Abstract
Tumor cells secrete factors that stimulate the migration of peripheral blood leukocytes and enhance tumor progression by affecting angiogenesis. In these studies, we investigated the effect of morphine, a known immunosuppressant, on leukocyte migration and recruitment to conditioned media derived from long-term cultures of mouse Lewis lung carcinoma cells. Our results indicate that morphine treatment reduced the migration and recruitment of tumor-infiltrating leukocytes into Matrigel plugs and polyvinyl alcohol sponges containing conditioned media derived from long-term cultures of mouse Lewis lung carcinoma cells when compared with placebo. A reciprocal increase in peripheral blood leukocytes was observed at the time of plug or sponge removal in morphine-treated mice. Decreased angiogenesis was observed in conditioned media derived from long-term cultures of mouse Lewis lung carcinoma cells Matrigel plugs taken from morphine-treated wild-type mice when compared with placebo but was abolished in morphine-treated μ-opioid receptor knockout mice. In addition, in vitro studies using trans-well and electric cell substrate impedance sensing system studies reveal for the first time morphine's inhibitory effects on leukocyte migration and their ability to transmigrate across an activated endothelial monolayer. Taken together, these studies indicate that morphine treatment can potentially decrease leukocyte transendothelial migration and reduce angiogenesis associated with tumor growth. The use of morphine for cancer pain management may be beneficial through its effects on angiogenesis.
Morphine has been used since ancient times for chronic pain. Today morphine is still used clinically for chronic pain associated with cancer growth and treatments with few alternative options. Understanding the contribution of morphine to cancer growth is still an important question because existing reports conflict.1,2 Underlying mechanisms associated with cancer cell initiation and progression to tumor formation are complex and often patient specific. However, one common event is angiogenesis, which is often driven by inflammation and is an important part of tumor progression, even in tumors of different cellular origins. For decades scientists have recognized leukocyte contribution to tumor growth. Both microbial and virus-initiated cancers have some level of leukocyte infiltration, and this is correlated with an increased incidence of tumor formation.3–6
During the early stages of solid tumor growth, polymorphonuclear cells, neutrophils, monocytes, macrophages, dendritic cells, and T lymphocytes infiltrate the tumor microenvironment.7,8 Initially leukocytes eradicate tumor cells but eventually contribute to blood vessel formation and maintenance.8 Tumor-derived leukocytes often display an immunosuppressive phenotype and limit the activation of effective T-cell tumor–killing responses.9 Chemokines and proangiogenic factors secreted from hypoxic, stressed, and dying tumor cells and surrounding stromal cells attract leukocytes that accumulate within many solid tumor microenvironments. Once recruited in the hypoxic tumor, these tumor-promoting leukocytes secrete vascular endothelial growth factor (VEGF), and VEGF secreted from hypoxic tumor and stromal cells display chemotactic activity for monocytes expressing VEGF receptor 1.10 Recruited leukocytes support tumor growth by secreting additional proangiogenic factors, matrix-remodeling enzymes, and chemotactic factors that further sustain leukocyte recruitment and maintain angiogenesis. On stimulation leukocytes release preformed intracellular granules that contain VEGF, which further enhance endothelial cell proliferation and tube formation within solid tumors.8 Cytokines, such as tumor necrosis factor (TNF)–α and IL-1, stimulate the expression of cell adhesion molecules on tumor-infiltrating leukocytes to increase the adhesiveness between leukocytes and the endothelium for transendothelial migration (TEM).11 Various leukocytes express integrin β2 (CD18) that combines with numerous other proteins to form different integrin complexes capable of engaging with specific intercellular adhesion molecules [intercellular adhesion molecule (ICAM) 1, 2, and 3]. ICAMs can be found clustered on activated endothelial cells exposed to proinflammatory stimulus and participate in leukocyte endothelial transmigration.11 Chronic inflammation leads to increased production of proinflammatory mediators that further stimulate and enhance inflammatory cell migration and tumor angiogenesis, promoting tumor cell proliferation and survival, invasion, and metastasis.12
Immune-modulating agents, such as morphine, are well known to alter leukocyte behavior. These alterations include effects on monocyte-mediated phagocytosis and activation, neutrophil and monocyte chemotaxis, macrophage activation, mitogen-induced proliferation, antimicrobial resistance, and antibody production.13 Morphine inhibits neutrophil chemotaxis to serum activated with Escherichia coli, casein, monocyte inflammatory protein 1α, and IL-8.14,15 In a cohort of opioid treatment–naive cancer patients, plasma concentration of monocyte inflammatory protein 1α significantly decreased during morphine treatment compared with baseline.16 Studies with mouse, monkey, and carp leukocytes revealed that morphine impairs leukocyte migration.17–19 In previous studies, we reported that morphine, through the μ-opioid receptor, inhibited tumor cell–induced angiogenesis in mice. We proposed that morphine suppressed hypoxia-induced tumor cell expression of VEGF, leading to a significantly reduced angiogenesis and tumor cell survival.20 The purpose of this study was to investigate the migration and recruitment of tumor-infiltrating leukocytes after morphine treatment and their contribution to morphine-mediated inhibition of tumor growth and angiogenesis. Strategies to target inflammatory cells and inhibit inflammation-associated angiogenesis may prove to be effective to reduce tumor growth and progression.
Materials and Methods
Animals, Cell Lines, and Reagents
Luciferase (Luc) mice expressing FVB background were crossed with μ-opioid receptor knockout (MORKO) mice (C57Bl/6 × 129 Ola background). Male or female C57BL/6 F2 wild-type (WT) mice were used as recipients (bred at the University of Minnesota specific pathogen free environment animal facility). In all experiments, animals were sex and aged matched (8 to 21 weeks). All experimental protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Conditioned media (CM) from spontaneous, mouse-derived Lewis lung carcinoma (LLC) cells or human ovarian cancer cell line (MA148-CM) was collected by growing cells in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS) and 1% Penn-Strep until confluence. CM was frozen in aliquots for use in subsequent experiments. Human umbilical vein endothelial cells (HUVECs; Lonza Group Ltd., Basel, Switzerland) and human microvascular endothelial cells (huMVECs; derived from human foreskin) were cultured in endothelial cell growth media (Lonza). Human PLB985 leukocytes are from a human myelomonocytic cell line and were cultured in RPMI 1640 medium supplemented with 5% FBS and 1% Penn-Strep.
Drug Administration
In all in vivo experiments, mice were administered either 75 mg of slow-release morphine or placebo pellet implanted s.c. (National Institute on Drug Abuse, National Institutes of Health) under ketamine and xylazine anesthesia.
LLC Tumor Growth Assay
Tumor growth assay was performed as previously described.20 Data are from one representative experiment from two independent experiments (n = 5 per group). Two to four sections of individual frozen tumors were acetone fixed, rehydrated, and blocked in 5% bovine serum albumin (BSA) at room temperature for 1 hour. Slides were then washed and stained at 4°C overnight with the fluorescently labeled primary antibodies [fluorescein isothiocyanate (FITC) for Gr1 and phycoerythrin (PE) for F4/80] in 1% BSA. Sections were counterstained with DAPI (Sigma, St. Louis, MO) before image capturing (Nikon, Chiyoda, Tokyo). Paraffin-embedded sections was heated at 65°C for 30 minutes, incubated in xylene (10 minutes, 2 times) followed by ethanol (5 minutes each at 100%, 80%, and 70%), and rehydrated (10 minutes and 5 minutes). A 1× universal retrieval antigen (R&D Systems, Minneapolis, MN) was applied to slides and heated (15 minutes). Cooled slides were preincubated at room temperature in 1% horse serum and 1% BSA (30 minutes at room temperature) and then primary antibodies (CD31, ICAM-1) resuspended in incubation buffer (1% horse serum, 1% BSA, and 0.03% Triton X-100) overnight at 4°C.
