Abstract
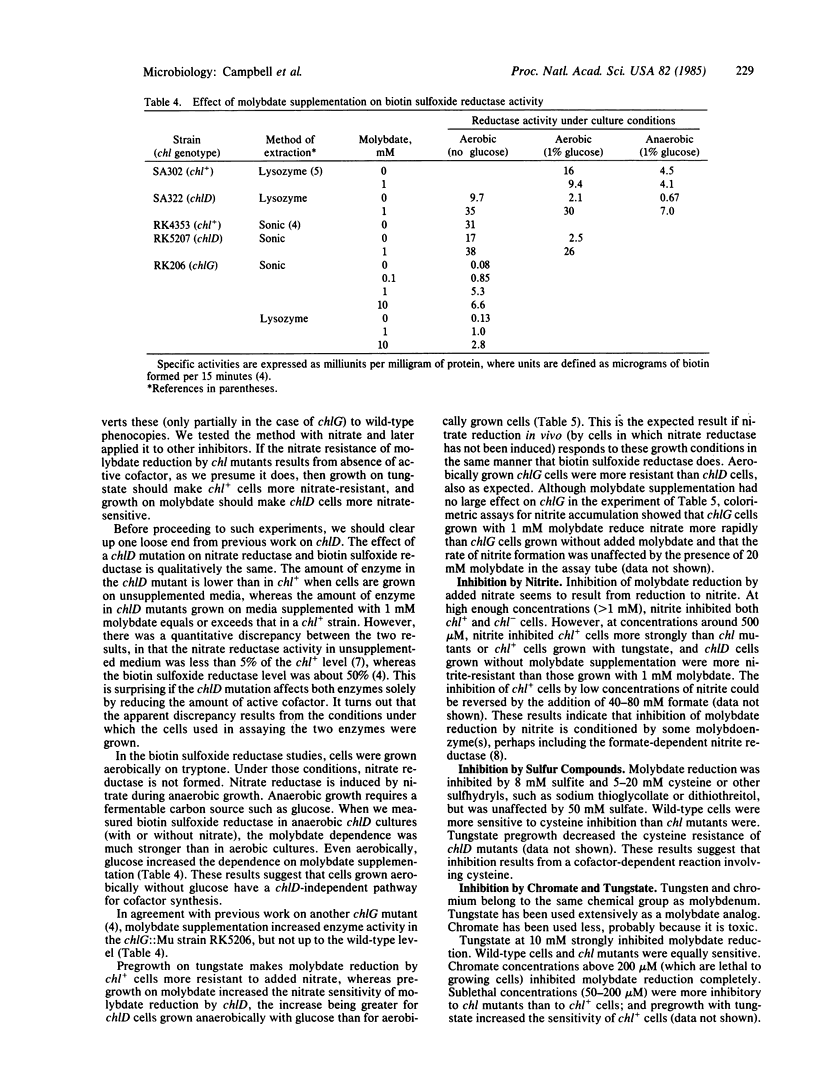
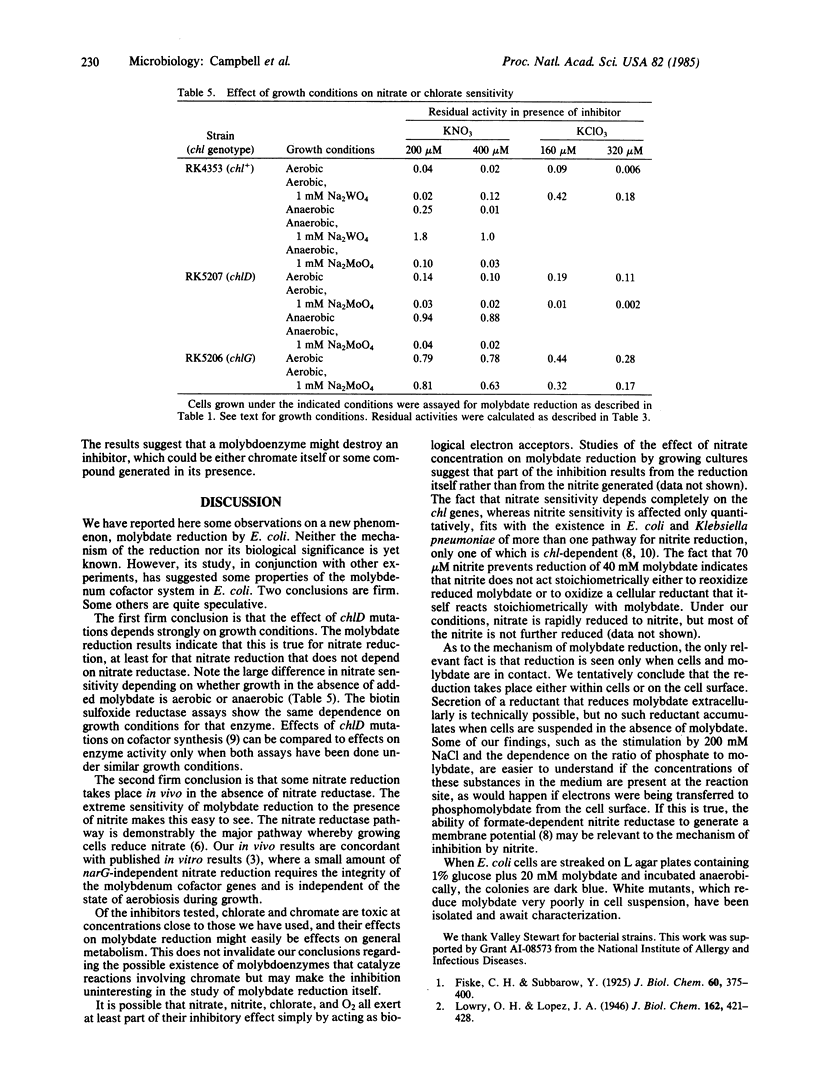
During anaerobic growth, Escherichia coli can reduce phosphomolybdate. The reduction can also be carried out by washed cells suspended in buffer at pH 5.7. Phosphate, molybdate, glucose, cells, and anaerobic conditions are required. Reduction is inhibited by 200 microM chromate, 290 microM nitrite, 10 mM tungstate, or 20 mM cysteine. Wild-type (chl+) cells are inhibited by addition of 200 microM nitrate, but chlA, chlB, and chlE mutants are not. The inhibition of chl+ cells results from reduction of nitrate to nitrite. This nitrate reduction is not catalyzed by nitrate reductase. Wild-type cells are more sensitive than chl mutants to inhibition by nitrite and cysteine but more resistant to chromate. Pregrowth of chlD cells in 1 mM Na2MoO4 increases their sensitivity to nitrite and cysteine, and pregrowth of chl+ cells in 1 mM Na2MoO4 increases their resistance to these agents. Assays of biotin sulfoxide reductase show that the tightness of the chlD block depends on growth conditions; chlD cells grown aerobically in tryptone broth make about 50% as much active enzyme as chl+ cells, whereas chlD cells grown anaerobically with tryptone plus glucose make less than 10%. The effect of anaerobic pregrowth on the inhibition of molybdate reduction by added nitrate indicates that in vivo nitrate reduction responds to growth conditions in the same manner as biotin sulfoxide reductase does.