Abstract
Cyclooxygenase-2 (COX-2) overexpression is implicated in increased risk and poorer outcomes in breast cancer in young women. We investigated COX-2 regulation in normal premenopausal breast tissue and its relationship to malignancy in young women. Quantitative COX-2 immunohistochemistry was performed on adjacent normal and breast cancer tissues from 96 premenopausal women with known clinical reproductive histories, and on rat mammary glands with distinct ovarian hormone exposures. COX-2 expression in the normal breast epithelium varied more than 40-fold between women and was associated with COX-2 expression levels in ductal carcinoma in situ and invasive cancer. Normal breast COX-2 expression was independent of known breast cancer prognostic indicators, including tumor stage and clinical subtype, indicating that factors regulating physiological COX-2 expression may be the primary drivers of COX-2 expression in breast cancer. Ovarian hormones, particularly at pregnancy levels, were identified as modulators of COX-2 in normal mammary epithelium. However, serial breast biopsy analysis in nonpregnant premenopausal women suggested relatively stable baseline levels of COX-2 expression, which persisted independent of menstrual cycling. These data provide impetus to investigate how baseline COX-2 expression is regulated in premenopausal breast tissue because COX-2 levels in normal breast epithelium may prove to be an indicator of breast cancer risk in young women, and predict the chemopreventive and therapeutic efficacy of COX-2 inhibitors in this population.
In 2010, approximately 13% of all breast cancers in the United States were diagnosed in women age 45 and younger, accounting for nearly 18,600 cases of invasive breast cancer and 6500 cases of ductal carcinoma in situ (DCIS).1 Furthermore, the proportion of advanced breast cancers diagnosed in young American women is increasing at a rate of 2% per year, making young women's breast cancer an emerging concern.2 Compared with breast cancer in older women, young breast cancer patients have increased recurrence and lower survival rates.3–8 Although a delayed diagnosis can contribute to poorer survival in some young patients,6,9 the primary factor driving poor prognosis is tumor biology. Young women's breast cancer has increased hormone-receptor negativity, tumor cell proliferation, and lymphovascular invasion compared with postmenopausal cases.4,6,10 Moreover, young age at the time of breast cancer diagnosis is an independent poor prognostic factor.4,6,7,11,12 These data provide compelling arguments to develop novel strategies to reduce breast cancer incidence and poor outcomes in young women.
One potential target for young women's breast cancer is cyclooxygenase-2 (COX-2),13 a key enzyme in the synthesis of homeostatic and proinflammatory prostanoids.14 In rodent breast cancer models, COX-2 overexpression induces mammary tumorigenesis and is associated with multiple tumor-promotional effects including increased angiogenesis, enhanced tumor cell migration and invasion, and reduced antitumor immunity.15–20 Conversely, COX-2 inhibition or loss in rodent models reduces mammary tumorigenesis and metastasis.17,21–23 Clinical data are consistent with similar roles for COX-2 in human breast cancer because COX-2 overexpression in breast cancer is associated with decreased disease-free and overall survival.24,25 In addition, regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the COX family of enzymes, can reduce overall breast cancer risk.26,27 To date, the function of COX-2 in young women's breast cancer has not been addressed. In a single study, high COX-2 expression in combination with increased collagen I is reported as a poor prognostic indicator in young-onset breast cancer patients (age, <45 years).16 One mechanism by which COX-2 may contribute to young-onset breast cancers is through its role in normal breast tissue remodeling. Importantly, windows of active breast tissue remodeling specific to young women, such as those associated with puberty, menstrual cycling, pregnancy, and postpartum breast involution, correlate with an increased risk for incidence and progression of breast cancer.28,29 Support for this has been shown in rodent models in which postpartum mammary gland involution promotes tissue remodeling, tumor progression, and metastasis, all of which are mitigated by anti–COX-2 treatment.16,30 Furthermore, COX-2 up-regulation has been observed in rat mammary glands after treatment with the ovarian hormones estrogen and progesterone,31 which is consistent with a role for COX-2 in physiological breast tissue remodeling associated with pregnancy and the menstrual cycle.
We hypothesized that if COX-2 is involved in breast tissue remodeling, then COX-2 inhibition may represent a particularly efficacious chemoprevention strategy for young women. One important step in addressing this hypothesis is to evaluate COX-2 expression in young women's breast tissue. We used human and rodent mammary tissues to investigate the effect of pregnancy and ovarian hormones on COX-2 expression in normal tissue as well as to explore the link between COX-2 expression in histologically normal adjacent breast tissue, DCIS, and invasive ductal carcinoma (IDC) in young-onset cases. We found that COX-2 expression primarily was epithelial and varied greatly between individual women, with evidence of modulation by ovarian hormones. In addition, analysis of COX-2 expression in paired normal adjacent breast epithelium, DCIS, and IDC within breast tissue from the same woman showed that COX-2 expression in the normal epithelium was associated with COX-2 expression in DCIS and IDC. Altogether, these data suggest that factors regulating COX-2 expression in normal breast epithelium influence COX-2 levels in breast cancer, and indicate that further research is warranted into whether women with high COX-2 expression may benefit preferentially from COX-2 inhibition strategies.
Materials and Methods
Human Tissue Acquisition
Breast specimens from 96 premenopausal women ages 20 to 45 years who underwent a clinically indicated biopsy (n = 10) or surgery (n = 86) were obtained under University of Colorado Institutional Review Board–approved protocols. Eighty-six specimens were obtained through a Health Insurance Portability and Accountability Act–exempt, consent-exempt retrospective cohort study with Institutional Review Board approval (University of Colorado Institutional Review Board 05-0958), and 10 specimens were obtained through a subsequent prospective full-consent cohort study (University of Colorado Institutional Review Board 09-0583 and 08-0104). Histologically normal tissue, DCIS, and IDC were identified by pathologic review. In 11 cases, COX-2 expression in the normal epithelium adjacent to cancer was compared with expression in a separate quadrant of the breast to determine whether COX-2 expression in the histologically normal epithelium was influenced by location. For reproductive stage and epithelial-stromal analyses, 28 cases with clinical reproductive histories were grouped by reproductive categories of nulliparous (n = 7), pregnant (n = 5), postpartum involuting (within 2 months of parturition or lactation, n = 7), and fully regressed parous (7 to 22 years after parturition, n = 9). Thirty-seven cases were used for comparison of paired normal adjacent breast epithelium, DCIS, and IDC within breast tissue from the same woman. For correlations, 46 cases were used for normal and DCIS, and 57 for normal and IDC. To determine COX-2 expression over time, an independent cohort of six premenopausal patients with serial biopsies 2 to 3 weeks apart were analyzed.