VEGF- and LLC-CM–Induced Matrigel Plug Assays
Matrigel plug assay was performed as previously described20 with few modifications using either 1:4 LLC-CM to Matrigel (n = 5 mice per group) (BD Biosciences, San Jose, CA) or 100 ng/mL of VEGF admixed in Matrigel (n = 4 mice per group). After 7 days, plugs were removed, photographed before fixation, sectioned, and stained for H&E (Fairview Riverside Pathology Services, Minneapolis, MN). Hemoglobin levels determined as a measure of angiogenesis using Drabkin's reagent (Sigma) (BMG Labtech FLUOstar Plate Reader, Cary, NC). Plug-infiltrated cells were identified by flow cytometry and immunohistochemistry using fluorescently labeled antibodies (F4/80, Gr1, Ly6C, Tie2, CD34, CD45, Ly6G). Peripheral blood was collected through cardiac puncture and red blood cells lysis. White blood cells were fixed, stained, and analyzed using flow cytometry (GUAVA xEasyCyte Cell Analysis Flow Cytometer, Millipore, Billerica, MA). CD31-morphometric analysis for blood vessel density was performed as described.20
LLC-CM–Induced PVA Sponge Assay for Leukocyte Migration
Polyvinyl alcohol (PVA) sponge assay was performed as previously described.23 WT Luc-negative (B6129PF1), positive (FVB), and MORKO Luc-positive (C57Bl/6Jx129/Ola) mice were used in these experiments. MORKO Luc-positive mice were crossed and bred in our laboratory (8 to 20 weeks). Bioluminescent images were captured after administration of 50 μL of 15 mg/mL of d-luciferin at sponge site using an IVIS Xenogen whole-body imaging system (University Imaging Center; University of Minnesota, Minneapolis, MN). Relative luminescence units of recruited sponge cells (days 1 and 5) were determined using a luminometer (Turner Designs, Sunnyvale, CA). Data are expressed as fold change relative to placebo-treated mice. The phenotype of PVA sponge-infiltrating cells was determined using flow cytometry. Data expressed as percentage of positive cells in total events were analyzed (n = 2).
Adoptive Transfer Tumor Cell–Induced Leukocyte Migration Assay
WT or MORKO mice were used as hosts or recipients. Donor bone marrow (BM) was isolated from drug-treated WT and MORKO Luc-expressing mice. At a ratio of one donor to two recipients, BM cells were injected into lateral tail veins 3 hours after pellet and PVA-LLC-CM sponge implantation. These experiments contained three to five mice per group. Eighteen hours after BM injections, the relative cell recruitment was quantified using bioluminescence (relative luminescence units).
TEM Using the ECIS System
To further investigate the effects of morphine on leukocyte-endothelial transmigration, we used the electric cell substrate impedance sensing (ECIS; Applied Biophysics, Troy, NY) system that monitors changes in transcellular resistance (TER). To assess the effect of morphine on the endothelial compartment, HUVECs were added to 8W10E and ECIS arrays (gelatin precoated). HUVEC monolayers were morphine pretreated (1.0 μmol/L for 16 hours) before interaction with naive huPLB985 cells and changes in TER monitored. To assess the effect of morphine on the leukocyte, huMVEC monolayers were pretreated with TNF-α and changes in TER monitored on interaction with saline- and morphine-pretreated huPLB985 cells (1.0 μm overnight).
Cell Migration Using Transwell Assay
To further investigate the effect of morphine on leukocyte migration in vitro, we used huPLB985 cells and tested chemotatic migration toward MA148-CM. Approximately, 1 × 106 cells/mL were pretreated with 1.0 μmol/L saline or morphine overnight in 5% FBS and RPMI 1640 medium. The next day, all cells were collected and resuspended in 1 mL of 5% FBS and RPMI 1640 medium and labeled with a 1 mmol/L carboxyfluorescein diacetate solution (Invitrogen, Carlsbad, CA) at 37°C for 10 minutes. Labeled cells were added to the top wells of collagen-coated 3.0-μm pore inserts (Becton Dickinson, Minneapolis, MN) at 5 × 105 cells/mL. Cells were allowed to migrate to bottom wells that contained 5% FBS and RPMI 1640 medium alone or a mix that contained 25% MA148 CM and 5% FBS and RPMI 1640 medium in a 37°C, 5% CO2 humidified incubator. After 6 hours, cells that had migrated to the bottom wells were collected and spun down. Supernatants were aspirated and cell pellets were resuspended in 100 μL of RPMI 1640 medium and placed into 96-well black fluorescent plates and fluorescence read at a 560-nm wavelength. Data are expressed as the percent change from RPMI 1640 medium control. To further evaluate the role of morphine on leukocyte migration, human blood was purchased from the Red Cross (Minneapolis, MN) and granulocytes separated using Histopaque-1077 as suggested by manufacturers (Sigma-Aldrich, St. Louis, MO). Cells were counted and incubated for 16 hours with 1.0 μmol/L saline or morphine before the migration assay as described. Data are shown as percent change in migration compared with 5% FBS and RPMI 1640 medium for each treatment. Data are shown from four different donors.
Cell Adhesion Using the ECIS System
To test the ability of BM cells to attach to fibronectin, 10 mg/mL of fibronectin solution was diluted in water (1:10) to coat ECIS/8W10E and plates at room temperature for 15 minutes. Freshly isolated mouse BM cells were pretreated overnight with 1.0 μmol/L saline or morphine. Approximately 5 × 105 saline- or morphine -treated mouse BM cells were plated into each well containing either 5% FBS and RPMI 1640 medium alone or 50% to 5% FBS and RPMI 1640 medium and 50% LLC mixture. The arrays were placed in the ECIS instrument and the resistance values collected under multiple frequency and time intervals settings for 24 hours. The graph data depict relative resistance derived from raw resistance values in 5% FBS and RPMI 1640 medium alone subtracted from 50% LLC resistance values.
Modified Under Agar Migration Using the ECIS System
To further assess the effect of morphine on cell migration, we performed a modified under agar assay in 8WCP plates as previously described.21 Briefly, a 0.5% final agarose concentration solution was made using 1 mL of a 3% agarose HBSS solution, 5 mL of prewarmed RPMI 1640 medium supplemented with 10% FBS, 40 mmol/L HEPES, and 0.075% sodium bicarbonate. ECIS/8WCP plates were pretreated with 10 mmol/L cysteine and water for 15 minutes at room temperature. Approximately 250 μL of the 0.5% agarose solution was added and allowed to solidify slowly at room temperature. Once solidified, gel was placed at 4°C for 15 minutes, and a 2-mm biopsy punch was used to make wells on each side, equidistant from the electrode. Agarose plugs were removed through low aspiration using a Pasteur pipette attached to a vacuum line. Approximately 15 μL containing 1 × 106 mouse BM cells pretreated overnight with 1.0 μmol/L saline or morphine was added to wells opposite that containing 5% FBS and RPMI 1640 medium or LLC-CM. The array was placed in the ECIS instrument and the resistance values collected under multiple frequency and time intervals settings. The graph data depict the relative resistance derived from raw resistance values collected (and after export into Microsoft Excel for analysis). Relative resistance values were derived by subtracting actual resistance recorded values at each time point for saline-treated BM cell migration (basal migration) toward 5% FBS and RPMI 1640 medium from saline-treated BM cell migration toward LLC-CM. Similarly, relative resistance values for morphine-treated BM cells were derived from subtracting 5% FBS and RPMI 1640 medium actual resistance recorded values from morphine-treated BM cell migration toward LLC-CM.
Adhesion Molecule Expression
To assess whether morphine altered the expression of adhesion molecules involved in transmigration, we treated naive huPLB985 cells with 1.0 μmol/L saline or morphine overnight. Pretreated cells were then incubated with 50 to 100 ng/mL of TNF-α and MA148-CM for 15 minutes to 24 hours. Cells were then fixed at various time points and stained with anti-human CD18 (β2) antibodies. HUVECs were grown in monolayers and then pretreated with 1.0 μmol/L saline or morphine overnight. Pretreated HUVECs were then treated with TNF-α or E. coli–derived lipopolysaccharide (LPS) for 4 hours. Cells were scraped and immediately fixed and then subsequently stained for vascular endothelial cadherin and ICAM-1. Antibody-stained cells were then r-suspended in PBS and azide and analyzed using a BD FACS Canto Flow Cytometer (Becton Dickinson). Data are expressed as a percentage of a fixed number of gated cells.
Statistical Analysis
Differences in mean values between the two groups were analyzed using the two-tailed Student's t-test. P < 0.05 was considered statistically significant.