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dvir S., Acad B. A., Sonn J., Furman E., Kedem J. Preservation of myocardial oxygen balance and functional reserve by coronary vasodilators. Arch Int Physiol Biochim. 1985 Sep;93(3):231–239. doi: 10.3109/13813458509069925. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116(1):1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- Miller J. B., Amy N. K. Molybdenum cofactor in chlorate-resistant and nitrate reductase-deficient insertion mutants of Escherichia coli. J Bacteriol. 1983 Aug;155(2):793–801. doi: 10.1128/jb.155.2.793-801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope N. R., Cole J. A. Generation of a membrane potential by one of two independent pathways for nitrite reduction by Escherichia coli. J Gen Microbiol. 1982 Jan;128(1):219–222. doi: 10.1099/00221287-128-1-219. [DOI] [PubMed] [Google Scholar]
- Satoh T., Hom S. S., Shanmugam K. T. Production of nitrous oxide from nitrite in Klebsiella pneumoniae: mutants altered in nitrogen metabolism. J Bacteriol. 1983 Aug;155(2):454–458. doi: 10.1128/jb.155.2.454-458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperl G. T., DeMoss J. A. chlD gene function in molybdate activation of nitrate reductase. J Bacteriol. 1975 Jun;122(3):1230–1238. doi: 10.1128/jb.122.3.1230-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campillo-Campbell A., Campbell A. Molybdenum cofactor requirement for biotin sulfoxide reduction in Escherichia coli. J Bacteriol. 1982 Feb;149(2):469–478. doi: 10.1128/jb.149.2.469-478.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