Animal Husbandry, Reproductive Staging, and Hormone Stimulation
Animal procedures were approved with ethical consideration by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee. Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed in static caging with 12-hour light-dark cycles and access to food and water ad libitum. To obtain distinct reproductive states, female rats approximately 70 days of age were bred, and mammary tissue was harvested from age-matched virgin, pregnant (days 18 to 20), lactating (days 10 to 11), involuting day 2, 4, 6, 8, and 10 (2 to 10 days after weaning), and fully regressed rats (4 weeks after weaning) as described.32 For estrous cycle studies, serial vaginal smears were performed and mammary glands were harvested at proestrus, estrus, and diestrus stages 1 and 2 as described.33 Vaginal smear data were confirmed by cervical histology.33 For estradiol treatment, virgin female rats approximately 65 days of age were injected subcutaneously with 5 μg 17-β-estradiol (Sigma-Aldrich, St. Louis, MO) in 250 μL sesame oil or sesame oil alone daily for 3 days, and sacrificed 3 days after treatment. For estradiol plus progesterone treatment, female rats approximately 56 days of age were injected subcutaneously with 5 μg 17-β-estradiol plus 1.5 mg progesterone (Sigma-Aldrich) in 50 μL sesame oil or sesame oil alone daily for 7 days, and sacrificed 24 to 48 hours after treatment.
IHC, Image Acquisition, and Quantification
Four-micrometer–thick, formalin-fixed, paraffin-embedded sections were deparaffinized, rehydrated, and sequentially subjected to Dako TRS Antigen Retrieval Solution (125°C under pressure for 5 minutes; Dako, Carpinteria, CA), Dual Endogenous Enzyme Block (10 minutes; Dako), and Protein Block (10 minutes; Dako). Tissue sections were incubated in primary antibody (1 hour at room temperature; human: Cayman Chemical 160112; rat: Cayman Chemical 160106; Ann Arbor, MI), followed by Envision Plus Rabbit (30 minutes at room temperature; Dako). Immunoreactivity was visualized using 3,3′-diaminobenzidine (Dako). Primary antibody specificity was confirmed in humans by incubating 1:1 with COX-2 blocking peptide before staining (Cayman Chemical) (Supplemental Figure S1A), and in rodents by staining mammary tissue from a COX-2 knockout mouse, kindly provided by Christopher Rivard, University of Colorado AMC (Supplemental Figure S1B).
COX-2–stained slides were acquired using a ScanScope T3 scanner (AperioTechnologies, Leica Biosystems) at 0.46 μ per pixel. Aperio analysis software (Leica Biosystems, Wetzlar, Germany) and a color deconvolution algorithm (thresholds: clear = 240, weak positive = 225, medium positive = 198, strong positive = 150) were used to quantify staining intensity (Supplemental Figure S2). For all COX-2 analyses, the analyst was blinded to study design. For COX-2 quantification of histologically normal adjacent breast tissue, lobules of representative COX-2 staining and size were quantified. Representative lobules were chosen based on pathologic review by a clinical pathologist blinded to the study design. When possible, 10 representative lobules per case were analyzed; however, in cases with limited epithelium as a result of the biopsy method used (ie, surgical versus needle), or high stroma or tumor content, at least five lobules per case were analyzed, for a total of 693 lobules across all cases. To quantify epithelial and stromal COX-2, the stroma within each lobule (intralobular stroma) and the stroma between lobules (interlobular stroma) were analyzed separately. To determine epithelial-only stain, the intralobular stroma signal was subtracted from the whole lobule signal. For alveolar:ductal COX-2 analysis, five to seven ducts and five alveoli per duct were analyzed per case. For quantification of COX-2 expression in rat mammary glands, 10 representative fields per animal were analyzed (n = 4 animals/reproductive stage). To control for changes in adipose tissue, lumen size, and cellular content in the rat mammary gland across the reproductive stages, COX-2 stain was normalized to the total number of nuclei.
Immunoblotting
Pooled rat mammary tissue lysates (n = 6 animals/reproductive group) were prepared as described.34 Forty micrograms of total protein was separated by SDS-PAGE and immunoblotting was performed using polyclonal rabbit anti–COX-2 (160106; Cayman Chemical) and monoclonal anti-actin (Chemicon, Billerica, MA), followed by anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA, and Santa Cruz Biotechnologies, Santa Cruz, CA, respectively) with detection using ECL Western Blotting Substrate (Thermo Fisher Scientific, Waltham, MA). COX-2 antibody specificity was confirmed using mammary tissue from COX-2 knockout mice described earlier (Supplemental Figure S1C). Densitometry was performed using ImageJ software version 1.42q (NIH, Bethesda, MD).
Statistical Analysis
For analysis of COX-2 expression across reproductive stages and after estrogen or estrogen plus progesterone treatment, unpaired one-tailed t-tests were used to test for increased COX-2 expression compared with nulliparous/virgin or vehicle. Two-tailed t-tests were used for comparison of low, medium, and high COX-2 levels in normal adjacent, DCIS, and IDC groups, as well as for analyses of COX-2 expression in the normal epithelium in relation to DCIS grade, tumor grade, tumor stage, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) status. The Welch correction was applied if variance between groups was unequal. For linear regression analyses, Gaussian distributions were assumed and Pearson correlation coefficients were calculated. Statistical analyses were performed using GraphPad Prism software (version 6; San Diego, CA).
