. 2011;23:269–274. doi: 10.1097/MOP.0b013e3283464b3e. [DOI] [PubMed] [Google Scholar]
- 39.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulbas Z., Erdogan Y., Balci S. Impaired glucose tolerance in pulmonary tuberculosis. Eur J Respir Dis. 1987;71:345–347. [PubMed] [Google Scholar]
- 41.Kapur A., Harries A.D. The double burden of diabetes and tuberculosis: public health implications. Diabetes Res Clin Pract. 2013;101:10–19. doi: 10.1016/j.diabres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Mugusi F., Swai A.B., Alberti K.G., McLarty D.G. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle. 1990;71:271–276. doi: 10.1016/0041-3879(90)90040-f. [DOI] [PubMed] [Google Scholar]
- 43.Oluboyo P.O., Erasmus R.T. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990;71:135–138. doi: 10.1016/0041-3879(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.H., Langston A.A., Gallis H.A. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12:583–590. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 45.Eum S.Y., Kong J.H., Hong M.S., Lee Y.J., Kim J.H., Hwang S.H., Cho S.N., Via L.E., Barry C.E., 3rd Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker E.H., Clark N., Brennan A.L., Fisher D.A., Gyi K.M., Hodson M.E., Philips B.J., Baines D.L., Wood D.M. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol. 2007;102:1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 47.Pezzulo A.A., Gutierrez J., Duschner K.S., McConnell K.S., Taft P.J., Ernst S.E., Yahr T.L., Rahmouni K., Klesney-Tait J., Stoltz D.A., Zabner J. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One. 2011;6:e16166. doi: 10.1371/journal.pone.0016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viswanathan V., Kumpatla S., Aravindalochanan V., Rajan R., Chinnasamy C., Srinivasan R., Selvam J.M., Kapur A. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao F., Chen T., Zhao Y., Zhang C., Bai B., Zhao S., Xu Z., Shi C. Insulin resistance: a potential marker and risk factor for active tuberculosis? Med Hypotheses. 2011;77:66–68. doi: 10.1016/j.mehy.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Kumar N.P., Sridhar R., Banurekha V.V., Jawahar M.S., Nutman T.B., Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–748. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nandi B., Behar S.M. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torrado E., Cooper A.M. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasheed Z., Akhtar N., Haqqi T.M. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford) 2011;50:838–851. doi: 10.1093/rheumatology/keq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan S.F., Ramasamy R., Schmidt A.M. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 55.Yeh C.L., Hu Y.M., Liu J.J., Chen W.J., Yeh S.L. Effects of supplemental dietary arginine on the exogenous advanced glycosylation end product-induced interleukin-23/interleukin-17 immune response in rats. Nutrition. 2012;28:1063–1067. doi: 10.1016/j.nut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Sterling T.R., Martire T., de Almeida A.S., Ding L., Greenberg D.E., Moreira L.A., Elloumi H., Torres A.P., Sant'Anna C.C., Calazans E., Paraguassu G., Gebretsadik T., Shintani A., Miller K., Kritski A., Lapa e Silva J.R., Holland S.M. Immune function in young children with previous pulmonary or miliary/meningeal tuberculosis and impact of BCG vaccination. Pediatrics. 2007;120:e912–e921. doi: 10.1542/peds.2006-3150. [DOI] [PubMed] [Google Scholar]
- 57.Tadokera R., Meintjes G., Skolimowska K.H., Wilkinson K.A., Matthews K., Seldon R., Chegou N.N., Maartens G., Rangaka M.X., Rebe K., Walzl G., Wilkinson R.J. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu K., Koo J., Jiang X., Chen R., Cohen S.N., Nathan C. Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R. PLoS One. 2012;7:e30512. doi: 10.1371/journal.pone.0030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torrado E., Robinson R.T., Cooper A.M. Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol. 2011;32:66–72. doi: 10.1016/j.it.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]