Results
Morphine Reduces Leukocyte Migration in an LLC Solid Tumor Model
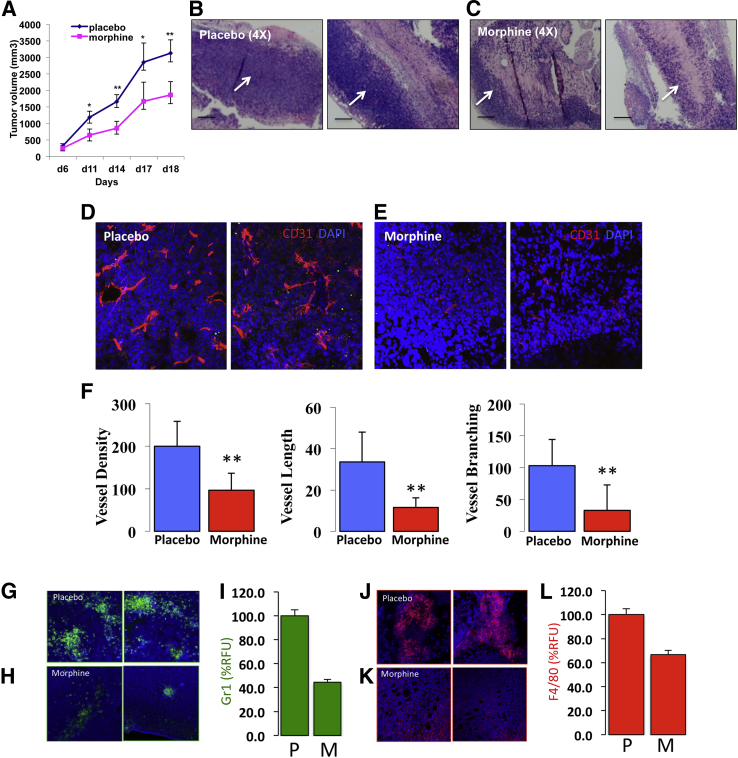
To study the effects of long-term morphine administration on tumor growth and angiogenesis, LLC cells were injected s.c. into placebo- or morphine-treated athymic mice. Tumor volume was recorded as a measure of tumor progression and tumor mass removed after 21 days of s.c. implantation. During the 21-day period, mice received supplemental doses of saline or morphine i.p. to prevent withdrawal and maintain morphine at analgesic levels. Morphine treatment decreased tumor growth progression when compared with placebo (Figure 1A, P < 0.05). Representative H&E-stained tumor sections taken from different placebo-treated mice (Figure 1B) showed successful tumor growth as indicated by an increased cell density, unlike tumors taken from morphine-treated mice (Figure 1C). Tumor sections were further quantified for angiogenesis using anti-CD31 (red) counterstained with nuclear DAPI stain (blue). As shown in Figure 1D, sections taken from individual placebo-treated mice showed more intense CD31 staining and developed vascular network compared with sections analyzed from morphine-treated mice (Figure 1E). Morphometric analysis using reindeer plug-in functions for Adobe PhotoShop (Adobe Systems Inc., San Jose, CA) confirmed that morphine treatment reduced the overall number of vessels formed within the solid tumors (vessel density), the average vessel length, and branching points (Figure 1F). To assess leukocyte infiltration, frozen tumor sections were grossly examined for the presence of leukocytes using fluorescently labeled antibodies against Gr1+ neutrophils (green) and F4/80+ macrophages (red). Figure 1, G, H, J, and K, shows fluorescent captured images showing DAPI nucleus (blue) merged with corresponding Gr1+ neutrophils (green) and F4/80+ macrophages (red), respectively. Gr1+ neutrophil and F4/80+ macrophage clusters were easily detected within tumor sections taken from placebo-treated mice (Figure 1, G and J). In contrast, sections from morphine-treated mice (Figure 1, H and K) displayed a marked reduction in Gr1+ neutrophil (Figure 1I; P < 0.011) and F4/80+ macrophage (Figure 1L; P < 0.025) infiltration, respectively. To further assess whether increased leukocyte infiltration coincided with increased angiogenesis, tumor sections were double stained for the presence of Gr1+ leukocytes and CD31+ endothelial cells. As shown in Supplemental Figure S1A, tumor sections from placebo-treated mice showed intense CD31 blood vessel (red) staining in close approximation to Gr1+ leukocyte clusters. In contrast, morphine tumor sections had reduced angiogenesis with fewer and smaller Gr1+ leukocyte clusters (Supplemental Figure S1B). These results suggest that morphine suppressed leukocyte infiltration, which may have contributed to the decrease in tumor growth.
Figure 1.
Effect of morphine on leukocyte infiltration into a solid tumor microenvironment. A: Graph shows morphine decreased tumor growth when compared with placebo treatment (n = 5 per group) observed during 21 days. H&E-stained LLC tumor sections taken from two distinct placebo-treated (B) and morphine-treated (C) mice on day 21. Fluorescent images show frozen tumor sections stained for CD31-positive endothelial cells (red) counterstained with DAPI [nuclear stain (blue)] from placebo-treated (D) and morphine-treated (E) mice. F: Morphometric analysis showing relative blood vessel density, length, and branching was determined using reindeer plug-in functions for Adobe Photoshop. The infiltration of Gr1+ neutrophils (green) from placebo-treated (G) and morphine-treated (H) mice are shown, as well as F4/80+ macrophages (red) from placebo (J) and morphine (K) merged with corresponding DAPI. Corresponding relative fluorescent units (RFU) between groups are shown (I) and (L). ∗P < 0.05, ∗∗P < 0.01 (A); P < 0.011 (I); P < 0.025 (L). Scale bar = 100 μm (B and C). Original magnification ×4 (B and C); ×20 (D and E); ×10 (G, H, J, and K).
Morphine Reduces Angiogenesis Induced by LLC-CM
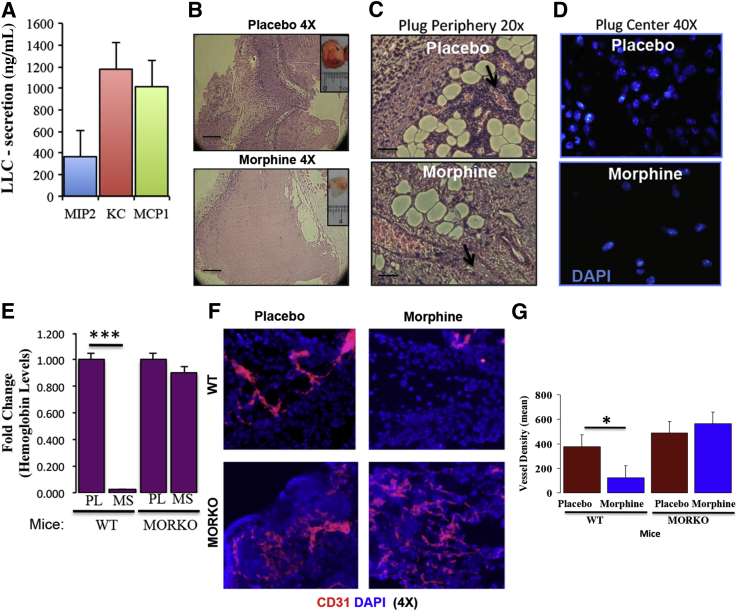
To study the effect of leukocyte infiltration on angiogenesis, we next performed a modified Matrigel plug angiogenesis assay in immune competent mice. Because hypoxic tumor cells secrete numerous chemotactic factors that can attract leukocytes into the tumor sites, we screened LLC cells for monocyte inflammatory protein 2, keratinocyte chemoattractant, and monocyte inflammatory protein 1 in cell culture supernatants. Indeed, ELISA confirmed that LLC cells secrete these proteins (Figure 2A). We therefore admixed LLC-CM into Matrigel and injected equal volumes s.c. in placebo- and morphine-pretreated WT mice. Analysis of H&E-stained plug sections from placebo-treated mice showed massive infiltration of leukocytes in response to tumor-derived chemokines (Figure 2B), suggesting that CM alone was sufficient to recruit host cells. In contrast to placebo, morphine treatment reduced host cell recruitment from blood vessels surrounding the Matrigel plugs (Figure 2C) and leukocyte infiltration into plug centers (Figure 2D). In subsequent experiments, hemoglobin levels were determined as a measure of angiogenesis, and morphine administration significantly decreased LLC-CM–induced angiogenesis (Figure 2E; P < 0.005) in vivo. To assess the involvement of μ-opioid receptors in morphine-induced inhibition of angiogenesis, similar experiments were performed in MORKO mice. Placebo- and morphine-treated MORKO mice had similar levels of LLC-CM–induced angiogenesis (Figure 2E). Because LLC-CM–induced angiogenesis was abolished in morphine-treated MORKO mice, this effect is likely mediated through a classical opioid receptor.