Results
Wide Range in COX-2 Expression across Premenopausal Human Breast Tissue
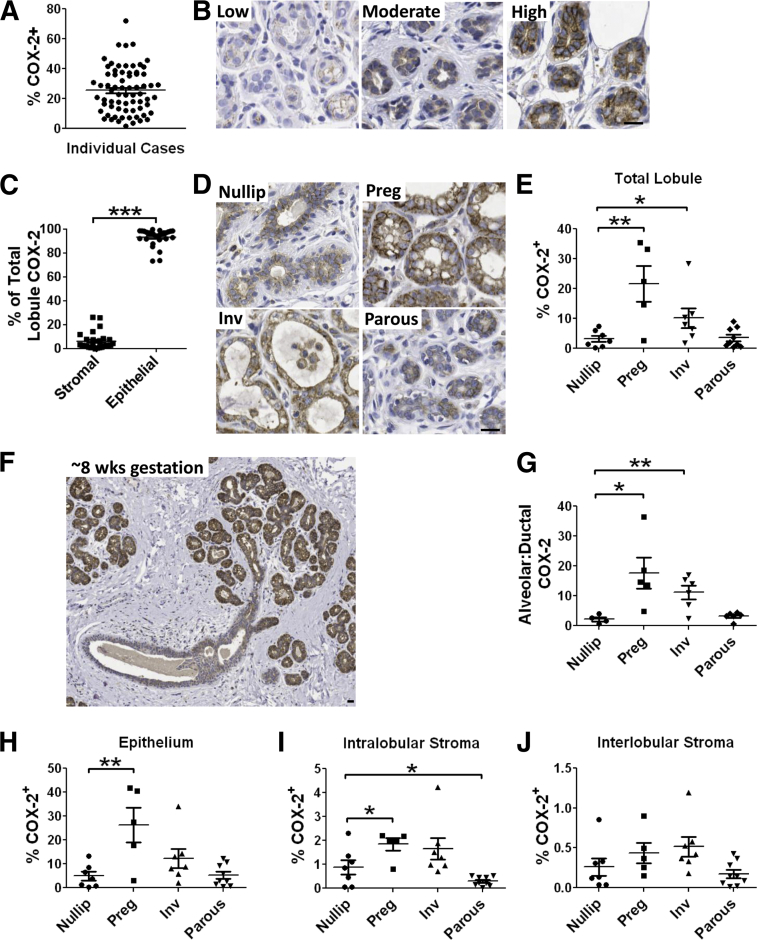
To investigate COX-2 expression in the premenopausal human breast, quantitative COX-2 immunohistochemical analyses were performed on breast tissue obtained from women 20 to 45 years of age. First, the effect of tumor proximity on COX-2 expression in normal adjacent epithelium was assessed because field effects in adjacent normal epithelium are well documented (reviewed by Heaphy et al35). In a subset analysis (n = 12), the proximity to tumor did not significantly affect adjacent normal COX-2 expression, indicating that COX-2 expression in normal breast tissue can be assessed in breast cancer cases (Supplemental Figure S3). In all cases containing normal epithelium (n = 66), the normal breast tissue stained positively for COX-2, although the percentage of positivity of the tissue varied more than 40-fold (1.8% to 71%) (Figure 1A). Furthermore, the mammary epithelium was the dominant source of COX-2, showing a range of low, moderate, and high expression, and accounting for approximately 94% of the total signal (Figure 1, B and C). Based on the wide variation in epithelial COX-2 expression observed, we explored whether the reproductive state contributes to COX-2 expression in normal breast tissue. Cases with clinical reproductive histories (Table 1) were separated into nulliparous, pregnant, postpartum involution, and fully regressed parous groups. A marked approximately sevenfold increase in total lobule COX-2 expression was observed in pregnant cases compared with nulliparous controls (Figure 1, D and E). During pregnancy, COX-2 up-regulation occurred predominantly in the alveoli (Figure 1, F and G). COX-2 expression in the normal breast also increased in postpartum involution cases, but to a lesser extent than during pregnancy (Figure 1, D and E). In fully regressed breast tissue from parous women, expression levels returned to nulliparous baseline levels (Figure 1, D and E). Furthermore, COX-2 expression was independent of patient age or body mass index (Supplemental Figure S4).
Figure 1.
Normal breast tissue COX-2 expression is primarily epithelial and is regulated by pregnancy and postpartum involution. A: COX-2 IHC expression varies greatly by case. B: COX-2 IHC (brown signal) images are representative of low-, moderate-, and high-expression cases. C: Relative stromal and epithelial contribution to the total lobular COX-2 signal. Representative COX-2 IHC (D) and percentage of lobular area positive for COX-2 (E) in breast tissue from nulliparous (nullip), pregnant (preg), postpartum involution (inv), and parous cases. F: COX-2 staining in human breast at approximately 8 weeks gestation shows up-regulation of COX-2 specific to hormone-responsive alveolar epithelium. G: Ratio of alveolar to ductal COX-2 stain in the breast across reproductive stages shows preferential up-regulation of COX-2 in alveoli with pregnancy and involution. Percentage of epithelium (H), intralobular stroma (I), and interlobular stroma (J) positive for COX-2. ∗P < 0.05, one-tail unpaired t-test; ∗∗P < 0.01, one-tailed unpaired t-test; ∗∗∗P < 0.0001, one-tailed paired t-test. Scale bar = 40 μm.
Table 1.
Clinical Characteristics of Cases Analyzed by Reproductive Stage
| Patient Parameters | Nulliparous | Pregnant | Postpartum involution∗ | Parous† |
|---|---|---|---|---|
| Cases (n) | 7 | 5 | 7 | 9 |
| Average age (years) | 35.6 ± 6.5 | 34.0 ± 7.7 | 28.9 ± 7.0 | 40.9 ± 4.1 |
| Average gravidity | 0 | 1.50 ± 0.7 | 2.3 ± 0.5 | 3.3 ± 1.4 |
| Average parity | 0 | 0.50 ± 0.7 | 1.8 ± 0.5 | 2.3 ± 1.1 |
| Race | ||||
| White | 7 | 1 | 1 | 6 |
| Hispanic | 0 | 0 | 0 | 2 |
| African American | 0 | 0 | 0 | 1 |
| Asian | 0 | 0 | 1 | 0 |
| Not reported | 0 | 4 | 5 | 0 |
Data correspond to Figure 1.
Within 2 months of parturition or lactation.
More than 7 years after parturition.
Based on the known role for COX-2 stromal cells during wound healing and inflammation (reviewed by Smith et al36), we investigated COX-2 expression specifically within the intralobular (within a lobule) and interlobular (between lobules) stromal compartments. These analyses confirmed that COX-2 induction during pregnancy is predominantly epithelial, and also identified a small, but significant, increase in the intralobular stroma (Figure 1, H and I, and Supplemental Figure S5); similar trends were observed during involution (Figure 1, H and I, and Supplemental Figure S5). Quantification of COX-2 in the interlobular stroma showed no significant changes across stages (Figure 1J and Supplemental Figure S5). These data are consistent with coordinated regulation of COX-2 within the epithelium and intralobular stroma during pregnancy, but not within the interlobular stroma.
