Abstract
β-lactam antibiotics are the most commonly used antibacterial agents and growing resistance to these drugs is a concern. Metallo-β-lactamases are a diverse set of enzymes that catalyze the hydrolysis of a broad range of β-lactam drugs including carbapenems. This diversity is reflected in the observation that the enzyme mechanisms differ based on whether one or two zincs are bound in the active site which, in turn, is dependent on the subclass of β-lactamase. The dissemination of the genes encoding these enzymes among Gram-negative bacteria has made them an important cause of resistance. In addition, there are currently no clinically available inhibitors to block metallo-β-lactamase action. This review summarizes the numerous studies that have yielded insights into the structure, function, and mechanism of action of these enzymes.
Keywords: β-lactamase, antibiotic resistance, carbapenem, zinc metallo-enzyme
Introduction
β-lactam antibiotics are among the most often used antimicrobial agents and an increasing incidence of resistance to these drugs is a public health concern. β-lactam antibiotics as a class have a broad spectrum of antibacterial activity, including important Gram-positive and Gram-negative pathogens. Because of their favorable characteristics, β-lactams are the most broadly used antibiotics worldwide.1, 2 These antibiotics act by inhibiting a set of transpeptidase enzymes (also called penicillin binding proteins or PBPs) that are essential for the synthesis of the peptidoglycan layer of the bacterial cell wall 3. The inhibition of peptidoglycan synthesis results in the death of growing bacteria and accounts for the antimicrobial effect of β-lactam antibiotics. In response, bacteria have evolved defense mechanisms to resist the lethal effects of these drugs4. Due to widespread β-lactam antimicrobial use, bacterial resistance has been increasing and now represents a serious threat to the continued use of antibiotic therapy.5
β-lactam antibiotics are characterized by a four-membered β-lactam ring that serves as a substrate for the transpeptidase target enzymes. The transpeptidase enzymes react via an active site serine residue with the D-Ala-D-Ala terminus of a pentapeptide that is attached to N-acetylmuramic acid of the peptidoglycan polymer to form an acyl-enzyme intermediate6. The carbonyl carbon of this intermediate is then attacked by a lysine-like residue from another pentapeptide to create a covalent bond between peptides which serves to cross-link the peptidoglycan. The four-membered ring of β-lactam antibiotics resembles the D-Ala-D-Ala structure and is able to bind in the active site of transpeptidase enzymes where it forms an acyl-enzyme with the active site serine. This acyl-enzyme, however, is sterically blocked for attack by the pentapeptide lysine residue and the covalently bound β-lactam is a long lived inhibitor of the transpeptidase, which blocks peptidoglycan cross-linking and leads to cell death7; 8 (Fig. 1).
Figure 1.
Illustration showing the acylation of β-lactam antibiotic by transpeptidases and β-lactamases and subsequent trapping of the transpeptidase versus deacylation and hydrolysis by the β-lactamases.
There are hundreds of different β-lactams, and they are classified into groups based on structure9; 10. Clinically important β-lactams include the penicillins, cephalosporins, carbapenems, and monobactams (Fig. 2). The penicillins and cephalosporins contain the β-lactam ring fused to five- and six-membered rings, respectively, which contain a carboxyl-group at the C-3 and C-4 positions. As a group, these antibiotics display a wide range of activity against Gram-positive and Gram-negative bacteria. The monobactams, in contrast, do not contain a fused ring system and consist of the β-lactam ring with a linked sulfonic acid group at the analogous position of the carboxylate group found in penicillins and cephalosporins11; 12. The monobactams are active against aerobic Gram-negative pathogens. Finally, the carbapenems possess a potent, broad spectrum of activity against Gram-positive and Gram-negative bacteria and are an increasingly essential group of antibiotics for the treatment of infections caused by multidrug resistant bacteria 13. The carbapenems consist of a β-lactam ring fused to a penicillin-like five-membered ring that has a carbon replacing the sulfur at C-1 and also contains a double bond between C-2 and C-3. Another important characteristic of the carbapenems is resistance to inactivation by β-lactamases. In fact, carbapenems act as inhibitors for many β-lactamases by reacting with an active site serine and forming a long-lived acyl-enzyme intermediate14–16. However, in the past decade β-lactamases able to hydrolyze carbapenems, including the class A carbapenemases and class B metallo-β-lactamases, have become an increasing source of resistance 13; 17.
Figure 2.
Classes of β-lactam antibiotics. A. Core structure of penicillin. Different R-groups distinguish various penicillins. B. Cephalosporin core structure. C. Monobactam core structure. D. Carbapenem core structure. Atoms are numbered for reference to discussion in the text.
There are several mechanisms by which bacteria acquire resistance to β-lactam antibiotics including efflux, reduced permeability, altered transpeptidases, and inactivation by β-lactamases. Alteration of the sequence of target transpeptidases by mutation or recombination to create enzymes that bind poorly to β-lactams is an important source of resistance in Streptococcus pneumoniae18. In addition, the acquisition of a new transpeptidase (PBP2a) that binds β-lactam antibiotics slowly is the basis for resistance in methicillin-resistant S. aureus (MRSA)19. However, despite these important examples, the production of β-lactamase enzymes is the most common mechanism of bacterial resistance to β-lactam antibiotics.