Figure 2.
A: Effect of morphine on leukocyte infiltration. LLC cells secrete chemotactic factors as determined using an ELISA. LLC-CM–induced leukocyte infiltration as seen in H&E-stained Matrigel plugs (inset) taken from placebo- and morphine-treated mice (B) (n = 5 per group); leukocyte accumulation near blood vessels (arrows) at the plug periphery (C) and cell nuclei using DAPI stain on sections showing plug centers (D). E: Graph shows relative hemoglobin levels of Matrigel plugs removed on day 7 and determined using a standard curve from WT and MORKO mice (n = 4 per group) implanted with either placebo or morphine. F: Fluorescent captured images of CD31-PE–stained (red) Matrigel plug (containing 100 ng/mL of VEGF) sections taken from WT and MORKO placebo and morphine pellet implanted mice. G: Quantification of blood vessel density using morphometric analysis. ∗P < 0.05; ∗∗∗P < 0.005. Scale bars: 100 μm (B); 20 μm (C and D). Original magnification ×4 (B); ×20 (C and D).
Morphine-Reduces VEGF Induced Angiogenesis
To further assess immune cell contribution to angiogenesis, we tested the effect of morphine on VEGF-induced angiogenesis using a modified Matrigel plug assay in immune competent mice. Matrigel admixed with recombinant VEGF (100 ng/mL) was injected s.c. in WT and MORKO drug pretreated mice (7 days). VEGF-plug sections from placebo-WT mice stained with anti-CD31 (red) revealed successful angiogenesis (Figure 2F). These studies also found that when compared with placebo morphine treatment decreased vessel formation within VEGF Matrigel plugs from WT mice (P < 0.0012; Figure 2G). Unlike WT mice, morphine treatment of MORKO mice did not affect overall vessel density because levels were comparable to placebo-treated WT mice (Figure 2, F and G). H&E-stained VEGF-plug sections from morphine-treated WT mice had reduced leukocyte infiltration (Supplemental Figure S2C) when compared with placebo (Supplemental Figure S2A). Infiltrated cells were identified as monocyte-macrophages [F4/80 (red) and MAC3 (green)] and endothelial cells [CD31 (red)] (Supplemental Figure S2, B, D, and E–H). These results suggest that morphine disrupts VEGF-induced angiogenesis of infiltrating leukocytes to reduce tumor growth in mice.
Morphine Reduces LLC-CM–Induced Leukocyte Migration in a PVA Sponge Assay in Mice
To investigate the effect of morphine on leukocyte migration, we first compared the recruitment of BM-derived myeloid cells expressing Luc+BM into inert PVA sponges and characterized the recruited cells.
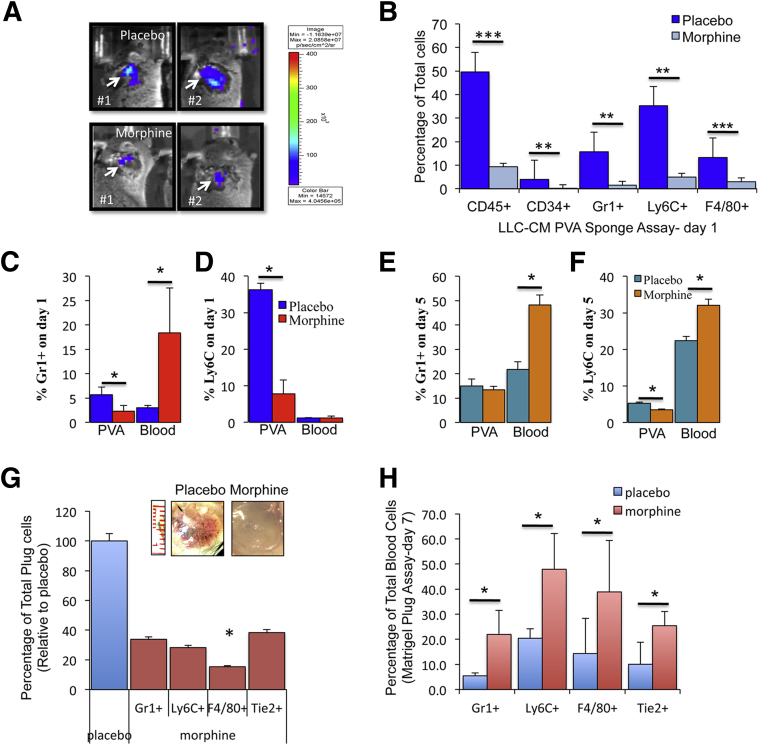
PVA sponges were presoaked with LLC-CM and implanted s.c. in WT mice preimplanted with either a placebo or morphine pellet. BM-Luc+ cells were injected i.p., and 24 hours later, mice were imaged after injection of luciferin substrate directly at the sponge site. Captured whole-body bioluminescent images showed successful recruitment of Luc+BM cells into LLC-CM PVA sponges from placebo-treated mice (Figure 3A); however, morphine-pretreated mice showed reduce bioluminescent signals (Figure 3A). Flow cytometry analysis of recruited cells, shown on day 1, recruited sponge cells consisted primarily of BM myeloid–derived cells (CD45+), undifferentiated progenitors or stem cells (CD34+), neutrophils (Gr1+), monocytes (Ly6C+), and macrophages (F4/80+). Sponges taken from morphine-treated mice had a significantly lower recruitment of CD45+ (P < 0.001), CD34+ (P < 0.03), Gr1+ (P < 0.017), Ly6C+ (P < 0.002), and F4/80+ (P < 0.001) Luc+BM cells (Figure 3B). These results clearly demonstrate that morphine decreased leukocyte migration and recruitment into the tumor microenvironment.
Figure 3.
Effect of morphine on leukocyte migration toward tumor-derived factors using a PVA sponge assay. A: Whole-body bioluminescent captured images showing infiltration of Luc-expressing BM cells (injected i.p.) into PVA-LLC-CM–containing sponges (arrow) from placebo (top row) and morphine pelleted mice (bottom row), n = 4 per group). B: Graph shows the percentage of total infiltrating leukocytes into PVA-LLC-CM sponges after 24 hours of implantation in placebo- and morphine-treated mice. Graphs show the relative percentages of Gr1+ neutrophils (C) and Ly6C+ monocytes (D) on day 1 and day 5 (E and F) from placebo- and morphine-treated mice (n = 5 per group). G: Graph shows the relative percentage of leukocytes that infiltrated LLC-CM Matrigel plugs (inset). H: The corresponding peripheral blood levels at the time of plug removal as determined using flow cytometry (n = 5 per group). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
To rule out the possibility that morphine was preventing leukocyte release from BM, we assessed leukocyte levels in peripheral blood collected at the time of LLC-CM sponge removal. As expected, there was reduced infiltration of Gr1+ neutrophils and granulocytes into sponges from morphine-treated mice (approximately 2.3%) when compared with placebo (approximately 5.7%) on day 1 (n = 4, P < 0.015, Figure 3C); however, there was a reciprocal increase in Gr1+ cells in peripheral blood from morphine-treated mice (approximately 18.4%). Our results also show levels of Gr1+ cells in peripheral blood was significantly greater after morphine when compared with placebo treatment (approximately 3.0%) (n = 4, P < 0.01, Figure 3D). By day 5 PVA sponges from placebo- and morphine-treated mice had similar levels of Gr1+ cells (P = 0.396, Figure 3E). However, peripheral blood Gr1+ cells was still significantly higher with morphine treatment when compared with placebo (P = 0.005, Figure 3F). LLC-CM recruited sponge cells had a granulocytic phenotype and expressed additional leukocyte markers—Ly6G [FITC (green)] and CXCR2 [PE (red)] (Supplemental Figure S2, I–K). Analysis of Ly6C+ monocytes revealed similar trends as samples from morphine-treated mice: reduced Ly6C+cell recruitment into LLC-CM sponges when compared with placebo on experimental day 1 and day 5 (Figure 3, D and F). On day 1, peripheral blood Ly6C+ monocyte levels revealed no difference with morphine treatment; however, on day 5 peripheral blood levels of Ly6C+ cells were significantly higher in morphine- than placebo-treated mice (P = 0.005, Figure 3F).