Evidence for Ovarian Hormone Regulation of COX-2
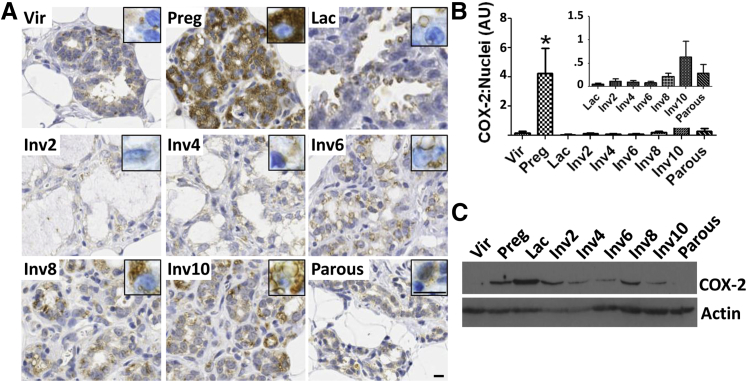
Having demonstrated that COX-2 is upregulated during pregnancy in women, the Sprague-Dawley rat model was used to investigate mammary tissue COX-2 expression across defined pregnancy, lactation, and postpartum involution time points. COX-2 expression in the mammary epithelium was low in virgin (nulliparous) rats, increased significantly during pregnancy, and was lost during lactation (Figure 2, A and B). After lactation, epithelial COX-2 gradually increased across mid- to late-involution (days 6, 8, and 10), and returned to baseline levels in the fully regressed gland (Figure 2, A and B). These data were verified by immunoblot for all stages except lactation (Figure 2C). By immunohistochemistry (IHC), COX-2 stain was low in the lactating gland and was restricted to apical, vesicle-like structures budding off the lactating mammary epithelium (Figure 2A); however, by immunoblot, COX-2 levels were highest during lactation (Figure 2C). Based on these disparate observations, we speculate that COX-2 is localized in the membrane of secreted vesicles during lactation and secreted with milk. Thus, the discrepancy between the IHC and immunoblot data may be explained by the fact that most milk is lost from the gland during IHC preparation, but remains in the tissue lysate used for immunoblot. Of note, a putative role for COX-2 in milk or milk production has not been reported, warranting additional investigation into this observation.
Figure 2.
COX-2 is up-regulated in the rat mammary gland during pregnancy and postpartum involution. A: Representative COX-2 IHC staining (brown signal) in rat mammary glands from virgin (vir), pregnancy (preg) days 18 to 20, lactation (lac) day 10, involution days 2 to 10 (inv2-10), and parous-4 weeks after weaning. Inset shows epithelial staining at a higher magnification. Scale bar = 10 μm. B: Percentage of COX-2+ area normalized to number of nuclei in rat mammary tissue across the reproductive cycle. C: Western blot for COX-2 and actin using pooled rat mammary tissue lysates. ∗P < 0.05 versus virgin, one-tailed unpaired t-test. AU, arbitrary units.
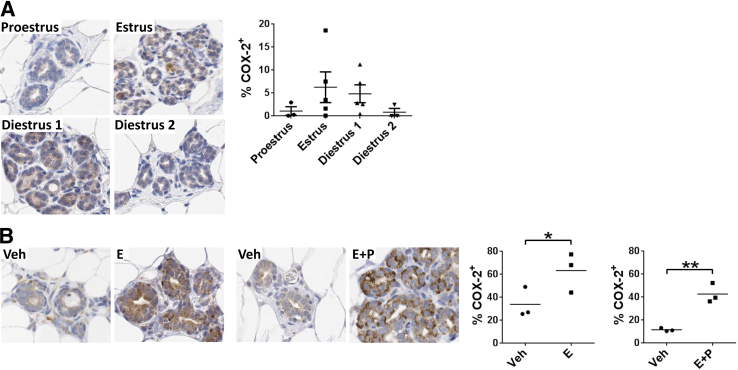
To explore the role of ovarian hormones in COX-2 regulation, COX-2 expression was evaluated by IHC across the rat estrous cycle. COX-2 expression in the mammary epithelium increased modestly during estrus and diestrus stage 1 of the estrous cycle (Figure 3A), consistent with estrogen and progesterone regulation. It is potentially relevant that the range of COX-2 expression levels in the nulliparous rat across estrous stages was similar to that observed in our premenopausal nulliparous and fully regressed parous human cohorts (Figure 1E). In rats, direct evidence for ovarian hormone regulation was shown by COX-2 up-regulation in the mammary epithelium after estradiol and estradiol plus progesterone treatment designed to mimic the biological effects of pregnancy (Figure 3B). The magnitude of COX-2 up-regulation after treatment was comparable with that observed in breast tissue of women during pregnancy (Figure 1, D and E).
Figure 3.
Evidence for ovarian hormone regulation of COX-2 in rat mammary epithelium. A: Representative IHC staining (brown signal) and quantification (right) of COX-2 expression in rat mammary tissue across the estrous cycle. P = 0.38, one-way analysis of variance. B: COX-2 IHC staining and quantification in rat mammary tissue after estradiol (E) or estradiol + progesterone (E + P) treatment. Veh, vehicle. ∗P < 0.05; ∗∗P < 0.01, one-tailed unpaired t-test.