β-lactamases catalyze the hydrolysis of the amide bond in the β-lactam ring to generate ineffective products. They are a frequent cause of resistance among Gram-negative bacteria and among certain Gram-positive species. Genes encoding β-lactamases can be found on the bacterial chromosome or on plasmids. Based on primary sequence homology, β-lactamases have been grouped into four classes20. Classes A, C, and D are active-site serine enzymes that catalyze, via a serine-bound acyl intermediate, the hydrolysis of the β-lactam 21. Class B enzymes require zinc for activity and catalysis does not proceed via a covalent intermediate but rather through direct attack of a hydroxide ion that is stabilized by the zinc in the active site 22; 23. The active-site serine β-lactamases belong to a larger family of penicillin-recognizing enzymes that includes the transpeptidases that crosslink bacterial cell walls 24–26. All of these enzymes contain the active-site serine as well as a conserved triad of K(S/T)G between the active-site serine and the C-terminus 24–26. The crystal structures of numerous class A, C, and D β-lactamases as well as transpeptidases show these enzymes have a similar three-dimensional structure, particularly around the active-site, suggesting a common evolutionary origin for the penicillin-recognizing enzymes 26. The structures of several class B enzymes confirm the lack of similarity with the serine β-lactamases and transpeptidases and indicate an independent evolutionary origin for these enzymes. 27–29
Class B metallo-β-lactamases
Spectrum and dissemination
Class B metallo-β-lactamases (MBLs) have a broad substrate spectrum and can catalyze the hydrolysis of virtually all β-lactam antibiotics with the exception of monobactams. They are not inhibited by mechanism-based inhibitors such asclavulanate, sulbactam, or tazobactam that are effective against serine-based, class A β–lactamases30; 31. In addition, they are not effectively inhibited by NXL-104, which is an inhibitor of class A and C enzymes that is in clinical trials32. Also, in contrast to serine-based enzymes, MBLs are inactivated by metal chelators such as EDTA30. MBLs were initially discovered over forty years ago but were not initially considered a serious problem for antibiotic therapy because they were found chromosomally encoded and in non-pathogenic organisms33; 34. This situation changed in the 1990s, however, with the spread of the IMP- and VIM-typemetallo-β-lactamases in Gram-negative pathogens, including Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter bumannii35; 36. In addition, the IMP- and VIM-type enzymes are encoded as gene cassettes and reside with other resistance genes within integron structures that are associated with transposons and can insert on the bacterial chromosome or within plasmids.35; 37 Integrons facilitate movement of resistance genes between integrons in plasmids and the plasmids allow transfer of genetic material to different bacteria38. Together, this is a formula for the spread of metallo-β-lactamase genes and, more generally, for the emergence of multidrug resistant bacteria.
The recently discovered NDM-1 β-lactamase provides an example of the potential for dissemination of MBLs. NDM-1 was first detected in 2008 in Klebsiella pneumonia and Escherichia coli in a patient returning to Sweden from India39. NDM-1 has been shown to be present at significant frequency within Enterobacteriacea in India and has subsequently been shown to be present in bacterial isolates in a number of countries worldwide39. The blaNDM-1 gene has been found on several plasmid types, including IncA/C, IncF, IncL/M, and it can be transferred among Gram-negative bacteria by conjugation. However, in contrast to the situation with the genes encoding IMP- and VIM-type MBLs, the blaNDM-1 gene has not been found in integron structures. Nevertheless, NDM-1 has spread broadly and rapidly40. The ISAba125 insertion element has been associated with the blaNDM-1 gene suggesting insertion sequences may contribute to transfer of NDM-1.
Metallo-β-lactamase structure and subclasses
As a family, the MBLs exhibit a diverse range of sequences with as little as 25% identity between some enzymes41.X-ray structures of multiple MBLs indicate, however, that the group is structurally similar and has a characteristic αβ/βα sandwich fold with the active site located at the interface between domains27; 29; 42–52. This scaffold supports up to six residues at the active site which coordinate either one or two zinc ions that are central to the catalytic mechanism. The MBL αβ/βαfold-type encompasses a larger superfamily of metalloproteins with diverse biological functions beyond β-lactam hydrolysis.53 Most non-β-lactamase superfamily members display a dinuclear metal center to catalyze hydrolysis of various substrates54. The coordination of zinc among non-β-lactamase superfamily members is broadly similar to MBLs except for the presence of an aspartate residue in non-MBLs that bridges the metal ions. Members of the superfamily are also known to bind a diverse set of metals including zinc, iron, and manganese 54. Enzymes with the MBL fold include glyoxalase II, arylsulfatase, cyclase/dihydrase, among others 53.
The MBLs are divided into three subclasses (B1, B2, B3) based on primary amino acid sequence homology55 (Table 1). There is relatively low sequence identity (< 20%) between the subclasses which can produce unreliable sequence alignments across subclasses 41. Within a subclass the sequence identity is higher and this, along with distinctive structural characteristics within the active sites of B1, B2, and B3 enzymes, is the basis for the establishment of the three subclasses 41.