We further assessed leukocyte recruitment by admixing LLC-CM in Matrigel and injecting s.c. in drug-pretreated mice (7 days, Figure 3G). Relative to placebo, Matrigel plugs removed from morphine-treated mice had lower infiltrating neutrophils (Gr1+, approximately 35%), monocytes (Ly6C+, approximately 30%), and endothelial progenitors (Tie2+, approximately 40%) and significant reduction in macrophages (F480+, approximately 15%, P = 0.025, Figure 3G). Analysis of peripheral blood collected at the time of plug removal also revealed significantly greater circulating neutrophils (Gr1+, P = 0.0000001), monocytes (Ly6C+, P = 0.0000004), macrophages (F4/80+, P = 0.002), and endothelial progenitors (Tie2+, P = 0.0005) with morphine- compared with placebo-treated mice (n = 5; Figure 3H). The observed reduction in Matrigel plug leukocyte infiltration and reciprocal increase in peripheral blood levels in the presence of morphine suggest that morphine reduced leukocyte recruitment through defects in leukocyte transmigration.
Morphine Alters Leukocyte Transmigration in Vivo and in Vitro
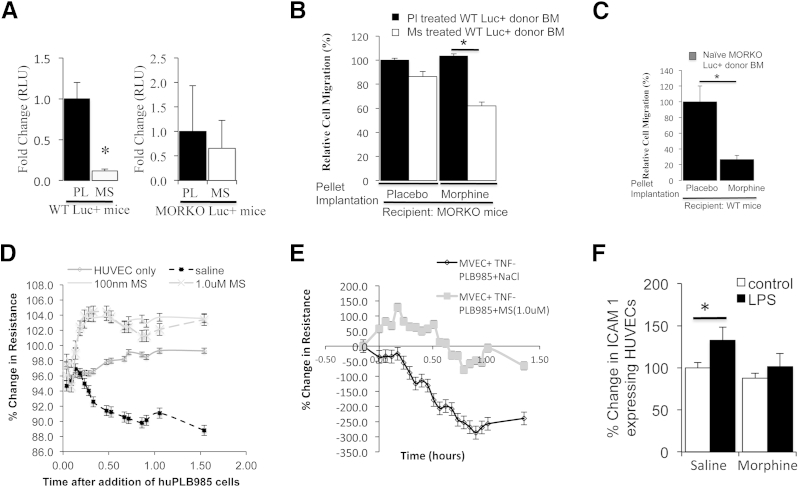
To further investigate the effect of morphine on leukocyte migration and the involvement of the μ-opioid receptors in morphine inhibition of leukocyte recruitment, we performed a LLC-CM sponge assay in WT and MORKO Luc-expressing mice (1 day). Morphine treatment reduced the recruitment of Luc+BM cells in WT mice when compared with placebo (P < 0.025) but had no significant effects in MORKO Luc+ mice (n = 4, Figure 4A).
Figure 4.
Effect of morphine on leukocyte migration into LCC-derived conditioned media–containing PVA sponge assay in mice. A: Graphs show the fold change in RLU. Luminescence was collected as a measure of Luc+BM infiltrating preimplanted LLC-CM-PVA sponges in WT and MORKO Luc-expressing mice (n = 4 per group) (∗P < 0.25). Graphs show the relative migration of WT Luc+BM cells isolated from placebo- and morphine-treated Luc-positive mice and injected via tail vein into non-Luc MORKO host mice (n = 4 per group) (∗P < 0.05) (B); the relative migration of naive MORKO Luc+BM cells in drug pretreated WT mice (n = 6 per group) (∗P < 0.005) (C); percent change in normalized TER from time on addition of naive human PLB985 cells to vehicle and morphine (1.0 μmol/L) pretreated HUVECs (D); and saline- or morphine-pretreated huPLB985 cells to human TNF-α (100 ng/mL every 2 hours)–pretreated huMVECs (E). LPS induces increase in ICAM-1 on HUVECs as determined using flow cytometry (∗P < 0.05) (F).
To test the possibility that morphine acted directly on the leukocytes to reduce trafficking into LLC-CM PVA sponges, placebo or morphine-pretreated WT Luc+BM cells were injected via tail vein into drug-pretreated MORKO recipients preimplanted with LLC-CM sponges. Our results indicate that morphine-pretreated WT Luc+BM donor cells, although not statistically significant, migrated to a lesser extent than placebo-treated WT Luc+BM donor cells in placebo-treated MORKO host mice (13.63%); this was further reduced when MORKO host mice were pretreated with morphine (41.4%, Figure 4B).
To evaluate the effect of morphine solely on the ability of the host endothelium to allow leukocyte transmigration, the infiltration of donor-derived MORKO Luc+BM cells into LLC-CM PVA sponges was compared in placebo and morphine-pretreated WT non-Luc recipient mice. Our results indicate that morphine significantly reduced the migration of MORKO BM-Luc+ donor cells in WT mice by 75% when compared with placebo treatment (P < 0.005, Figure 4C). Taken together, these results suggest that morphine's inhibition of leukocyte recruitment involves a classic opioid receptor and occurs as a result of its effects on the host endothelium.
To further evaluate the effect of morphine on TEM, we used the ECIS system that monitors changes in TER or TER in real time in vitro. HUVECs were grown on gelatin precoated ECIS arrays. As the HUVECs attached, the TER increased and plateaued at maximum spreading when cell-cell contact was achieved. At this point, naive huPLB985 cells, a monocytic granulocytic cell line, were added to vehicle or morphine-pretreated HUVECs. Compared with HUVEC-only wells, the addition of naive huPLB985 cells to saline-pretreated HUVECS resulted in a notable loss in TER, which was indicative of successful leukocyte-endothelial interaction in preparation for TEM. Unlike saline-pretreated HUVECs, 100 nmol/L to 1.0 μmol/L morphine pretreatment resulted in a notable increase in TER on addition of huPLB985 cells (Figure 4D).
To test the effect of morphine solely on the huPLB985 cells' ability to undergo TEM, huMVEC monolayers (at maximum TER) were first activated with 100 ng/mL of human TNF-α for 2 hours to mimic an inflammatory state. Saline- and morphine-pretreated huPLB985 cells were then added and the TER monitored over time. Saline-pretreated huPLB985 cells interacted successfully with the huMVEC monolayers to produce a notable decrease in TER (Figure 4E). Unlike saline, initially morphine-treated huPLB985s were unable to transmigrate as indicated by the increased TER generated by huMVECs during the initial interaction. Morphine-pretreated huPLB985 cells eventually transmigrated, but the loss in TER generated was not to the extent of that seen with saline-pretreated huPLB985 cells. These results support our in vivo observations and suggest that morphine can exert independent effects on leukocytes and endothelial cells to result in a net reduction in leukocyte TEM.
To further investigate the effects of morphine on transmigration, we assessed the expression of known leukocyte and endothelial cell adhesion molecules involved in leukocyte endothelial transmigration. To do this, HUVEC monolayers were pretreated with 1.0 μmol/L morphine for 18 hours before 100 ng/mL of LPS treatment (2 hours). HUVECs were analyzed for ICAM-1 and vascular endothelial cadherin expression using flow cytometry. LPS treatment of HUVECS in vitro increased ICAM-1 expression; however, this was reduced in the presence of morphine (Figure 4F). Additional examination of day 21 tumor sections revealed abundant ICAM-1 expression within CD31+ blood vessels at the tumor periphery. As shown previously, morphine treatment reduced CD31 staining, and ICAM-1 was weakly present in blood vessels at the tumor periphery (Supplemental Figure S2L). In addition, LPS and TNF-α treatment decreased vascular endothelial cadherin levels when compared with untreated controls (P = 0.0505 for LPS and P = 0.025 for TNF-α). In contrast, morphine pretreatment prevented the LPS and TNF- α reduction in vascular endothelial cadherin in HUVECS cultured in vitro (Supplemental Figure S3A, P ≤ 0.05). To assess cell adhesion molecules on leukocytes, saline- and morphine-pretreated huPLB985 cells were treated with 50 to 100 ng/mL of TNF-α for 24 to 48 hours. At 48 hours, 100 ng/mL of TNF-α was sufficient to increase the total percentage of cells expressing CD18 by 20.34% (P = 0.007). Interestingly, morphine pretreatment prevented the TNF-α–induced increase in CD18 expression (Supplemental Figure S3B, P = 0.0009). To further assess leukocyte infiltration, tumor sections were stained for CD18+ BM-derived myeloid cells using monoclonal antibodies against CD45-PE (red) and CD18-FITC (green) on frozen tumor sections from different experimental mice. CD45+/CD18+ double positive cells were easily identifiable in sections from individual mice treated with placebo (Supplemental Figure S3C). Unlike placebo, morphine treatment reduced the accumulation of CD45+/CD18+ leukocytes within the tumor microenvironment. These results suggest that morphine can potentially alter the expression of cell adhesion molecules, reducing the ability to slow or adhere firmly before TEM and thus reduce extravasation into the tumor site.