Coordinated COX-2 Expression in Normal Epithelium, DCIS, and Breast Cancer
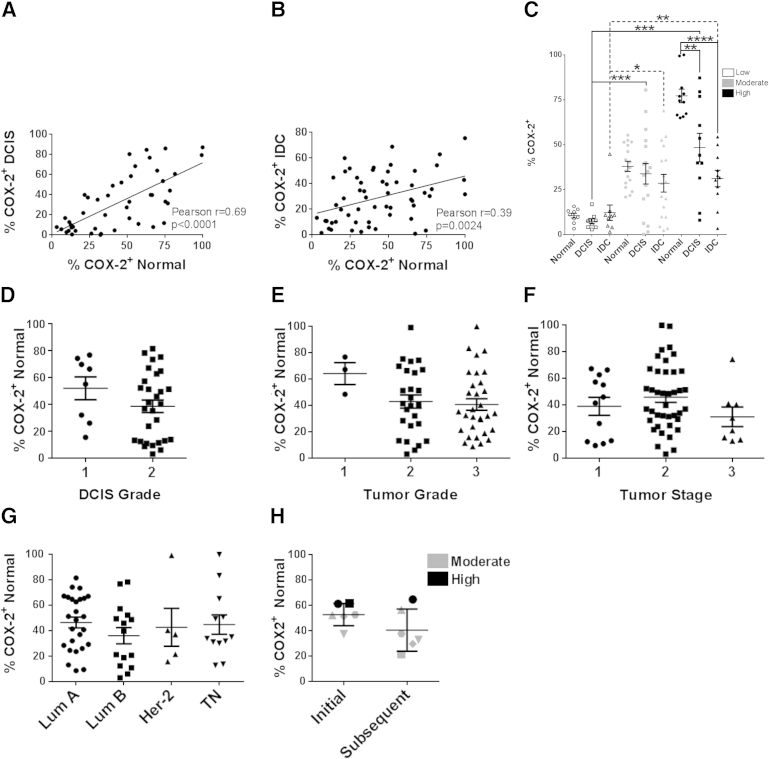
Given the wide range of COX-2 expression in the normal premenopausal human breast (Figure 1, A and B), and the reported poor prognosis associated with COX-2 up-regulation in breast cancer,24,25 we investigated the relationships between COX-2 expression in normal adjacent breast tissue, DCIS, and IDC. A quantitative assessment of COX-2 expression was performed using matched normal adjacent and DCIS lesions within a single tissue section from the same case (n = 46), as well as matched normal adjacent and IDC, also from a single tissue section (n = 57), obtained from the cohort described in Table 2. COX-2 expression in the normal adjacent epithelium correlated strongly with DCIS COX-2 expression (Figure 4A), and correlated moderately with COX-2 expression in IDC (Figure 4B). Thirty-seven cases containing normal, DCIS, and IDC on the same tissue section then were stratified into categories of low, moderate, and high normal epithelium COX-2 expression. We found that cases with low adjacent normal COX-2 expression also had low expressing DCIS and IDC (Figure 4C). Similarly, cases with moderate and high adjacent normal COX-2 expression had moderate and high DCIS and IDC expression, respectively (Figure 4C). When comparing normal adjacent, DCIS, and IDC within the same case, COX-2 expression was highest in the adjacent normal tissue, decreased in the matched DCIS, and further decreased in the matched IDC lesion (Figure 4C). Importantly, COX-2 expression in the normal adjacent epithelium was not associated with DCIS or IDC grade, stage, or estrogen receptor, progesterone receptor, or HER2 status (Figure 4, D–G), indicating that, in our cohort, COX-2 expression in the normal epithelium was not driven by the cancer state. This observation was supported further by evidence that COX-2 expression in the normal epithelium also did not differ significantly with proximity to the tumor (Supplemental Figure S3). These data suggest that women can be segregated into groups of low, moderate, and high COX-2 expression based on the expression levels in their normal breast epithelium, and that factors underlying COX-2 expression in the normal epithelium may influence COX-2 expression in DCIS and IDC.
Table 2.
Clinical Characteristics of Cases Used for Normal Adjacent, DCIS, IDC, and Serial Biopsy Analyses
| Patient Parameters | Normal adjacent, DCIS, and IDC analysis | Serial biopsy analysis |
|---|---|---|
| Cases (n) | 83 | 6 |
| Average age (years) | 37.5 ± 5.6 | 35.8 ± 10.5 |
| Average body mass index | 25.4 ± 6.1 | 24.8 ± 1.5 |
| Average gravidity | 2.2 ± 1.6 | 1.8 ± 1.3 |
| Average parity | 1.7 ± 1.3 | 1.8 ± 1.3 |
| Race | ||
| White | 59 | 3 |
| Hispanic | 13 | 0 |
| African American | 3 | 0 |
| Not reported/other | 8 | 3 |
| Histologic subtype | ||
| Ductal | 74 | 5 |
| Lobular | 5 | 0 |
| Ductal + lobular | 2 | 1 |
| Other | 2 | 0 |
| Stage | ||
| 0 | 4 | 1 |
| I | 14 | 1 |
| II | 53 | 2 |
| III | 11 | 2 |
| Unknown | 1 | 0 |
| Tumor subtype | ||
| Luminal A | 37 | 2 |
| Luminal B | 17 | 2 |
| Triple negative | 16 | 0 |
| HER2 | 7 | 1 |
| Unknown (including stage 0) | 6 | 1 |
| Tumor grade | ||
| 1 | 4 | 0 |
| 2 | 31 | 2 |
| 3 | 39 | 3 |
| Unknown | 5 | 0 |
| DCIS grade | ||
| 1 | 0 | 0 |
| 2 | 3 | 0 |
| 3 | 1 | 1 |
| Tumor size | ||
| <2 cm | 17 | 3 |
| ≥2 cm | 51 | 2 |
| Unknown (including stage 0) | 15 | 1 |
| Lymphovascular invasion | ||
| Present | 28 | 3 |
| Absent | 31 | 1 |
| Unknown (including stage 0) | 24 | 1 |
| Lymph node involvement | ||
| Present | 40 | 4 |
| Absent | 37 | 1 |
| Unknown (including stage 0) | 6 | 1 |
Figure 4.
COX-2 expression in normal breast epithelium is associated with COX-2 expression in matched DCIS and IDC. A: Correlation between COX-2 expression in matched adjacent normal and DCIS. B: Correlation between COX-2 expression in matched adjacent normal and IDC. C: Stratification of cases based on low, moderate, and high COX-2 expression in the normal adjacent epithelium. COX-2 expression in normal epithelium is not associated with DCIS grade (D), tumor grade (E), tumor stage (F), or biological subtype (G). H: COX-2 expression in serial biopsies separated in time by 2 to 3 weeks. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001, two-tailed unpaired t-test. Lum A, luminal A; Lum B, luminal B; TN, triple negative.
For COX-2 expression in the normal breast epithelium to be considered a potential risk factor for breast cancer in young women, the distinct baseline levels of low, moderate, and high COX-2 expression in the normal epithelium would need to remain relatively stable over time. However, an important potential caveat raised by our data showing ovarian hormone modulation is whether a single breast biopsy can predict overall COX-2 expression in young premenopausal women. To begin to address this question, COX-2 expression was analyzed in a distinct cohort of premenopausal cases (n = 6) who underwent serial breast biopsies 2 to 3 weeks apart, allowing for analysis of COX-2 expression in a single case at two different time points during physiological menstrual cycling. The patient characteristics of this smaller cohort were reflective of the larger cohort used for the normal adjacent, DCIS, and IDC analysis in terms of age, body mass index, parity, histologic and tumor subtype, and stage (Table 2). In five of six cases, the relative COX-2 expression (ie, low, moderate, and high categories) remained within the same category between biopsies, although one case moved from the high to the moderate category (Figure 4H). Although this was a small cohort, these data suggest that COX-2 expression data obtained from a single breast biopsy may prove to be a valid indicator of individual baseline COX-2 expression levels in young women.
Discussion
A young women's breast tissue cohort and rat models were used to investigate COX-2 expression in histologically normal mammary epithelium and the relationship between normal adjacent and breast cancer COX-2 expression. This was the first study to address COX-2 expression exclusively in breast tissue of premenopausal women, ages 45 years and younger, and to use computer-based analysis software to quantify COX-2 expression separately in the mammary epithelium and stroma. We found the epithelium to be the dominant source of COX-2 in normal adjacent breast tissue, with an approximately 40-fold range in expression across cases. Cumulatively, our studies implicate differential baseline levels of COX-2 expression between women, which can be influenced by ovarian hormones. Moreover, COX-2 expression in the normal breast epithelium paralleled COX-2 expression in DCIS and IDC. These data are consistent with results obtained from predominantly postmenopausal cohorts in which COX-2 expression also was observed in the breast epithelium and correlated with DCIS and invasive cancer expression.24,37–39 It previously was proposed that field effects emanating from premalignant tissue or overt cancer are responsible for high COX-2 expression in normal adjacent epithelium38,40; however, our data showed that COX-2 expression in the normal epithelium is independent of known clinical prognostic features and support an alternative hypothesis in which physiological regulators of COX-2 expression in the normal breast epithelium influence DCIS and IDC COX-2 expression levels.
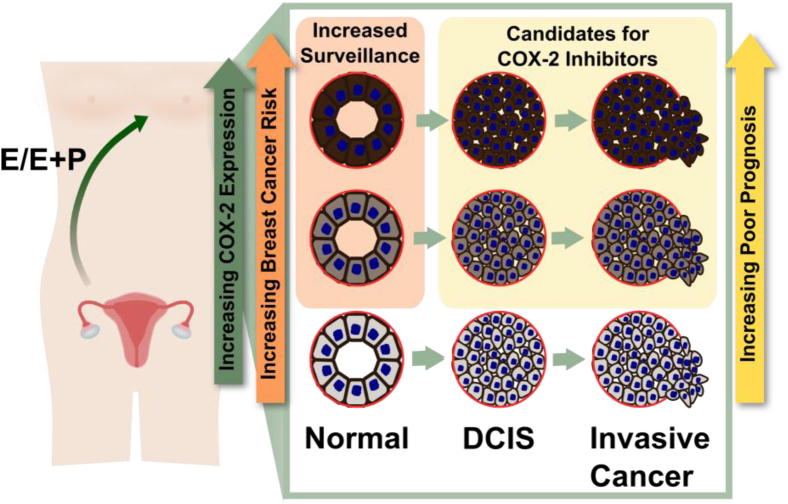
The observation that baseline COX-2 levels in the breast vary dramatically between young women raises two provocative but unanswered questions: whether high baseline COX-2 expression can predict risk of young-onset breast cancer, and whether women with high baseline COX-2 expression would preferentially benefit from COX-2 inhibition strategies (modeled in Figure 5). Consistent with a promotional role for COX-2 in breast cancer risk, increased COX-2 expression in the breast epithelium is associated with premalignancy and induces mammary tumor formation in mice.20,41 Furthermore, in women, high COX-2 expression in atypia is associated with an increased breast cancer risk.41 Importantly, a requisite for normal breast COX-2 expression to be predictive for breast cancer risk is that COX-2 expression remains relatively constant across time. Our data showing ovarian hormone modulation of COX-2 in normal breast epithelium would be expected to reduce the predictive value of COX-2 expression in young women. To address this concern, we performed a serial biopsy analysis in a small cohort of individual premenopausal women that showed relative stability in COX-2 expression levels over time. One interpretation of these data is that menstrual cycle–driven fluctuations in COX-2 expression occur within the context of a relatively stable baseline level of COX-2 expression. A larger sample size is necessary to address this key point, as well as to address potential mechanisms determining baseline COX-2 expression levels. Clinical relevance lies in the fact that 70% to 80% of the approximately 1 million clinical breast biopsies per year in the United States alone are given a benign diagnosis.42,43 For the vast majority of these women, molecular strategies to identify high-risk populations are absent, leaving populations of high-risk women unidentified. Data from our cohort suggest that as many as 30% of young women have high baseline COX-2 expression. The validation of our results showing stable categories of low, medium, and high baseline COX-2 expression would argue for future studies to investigate the relationship between normal epithelial COX-2 expression and breast cancer risk.
Figure 5.
Model of the relationships between COX-2 expression in the normal epithelium, DCIS, and invasive breast cancer in premenopausal women. Baseline COX-2 expression in the normal breast epithelium varies across individual premenopausal women and can be modified by estrogen (E) and progesterone (P) exposure. Young women with increased COX-2 expression in the normal epithelium are predicted to have an increased risk for breast cancers with a poor prognosis, and may be candidates for chemoprevention strategies targeting COX-2.
The association between COX-2 staining in the normal adjacent epithelium and breast cancer implies that if young women with high baseline COX-2 levels develop breast cancer, their tumors also will express high levels of COX-2 (Figure 5). This is of prognostic significance because COX-2 expression is an independent predictor of decreased disease-free and overall survival.24,25 Thus, understanding mammary COX-2 regulation and expression in young women may aid in identifying novel treatments for patients whose tumors express increased COX-2. This concept also is supported by other investigators who have proposed that understanding COX-2 expression in the normal breast epithelium is necessary to gain insight into COX-2 expression in cancer.24,37,38 Our data showing regulation of COX-2 by estrogen and progesterone raise intriguing questions such as whether increased baseline COX-2 expression in young women's breast tissue is associated with early menarche, contraceptive use, or pregnancy history. High COX-2 levels have been implicated in breast cancers diagnosed after pregnancy,16 providing additional support for understanding how reproductive history influences COX-2 expression in young women's breast cancer. Somewhat surprisingly, a relationship between body mass index and COX-2 expression in the normal adjacent breast epithelium was not observed in our cohort, although additional work is warranted to address this relationship further.
Data from colorectal cancer indicate that identifying patients whose tumors have high COX-2 expression may be a key step in achieving a survival benefit with NSAID treatment44; however, data from the Nurses' Health Study indicate that the survival benefit of NSAID use in breast cancer patients does not depend on tumor COX-2 expression levels.45 However, it remains unexplored whether young premenopausal breast cancer patients present a unique population in whom baseline COX-2 levels do impact outcomes. Given the high level of physiological tissue remodeling that occurs in the breasts of young women28 and the links between COX-2, tissue remodeling, and breast cancer, further investigations into the potential benefits of NSAID treatment in young women's breast cancer are warranted.