To further investigate the mechanisms associated with morphine's inhibition of leukocyte migration into the tumor microenvironment in vivo, we performed a series of in vitro chemotaxis and cell adhesion assays. Our results indicate that morphine treatment blunted the chemotactic migration of huPLB985 cells toward CM derived from a human ovarian cancer cell line, MA-148 (Supplemental Figure S4A). Experiments assessing the migration of human-derived peripheral blood granulocytes toward MA-148 CM revealed that in 3 of 4 donors morphine significantly reduced chemotaxis when compared with saline treatment (Supplemental Figure S4B, P < 0.05). To determine whether morphine altered leukocyte adhesion, we investigated the effect of morphine on the LLC-CM–induced adhesion of mouse BM cells to fibronectin (FN) in vitro. Our results indicate that morphine reduced LLC-CM–induced ability to adhere to FN in vitro when compared with saline (Supplemental Figure S4C, P < 0.05). Using The ECIS system, we performed a modified under agar assay to assess chemotactic migration that is dependent on adhesion to matrix substrates. In this assay, we found that saline-treated mouse BM cells were capable of migrating toward LLC-CM as indicated by the increased resistance generated as the cells adhered and migrated across the electrodes. In contrast, morphine-treated cells migrated, to a lesser extent, because resistance generated was lower than that of saline-treated cells (Supplemental Figure S4D). The data from these in vitro studies suggest that morphine decreased chemotactic migration and leukocyte adhesion to alter transmigration, further supporting our in vivo observations.
Discussion
Angiogenesis, the formation of new blood vessels within the growing tumor, occurs through the cooperative efforts of tumor- and myeloid-derived BM cells.8 In previous studies, we demonstrated that hypoxia-induced tumor cell expression of VEGF is altered in the presence of morphine treatment, resulting in decreased angiogenesis, tumor cell proliferation, and growth.20 Morphine, a known immunosuppressant, has profound effects on immune cell trafficking, cytokine production, and functionality.13 In this study, we found that in a s.c. LCC tumor model, long-term morphine administration reduced tumor growth and immune cell infiltration (Gr1+ neutrophils and F4/80+ macrophages). In addition, morphine-suppressed tumor cell CM induced host leukocyte recruitment and angiogenesis in WT but not MORKO mice. Because morphine's inhibition of leukocyte recruitment was not observed in MORKO mice, this suggests that this effect is likely mediated through classic opioid receptors.
Tumor cells secrete a host of factors, many of which attract immunosuppressive, BM-derived macrophage cells that contribute to angiogenesis within the developing tumor microenvironment. We report for the first time that long-term morphine administration disrupted the chemotactic migration of BM-derived macrophage cells with known roles in tumor progression and angiogenesis. These results are in agreement with previous studies assessing the effect of morphine on leukocyte migration in carp, mice, and monkeys.17–19 Studies analyzing the effect of morphine on BM-derived tumor-infiltrating leukocytes are scarce. We have identified infiltrating leukocytes as primarily BM-derived progenitors, granulocytes, monocytes, and macrophages. We also identified a subset of Tie-2 monocytes, an endothelial cell precursor shown to integrate into developing vasculature within the tumors.8 In support of these observations, Lam et al22 reported in an in vivo excisional wound model that long-term morphine administration reduced endothelial progenitors (identified as CD34+/CD133+) in circulation, leading to impaired angiogenesis in mice. Our results are consistent with their findings that morphine can also impair the mobilization of endothelial progenitors to suppress angiogenesis within a tumor microenvironment and an excisional wound model.20,23 Furthermore, adoptive transfer experiments examining donor WT leukocyte recruitment in MORKO host mice and donor MORKO leukocyte recruitment in WT host mice suggest that morphine exerts independent inhibitory effects on both the circulating leukocytes and host vascular endothelium to reduce leukocyte recruitment. In an attempt to understand why morphine decreased LLC-CM–induced BM cell recruitment in vivo, we assessed peripheral blood to rule out the possibility that morphine was preventing leukocyte release. Our results indicate that morphine treatment significantly suppressed leukocyte infiltration into the PVA sponges with a reciprocal increase in circulating leukocytes. These results suggested that the morphine-induced inhibition in leukocyte recruitment was a result of defects in transmigration and not due to defects in BM leukocyte release. Studies in mice show that inhibiting or depleting neutrophils reduces overall solid tumor growth and metastasis.24 Neutrophils secrete proangiogenic factors stored within granules.25,26 Experiments inhibiting neutrophil elastase resulted in reduced VEGF and platelet-derived growth factor release to inhibit tumor cell proliferation and cell invasion.27 The angiogenic response induced by IL-8 was found to be neutrophil dependent and was reversed on neutrophil depletion.28 Once recruited to the tumor site, polymorphonuclear leukocytes release chemoattractants that recruit more proinflammatory leukocytes.29 The continued recruitment of leukocytes generates a nonhealing wound. The possibility that morphine inhibited the initial neutrophil recruitment and therefore dampened the further recruitment of proinflammatory cytokines is plausible; however, more studies are necessary.
Morphine decreases the tight junction protein zonula occludens protein 1 expression in human brain–derived microvascular endothelial cells, and this may be one mechanism by which morphine alters vascular permeability.30 It is important for us to distinguish between passive and active migration of cells out of circulation and into tissues (extravasation). Leukocyte extravasation is a well-studied and tightly orchestrated process that involves leukocyte rolling, adhesion, tight binding, and transmigration in response to chemokine secretion, integrin and selectin activation, and interaction producing cytoplasmic changes for cell migration. Because morphine inhibits tight junction protein expression inducing permeability changes, one might predict that morphine should allow greater leukocyte infiltration. However, in our model we see a reduction in leukocyte extravasation, suggesting that morphine-induced changes in vascular permeability and leukocyte extravasation differ considerably and may operate independently. Morphine reduces embryonic kidney cell and tumor cell adhesion to extracellular matrix substrates.31,32 To investigate the mechanisms involved in morphine's inhibition of leukocyte trafficking from peripheral blood circulation into the tumor microenvironment in vivo, we performed a series of in vitro chemotaxis and cell adhesion assays. Morphine treatment blunted the migration and altered leukocyte adhesion and TEM, supporting our in vivo observations. Serum from human patients with myocardial infarction has high ICAM-1 and l-selectin levels that are reduced with morphine treatment.32 Similarly, other independent investigations have found an inhibitory effect of morphine on inflammation-induced ICAM-1, vascular cell adhesion molecule 1, and E-selectin using HUVECs as an in vitro model.33–35 To get a better understanding of the cellular mechanisms by which morphine altered leukocyte transmigration, we also assessed the effect of morphine on the expression of known leukocyte and endothelial cell adhesion molecules, critical for successful transmigration in vivo. Morphine alters the LPS (derived from E. coli)–induced up-regulation of ICAM-1 in HUVECs. Results from our experiments are consistent with previous studies because morphine pretreatment altered the expression of CD18 (β subunit; pairs with CD11a and known as lymphocyte function–associated antigen 1; interacts with ICAM-1 clusters found on endothelial cells) using the huPLB985 granulocyte-monocyte cells exposed to inflammatory signals (LPS or TNF-α). These findings suggest that morphine can alter leukocyte TEM into the tumor microenvironment by altering cell adhesion molecule expression on both the leukocyte and endothelial cells. Further studies are necessary to determine the extent of this inhibition and intracellular signaling associated with these defects.