In summary, we show high variability in COX-2 expression in normal breast epithelium between young women and provide evidence for hormone regulation. Furthermore, we provide evidence that the mediators of physiological COX-2 expression also influence expression in DCIS and breast cancer. These data provide impetus to further determine how COX-2 expression levels are regulated in the normal human breast because baseline COX-2 expression levels may inform breast cancer risk assessment, chemopreventive efficacy of NSAIDs, and utility of COX-2–targeted therapies in young premenopausal women.
Acknowledgments
We thank Patricia Bell, Traci Lyons, and Mona Hamermesh for technical assistance; Christopher Rivard for COX-2 knockout mouse tissue; Jenean O’Brien for generating mammary tissue from estrogen-treated rats; and Arthur Gutierrez-Hartmann, Jenean O’Brien, and Virginia Winn for insightful discussion and critical review of this manuscript. We also thank the patients for their contribution to this research.
Footnotes
Supported by a grant from the Tissue Biobanking and Processing Shared Resource of Colorado's NIH/National Cancer Institute Cancer Center (P30 CA046934), Department of Defense grants (BC104000 and BC10400P1 to P.S. and V.F.B.), a Department of Defense Predoctoral Traineeship Award (BC093130 to J.F.), a Cancer Center grant (P30 CA016056-27), and by the Avon, Men for the Cure, Grohne Family, and Glass Family Foundations.
Disclosures: None declared.
Supplemental Data
Confirmation of human and rodent COX-2 antibody specificity. A: Human COX-2 IHC staining in normal adjacent tissue and tumor using primary antibody alone or primary antibody in combination with COX-2 blocking peptide. B: Rodent COX-2 IHC staining using mammary tissue from the COX-2 knockout mouse. C: Western blot for rodent COX-2 using C57Bl/6 primary macrophage lysate as a positive control (Ctrl) and mammary tissue lysate from a COX-2 knockout (KO) mouse. Ab, antibody; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Brown, COX-2--–positive cells.
Quantification of COX-2 staining intensity using Aperio analysis software. Regions of interest were annotated positively on whole slide images, and staining intensity was analyzed for negative (blue), weak (yellow), medium (orange), and strong (red) staining intensity.
COX-2 expression in histologically normal epithelium does not differ with proximity to tumor. The percentage of COX-2+ epithelium in breast tissue adjacent to tumor (adjacent) and in a quadrant of the breast not containing tumor (distant) (n = 11) is shown. P = 0.16, one-tailed paired t-test.
COX-2 (IHC staining) expression in normal adjacent breast epithelium is independent of age (A) and body mass index (BMI) (B). Pearson r = 0.039, P = 0.76, two-tailed (A); Pearson r = −0.0067, P = 0.96, two-tailed (B).
COX-2 IHC staining in intralobular stroma increases during pregnancy, whereas interlobular COX-2 expression remains relatively constant across reproductive stage. nullip, nulliparous; preg, pregnant; inv, involution. Arrows show COX-2–positive stromal cells (brown signal).
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.12.026.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures 2009-2010. Atlanta: American Cancer Society, Inc. 2009
- 2.Johnson R.H., Chien F.L., Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309:800–805. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 3.Fowble B.L., Schultz D.J., Overmoyer B., Solin L.J., Fox K., Jardines L., Orel S., Glick J.H. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23–33. doi: 10.1016/0360-3016(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 4.Nixon A.J., Neuberg D., Hayes D.F., Gelman R., Connolly J.L., Schnitt S., Abner A., Recht A., Vicini F., Harris J.R. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 5.Elkhuizen P.H., van de Vijver M.J., Hermans J., Zonderland H.M., van de Velde C.J., Leer J.W. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 6.Maggard M.A., O'Connell J.B., Lane K.E., Liu J.H., Etzioni D.A., Ko C.Y. Do young breast cancer patients have worse outcomes? J Surg Res. 2003;113:109–113. doi: 10.1016/s0022-4804(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 7.Han W., Kim S.W., Park I.A., Kang D., Youn Y.K., Oh S.K., Choe K.J., Noh D.Y. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong C., Boyages J., Jayasinghe U.W., Bilous M., Ung O., Chua B., Salisbury E., Wong A.Y. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004;100:1823–1832. doi: 10.1002/cncr.20153. [DOI] [PubMed] [Google Scholar]
- 9.Gajdos C., Tartter P.I., Bleiweiss I.J., Bodian C., Brower S.T. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523–529. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 10.Walker R.A., Lees E., Webb M.B., Dearing S.J. Breast carcinomas occurring in young women (< 35 years) are different. Br J Cancer. 1996;74:1796–1800. doi: 10.1038/bjc.1996.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Rochefordière A., Campana F., Fenton J., Vilcoq J.R., Fourquet A., Asselain B., Scholl S.M., Pouillart P., Durand J.C., Magdelenat H. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 12.Dubsky P.C., Gnant M.F., Taucher S., Roka S., Kandioler D., Pichler-Gebhard B., Agstner I., Seifert M., Sevelda P., Jakesz R. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3:65–72. doi: 10.3816/CBC.2002.n.013. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J., Otto F., Baron J.A., Brown P.H., Burn J., Greenwald P., Jankowski J., La Vecchia C., Meyskens F., Senn H.J., Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 14.Smith W.L., Garavito R.M., DeWitt D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and −2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 15.Larkins T., Nowell M., Singh S., Sanford G. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons T.R., O'Brien J., Borges V.F., Conklin M.W., Keely P.J., Eliceiri K.W., Marusyk A., Tan A.-C., Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karavitis J., Hix L.M., Shi Y.H., Schultz R.F., Khazaie K., Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang S.H., Liu C.H., Conway R., Han D.K., Nithipatikom K., Trifan O.C., Lane T.F., Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M., Peluffo G., Chen H., Gelman R., Schnitt S., Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C.H., Chang S.-H., Narko K., Trifan O.C., Wu M.-T., Smith E., Haudenschild C., Lane T.F., Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 21.Na Y.R., Yoon Y.N., Son D.I., Seok S.H. Cyclooxygenase-2 inhibition blocks m2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS One. 2013;8:e63451. doi: 10.1371/journal.pone.0063451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howe L.R., Chang S.-H., Tolle K.C., Dillon R., Young L.J.T., Cardiff R.D., Newman R.A., Yang P., Thaler H.T., Muller W.J., Hudis C., Brown A.M.C., Hla T., Subbaramaiah K., Dannenberg A.J. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 23.Markosyan N., Chen E.P., Ndong V.N., Yao Y., Sterner C.J., Chodosh L.A., Lawson J.A., Fitzgerald G.A., Smyth E.M. Deletion of cyclooxygenase 2 in mouse mammary epithelial cells delays breast cancer onset through augmentation of type 1 immune responses in tumors. Carcinogenesis. 2011;32:1441–1449. doi: 10.1093/carcin/bgr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denkert C., Winzer K.-J., Müller B.-M., Weichert W., Pest S., Köbel M., Kristiansen G., Reles A., Siegert A., Guski H., Hauptmann S. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 25.Ristimäki A., Sivula A., Lundin J., Lundin M., Salminen T., Haglund C., Joensuu H., Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 26.Kwan M., Habel L., Slattery M., Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control. 2007;18:613–620. doi: 10.1007/s10552-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes M.D., Chen W.Y., Li L., Hertzmark E., Spiegelman D., Hankinson S.E. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinson H.A., Lyons T.R., Giles E.D., Borges V.F., Schedin P. Developmental windows of breast cancer risk provide opportunities for targeted chemoprevention. Exp Cell Res. 2013;319:1671–1678. doi: 10.1016/j.yexcr.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pike M.C., Krailo M.D., Henderson B.E., Casagrande J.T., Hoel D.G. 'Hormonal' risk factors, 'breast tissue age' and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien J.H., Vanderlinden L.A., Schedin P.J., Hansen K.C. Rat mammary extracellular matrix composition and response to ibuprofen treatment during postpartum involution by differential GeLC-MS/MS analysis. J Proteome Res. 2012;11:4894–4905. doi: 10.1021/pr3003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badawi A.F., Archer M.C. Effect of hormonal status on the expression of the cyclooxygenase 1 and 2 genes and prostaglandin synthesis in rat mammary glands. Prostaglandins Other Lipid Mediat. 1998;56:167–181. doi: 10.1016/s0090-6980(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien J., Lyons T., Monks J., Lucia M.S., Wilson R.S., Hines L., Man Y.G., Borges V., Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schedin P., Mitrenga T., Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–225. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- 34.Schedin P., Mitrenga T., McDaniel S., Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- 35.Heaphy C.M., Griffith J.K., Bisoffi M. Mammary field cancerization: molecular evidence and clinical importance. Breast Cancer Res Treat. 2009;118:229–239. doi: 10.1007/s10549-009-0504-0. [DOI] [PubMed] [Google Scholar]
- 36.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 37.Leo C., Faber S., Hentschel B., Höckel M., Horn L.-C. The status of cyclooxygenase-2 expression in ductal carcinoma in situ lesions and invasive breast cancer correlates to cyclooxygenase-2 expression in normal breast tissue. Ann Diagn Pathol. 2006;10:327–332. doi: 10.1016/j.anndiagpath.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Shim V., Gauthier M.L., Sudilovsky D., Mantei K., Chew K.L., Moore D.H., Cha I., Tlsty T.D., Esserman L.J. Cyclooxygenase-2 expression is related to nuclear grade in ductal carcinoma in situ and is increased in its normal adjacent epithelium. Cancer Res. 2003;63:2347–2350. [PubMed] [Google Scholar]
- 39.Boland G.P., Butt I.S., Prasad R., Knox W.F., Bundred N.J. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–429. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford Y.G., Gauthier M.L., Joubel A., Mantei K., Kozakiewicz K., Afshari C.A., Tlsty T.D. Histologically normal human mammary epithelia with silenced p16(INK4a) overexpress COX-2, promoting a premalignant program. Cancer Cell. 2004;5:263–273. doi: 10.1016/s1535-6108(04)00023-6. [DOI] [PubMed] [Google Scholar]
- 41.Visscher D.W., Pankratz V.S., Santisteban M., Reynolds C., Ristimäki A., Vierkant R.A., Lingle W.L., Frost M.H., Hartmann L.C. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J Natl Cancer Inst. 2008;100:421–427. doi: 10.1093/jnci/djn036. [DOI] [PubMed] [Google Scholar]
- 42.Flowers C.I., O'Donoghue C., Moore D., Goss A., Kim D., Kim J.H., Elias S.G., Fridland J., Esserman L.J. Reducing false-positive biopsies: a pilot study to reduce benign biopsy rates for BI-RADS 4A/B assessments through testing risk stratification and new thresholds for intervention. Breast Cancer Res Treat. 2013;139:769–777. doi: 10.1007/s10549-013-2576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker S.H., Burbank F., Jackman R.J., Aucreman C.J., Cardenosa G., Cink T.M., Coscia J.L., Jr., Eklund G.W., Evans W.P., 3rd, Garver P.R., Gramm H.F., Has D.K., Jacob K.M., Kelly K.M., Killebrew L.K., Lechner M.C., Perlman S.J., Smid A.P., Tabar L., Taber F.E., Wynn R.T. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193:359–364. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 44.Chan A.T., Ogino S., Fuchs C.S. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes M.D., Chen W.Y., Schnitt S.J., Collins L., Colditz G.A., Hankinson S.E., Tamimi R.M. COX-2 expression predicts worse breast cancer prognosis and does not modify the association with aspirin. Breast Cancer Res Treat. 2011;130:657–662. doi: 10.1007/s10549-011-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confirmation of human and rodent COX-2 antibody specificity. A: Human COX-2 IHC staining in normal adjacent tissue and tumor using primary antibody alone or primary antibody in combination with COX-2 blocking peptide. B: Rodent COX-2 IHC staining using mammary tissue from the COX-2 knockout mouse. C: Western blot for rodent COX-2 using C57Bl/6 primary macrophage lysate as a positive control (Ctrl) and mammary tissue lysate from a COX-2 knockout (KO) mouse. Ab, antibody; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Brown, COX-2--–positive cells.
Quantification of COX-2 staining intensity using Aperio analysis software. Regions of interest were annotated positively on whole slide images, and staining intensity was analyzed for negative (blue), weak (yellow), medium (orange), and strong (red) staining intensity.
COX-2 expression in histologically normal epithelium does not differ with proximity to tumor. The percentage of COX-2+ epithelium in breast tissue adjacent to tumor (adjacent) and in a quadrant of the breast not containing tumor (distant) (n = 11) is shown. P = 0.16, one-tailed paired t-test.
COX-2 (IHC staining) expression in normal adjacent breast epithelium is independent of age (A) and body mass index (BMI) (B). Pearson r = 0.039, P = 0.76, two-tailed (A); Pearson r = −0.0067, P = 0.96, two-tailed (B).
COX-2 IHC staining in intralobular stroma increases during pregnancy, whereas interlobular COX-2 expression remains relatively constant across reproductive stage. nullip, nulliparous; preg, pregnant; inv, involution. Arrows show COX-2–positive stromal cells (brown signal).