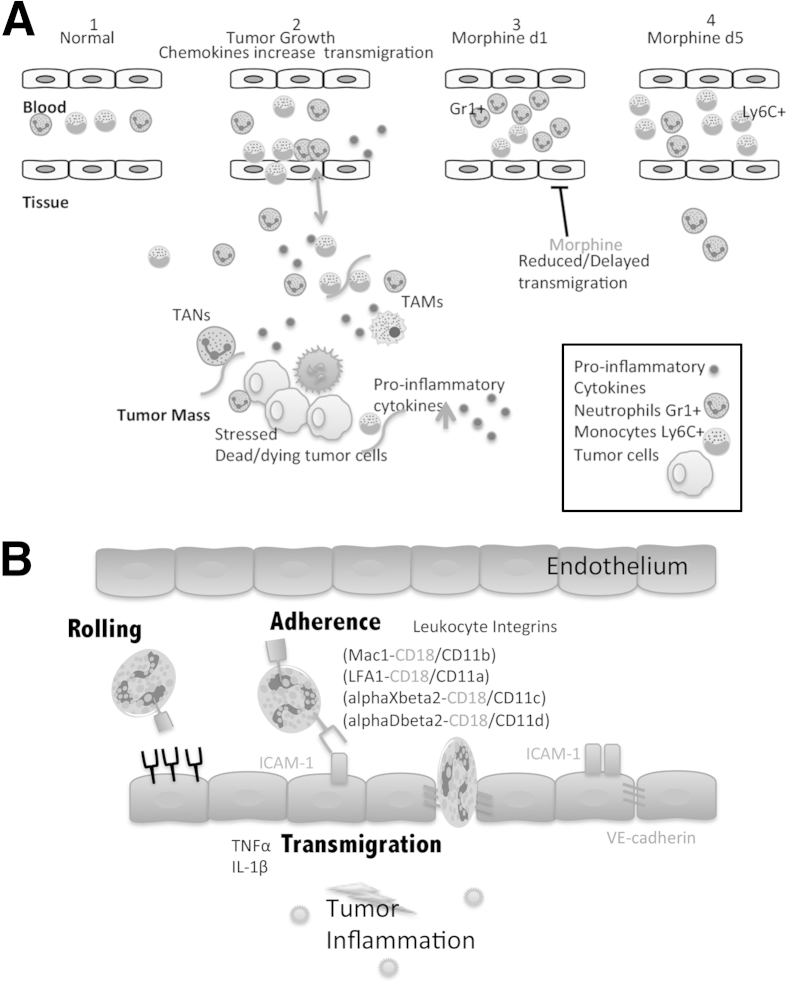
In summary, our studies indicate for the first time that long-term morphine treatment reduces leukocyte migration and recruitment to reduce angiogenesis and tumor growth (Figure 5). Strategies to target inflammatory cells, and inhibit inflammation-associated angiogenesis to potentially reduce neovascularization, associated with increasing tumor growth and progression, are highly desirable. Morphine administered for cancer pain management in humans may not be as detrimental but useful in the disease treatment itself.
Figure 5.
A: Schematic shows the working model for the effect of morphine on leukocyte migration. Morphine suppresses leukocyte infiltration to reduce angiogenesis associated with tumor growth. Leukocytes normally circulate freely in peripheral blood. B: Once an infection or injury occurs, damaged and stressed cells secrete a host of cytokines and chemokines that alter cell motility and promote slow rolling of activated leukocytes for firm binding, adhesion, and transmigration through the receptive endothelium. Data suggest that morphine alters leukocyte and endothelial cell expression of cell adhesion molecules necessary for effective TEM, leading to either slowed and/or delayed infiltration into the tumor tissue.
Acknowledgments
We thank the University of Minnesota Biomedical Imaging and Processing Laboratory for use and guidance in image capturing and Fairview University Pathology Services for tissue sectioning and staining.
Footnotes
Supported by research funding from National Institute on Drug Abuse grant F31-DA021005-01 and National Institute of Dental and Craniofacial Research grant T32DE007288 (L.K.); CA114340/CA/NCI NIH HHS grant CA114340 (S.Ra.); and National Institute on Drug Abuse grants RO1 DA 12104, RO1 DA 022935, RO1 DA031202, KO2 DA 015349, and P50 DA 011806 (S.Ro.).
Disclosures: None declared.
Supplemental Data
Tumor sections taken from placebo-treated (A) and morphine-treated (B) mice (n = 5 mice per group) were stained using fluorescent conjugated monoclonal antibodies for blood vessels using CD31-PE (red) and granulocytes using Gr1-FITC (green).
H&E-stained sections show a reduced cell infiltration in VEGF Matrigel plugs taken from morphine-treated (A) WT mice when compared with MORKO mice (C) (n = 4 mice per group). Infiltrating cells were identified as endothelial cells (B and D) [anti-CD31 (red)], macrophage-monocytes (E–H) [anti-F4/80 (red), anti-Mac3 (green), counterstained with DAPI nuclear stain (blue)]. Cells infiltrating sponges that contained PVA-LLC-CM were isolated and stained (n = 5 mice per group). Bright light images of infiltrated cells display a granular phenotype (I). Corresponding fluorescent captured images for neutrophil markers Ly6G [FITC (green)] (J) and CXCR2 [PE (red)] (K) and counterstained with DAPI (blue). Fluorescent captured images of LLC tumor sections (day 21) stained with anti–CD31-PE–conjugated antibody (red) and ICAM-1 (anti–ICAM-1, secondary IgG FITC conjugated) (L).
HUVECs were treated with 100 ng/mL of LPS for 24 hours and 100 ng/mL of TNF-α for 2 hours. A: Graph shows that morphine prevents the LPS- and TNF-α–induced down-regulation of vascular endothelial cadherin expression on HUVECs. Human PLB985 cells, from a monocytic-granulocytic leukemia–derived cell line, were pretreated with 1.0 μmol/L morphine for 16 hours before stimulation with 50 to 100 ng/mL of TNF-α for 2 hours at 37°C. B: Graph shows that morphine suppresses the TNF-α–induced increase in CD18 (β2) expression on human PLB-985 cells. C: Frozen tumor sections (from different mice) stained for anti–CD45-PE (red) and anti–CD18-FITC. ∗P ≤ 0.05, ∗∗P ≤ 0.001.
Graph shows the percentage change in chemotactic migration of the monocytic-granulocytic human cell line huPLB985s (A) and freshly isolated human granulocytes (peripheral blood) (B) toward CM derived from a human ovarian cancer cell line, 25% MA148, and compared with 5% FBS RPMI 1640 medium using a transwell assay. The ECIS system was used to generate the following graphs showing relative resistance generated by adhesion of mouse BM cells to fibronectin in the presence of CM derived from LLC cells (C) and chemotactic migration of mouse BM cells pretreated with either 1.0 μmol/L saline or morphine toward the LLC-CM using a modified under agar assay (D). ∗P < 0.05.
References
- 1.Eyelade O.R., Ajayi I.O., Elumelu T.N., Soyannwo O.A., Akinyemi O.A. Oral morphine effectiveness in Nigerian patients with advanced cancer. J Pain Palliat Care Pharmacother. 2012;26:24–29. doi: 10.3109/15360288.2011.650351. [DOI] [PubMed] [Google Scholar]
- 2.Jeon Y.S., Lee J.A., Choi J.W., Kang E.G., Jung H.S., Kim H.K., Shim B.Y., Park J.H., Joo J.D. Efficacy of epidural analgesia in patients with cancer pain: a retrospective observational study. Yonsei Med J. 2012;53:649–653. doi: 10.3349/ymj.2012.53.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohshima H., Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 4.Syrjänen K.J. HPV infections and lung cancer. J Clin Pathol. 2002 Dec;55:885–891. doi: 10.1136/jcp.55.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y.C., Chen J.H., Richard K., Chen P.Y., Christiani D.C. Lung adenocarcinoma and human papillomavirus infection. Cancer. 2004;101:1428–1436. doi: 10.1002/cncr.20538. [DOI] [PubMed] [Google Scholar]
- 6.Shukla S., Bharti A.C., Mahata S., Hussain S., Kumar R., Hedau S., Das B.C. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. 2009;130:222–233. [PubMed] [Google Scholar]
- 7.Joag S., Zychlinsky A., Young J.D. Mechanisms of lymphocyte-mediated lysis. J Cell Biochem. 1989;39:239–252. doi: 10.1002/jcb.240390304. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch C., Muthana M., Coffelt S.B., Lewis The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 9.Ueha S., Shand F.H., Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Barleon B., Sozzani S., Zhou D., Weich H.A., Mantovani A., Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 11.Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 12.Sethi G., Shanmugam M.K., Ramachandran L., Kumar A.P., Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1–15. doi: 10.1042/BSR20100136. [DOI] [PubMed] [Google Scholar]
- 13.Roy S., Ninkovic J., Banerjee S., Charboneau R.G., Das S., Dutta R., Kirchner V.A., Koodie L., Ma J., Meng J., Barke R.A. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6:442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasotti D., Mazzone A., Lecchini S., Frigo G.M., Ricevuti G. The effect of opioid peptides on peripheral blood granulocytes. Riv Eur Sci Med Farmacol. 1993;15:71–81. [PubMed] [Google Scholar]
- 15.Grimm M.C., Ben-Baruch A., Taub D.D., Howard O.M., Wang J.M., Oppenheim J.J. Opiate inhibition of chemokine-induced chemotaxis. Ann N Y Acad Sci. 1998;840:9–20. doi: 10.1111/j.1749-6632.1998.tb09544.x. [DOI] [PubMed] [Google Scholar]
- 16.Makimura C., Arao T., Matsuoka H., Takeda M., Kiyota H., Tsurutani J., Fujita Y., Matsumoto K., Kimura H., Otsuka M., Koyama A., Imamura C.K., Yamanaka T., Tanaka K., Nishio K., Nakagawa K. Prospective study evaluating the plasma concentrations of twenty-six cytokines and response to morphine treatment in cancer patients. Anticancer Res. 2011;31:4561–4568. [PubMed] [Google Scholar]
- 17.Choi Y., Chuang L.F., Lam K.M., Kung H.F., Wang J.M., Osburn B.I., Chuang R.Y. Inhibition of chemokine-induced chemotaxis of monkey leukocytes by mu-opioid receptor agonists. In Vivo. 1999;13:389–396. [PubMed] [Google Scholar]
- 18.Chadzinska M., Kolaczkowska E., Seljelid R., Plytycz B. Morphine modulation of peritoneal inflammation in Atlantic salmon and CB6 mice. J Leukoc Biol. 1999;65:590–596. doi: 10.1002/jlb.65.5.590. [DOI] [PubMed] [Google Scholar]
- 19.Chadzinska M., Savelkoul H.F., Verburg-van Kemenade B.M. Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev Comp Immunol. 2009;33:88–96. doi: 10.1016/j.dci.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Koodie L., Ramakrishnan S., Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol. 2010;177:984–997. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadjout N., Yin X., Knecht D.A., Lynes M.A. Automated real-time measurements of leukocyte chemotaxis. J Immunol Methods. 2007;320:70–80. doi: 10.1016/j.jim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam C.F., Chang P.J., Huang Y.S., Sung Y.H., Huang C.C., Lin M.W., Liu Y.C., Tsai Y.C. Prolonged use of high-dose morphine impairs angiogenesis and mobilization of endothelial progenitor cells in mice. Anesth Analg. 2008;107:686–692. doi: 10.1213/ane.0b013e31817e6719. [DOI] [PubMed] [Google Scholar]
- 23.Martin J.L., Koodie L., Krishnan A.G., Charboneau R., Barke R.A., Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol. 2010;176:786–799. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasama T., Miwa Y., Isozaki T., Odai T., Adachi M., Kunkel S.L. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 25.Gaudry M., Brégerie O., Andrieu V., El Benna J., Pocidalo M.A., Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 26.Gong Y., Koh D.R. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339:437–448. doi: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 27.Wada Y., Yoshida K., Tsutani Y., Shigematsu H., Oeda M., Sanada Y., Suzuki T., Mizuiri H., Hamai Y., Tanabe K., Ukon K., Hihara J. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol Rep. 2007;17:161–167. [PubMed] [Google Scholar]
- 28.Benelli R., Morini M., Carrozzino F., Ferrari N., Minghelli S., Santi L., Cassatella M., Noonan D.M., Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- 29.Negus R.P., Stamp G.W., Hadley J., Balkwill F.R. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–1734. [PMC free article] [PubMed] [Google Scholar]
- 30.Wen H., Lu Y., Yao H., Buch S. Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One. 2011;6:e21707. doi: 10.1371/journal.pone.0021707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeks B.S., Goldman S., Touma S., Payne M., Cadet P., Stefano G.B. Morphine inhibits indolactam V-induced U937 cell adhesion and gelatinase secretion. J Cell Physiol. 2001;189:179–188. doi: 10.1002/jcp.10015. [DOI] [PubMed] [Google Scholar]
- 32.Debruyne D.J., Mareel M.M., Bracke M.E. Opioids affect focal contact-mediated cell-substrate adhesion. Eur J Cancer Prev. 2010;19:227–238. [PubMed] [Google Scholar]
- 33.Wang T.L., Chang H., Hung C.R., Tseng Y.Z. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997;80:1532–1535. doi: 10.1016/s0002-9149(97)00788-1. [DOI] [PubMed] [Google Scholar]
- 34.Min T.J., Kim J.I., Kim J.H., Noh K.H., Kim T.W., Kim W.Y., Lee Y.S., Park Y.C. Morphine postconditioning attenuates ICAM-1 expression on endothelial cells. J Korean Med Sci. 2011;26:290–296. doi: 10.3346/jkms.2011.26.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber N.C., Kandler J., Schlack W., Grueber Y., Frädorf J., Preckel B. Intermitted pharmacologic pretreatment by xenon, isoflurane, nitrous oxide, and the opioid morphine prevents tumor necrosis factor alpha-induced adhesion molecule expression in human umbilical vein endothelial cells. Anesthesiology. 2008;108:199–207. doi: 10.1097/01.anes.0000299441.32091.ed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tumor sections taken from placebo-treated (A) and morphine-treated (B) mice (n = 5 mice per group) were stained using fluorescent conjugated monoclonal antibodies for blood vessels using CD31-PE (red) and granulocytes using Gr1-FITC (green).
H&E-stained sections show a reduced cell infiltration in VEGF Matrigel plugs taken from morphine-treated (A) WT mice when compared with MORKO mice (C) (n = 4 mice per group). Infiltrating cells were identified as endothelial cells (B and D) [anti-CD31 (red)], macrophage-monocytes (E–H) [anti-F4/80 (red), anti-Mac3 (green), counterstained with DAPI nuclear stain (blue)]. Cells infiltrating sponges that contained PVA-LLC-CM were isolated and stained (n = 5 mice per group). Bright light images of infiltrated cells display a granular phenotype (I). Corresponding fluorescent captured images for neutrophil markers Ly6G [FITC (green)] (J) and CXCR2 [PE (red)] (K) and counterstained with DAPI (blue). Fluorescent captured images of LLC tumor sections (day 21) stained with anti–CD31-PE–conjugated antibody (red) and ICAM-1 (anti–ICAM-1, secondary IgG FITC conjugated) (L).
HUVECs were treated with 100 ng/mL of LPS for 24 hours and 100 ng/mL of TNF-α for 2 hours. A: Graph shows that morphine prevents the LPS- and TNF-α–induced down-regulation of vascular endothelial cadherin expression on HUVECs. Human PLB985 cells, from a monocytic-granulocytic leukemia–derived cell line, were pretreated with 1.0 μmol/L morphine for 16 hours before stimulation with 50 to 100 ng/mL of TNF-α for 2 hours at 37°C. B: Graph shows that morphine suppresses the TNF-α–induced increase in CD18 (β2) expression on human PLB-985 cells. C: Frozen tumor sections (from different mice) stained for anti–CD45-PE (red) and anti–CD18-FITC. ∗P ≤ 0.05, ∗∗P ≤ 0.001.
Graph shows the percentage change in chemotactic migration of the monocytic-granulocytic human cell line huPLB985s (A) and freshly isolated human granulocytes (peripheral blood) (B) toward CM derived from a human ovarian cancer cell line, 25% MA148, and compared with 5% FBS RPMI 1640 medium using a transwell assay. The ECIS system was used to generate the following graphs showing relative resistance generated by adhesion of mouse BM cells to fibronectin in the presence of CM derived from LLC cells (C) and chemotactic migration of mouse BM cells pretreated with either 1.0 μmol/L saline or morphine toward the LLC-CM using a modified under agar assay (D). ∗P < 0.05.