Table 1.
Metallo-β-lactamases from subclasses B1, B2, and B3
| Subclass | Enzymea | Organismb | Genbank accession number |
PDB codec |
|---|---|---|---|---|
| B1 | BcII | Bacillus cereus | M11189 | 1BMC28 |
| CcrA | Bacteroidesfragilis | M63556 | 1ZNB29 | |
| IMP-1 | Serratiamarcescens, Pseudomonas aeruginosa | S71932 | 1DD689 | |
| VIM-2 | Pseudomonas aeruginosa, Acinetobacterbaumanii | AF191564 | 2YZ3, 1K0248,107 | |
| VIM-4 | Pseudomonas aeruginosa, Acinetobacterbaumanii | AY135661 | 2WHG108 | |
| VIM-7 | Pseudomonas aeruginosa, Acinetobacterbaumanii | AM778842 | 2Y87109 | |
| BlaB | Chryseobacteriummeningoseptica | AF189298 | 1M2X49 | |
| SPM-1 | Pseudomonas aeruginosa | AY341249 | 2FHX51 | |
| NDM-1 | Klebsiellapneumonia, Escherichia coli | JN420336 | 3Q6X, 3SPU, 3RKJ52,56,57 | |
| VIM-1 | Pseudomonas aeruginosa, Acinetobacterbaumanii | T18050 | ||
| GIM-1 | Pseudomonas aeruginosa | AJ620678 | ||
| SIM-1 | Acinetobacterbaumanii | AY887066 | ||
| DIM-1 | Pseudomonas stutzeri | GU323019 | ||
| TMB-1 | Achromobacterxylobacter | FR771847 | ||
| Bla2 | Bacillus anthracis | CP001598 | ||
| KHM-1 | Citrobacterfreundii | AB364006 | ||
| B2 | CphA | Aeromonashydrophila | X57102 | 1X8G, 3F9O47,84 |
| Sfh-1 | Serratiafonticola | AF197943 | 3SD983 | |
| ImiS | Aeromonasveronii | Y01415 | ||
| B3 | L1 | Stenotrophomonasmaltophilia | AB294542 | 1SML27 |
| FEZ-1 | Legionella gormannii | Y17896 | 1K0750 | |
| BJP-1 | Bradyrhizobiumjaponicum | NP772870 | 3LVZ45 | |
| AIM-1 | Pseudomonas aeruginosa | AM998375 | 4AWY110 | |
| THIN-B | Janthinobacteriumlividum | CAC33832 | ||
| GOB-1 | Chryseobacteriummeningoseptica | ABO21417 | ||
| CAU-1 | Caulobactercrescentus | CAC87665 | ||
| CAR-1 | Erwiniacaratovora | Q6D395 | ||
| SMB-1 | Serratiamarcescens | AB636283 | ||
| POM-1 | Pseudomonas otitidis | ADC79555 | ||
| CRB11 | Uncultured bacterium | ACS83724 |
Enzyme variants are not included in this table unless an X-ray structure of the variant is available. For example, there are many variants of IMP-1 that are not listed in the table.
The strains listed represent the original strain(s) for which the enzyme was identified and does not include the bacteria to which the gene has spread.
Protein data bank codes are listed for all enzymes for which X-ray structures have been determined.
X-ray crystal structures of B1 enzymes including BcII28; 46, CcrA29, IMP-1 44, VIM-2 48, VIM-7 42, BlaB49, NDM-1 52; 56; 57, SPM-1 51, among others, have been solved and reveal two zinc binding sites (labeled Zn1 and Zn2). The Zn1 binding site, also known as the 3H site, contains three histidines (His116, His118, and His196), while the ligands for the Zn2 or DCH site includes Asp120, Cys221, and His263. The zinc ligand residues in both the 3H and DCH sites are strictly conserved among B1 enzymes. These zinc binding motifs around the active site can distinguish MBL subgroups as B1 (zinc ligands at H116-H118-H196 and D120-C221-H263), B2 (N116-H118-H196 and D120-C221-H263)47 or B3 enzymes (H/G116-H118-H196 and D120-H121-H262) (Fig. 3 and Table 1). 27; 41; 45; 50; 58 The diverged amino acid sequences of the subclasses are reflected in somewhat different catalytic properties of the enzymes. For example, the B1 and B3 enzymes are most active with two zinc ions (Zn1 and Zn2) bound in the active site while the binding of the second zinc in the B2 enzymes inhibits catalysis. The B1 and B3 subclasses have a broad-spectrum substrate profile that includes penicillins, cephalosporins, and carbapenems while the B2 enzymes exhibit a narrow profile that includes carbapenems.
Figure 3.
Schematic illustration of the amino acid residues that serve as zinc binders in the active sites of subclass B1, B2, and B3 metallo-β-lactamases. A. Active site zinc chelator residues for the Bacteroidesfragilis subclass B1 CcrA enzyme (pdb ID: 1ZNB) 29. B. Active site zinc binding residues for Aeromonashydrophilamonozinc enzyme of subclass B2 (pdb ID: 1X8G)47. C. Active site zinc binding residues for Stenotrophomonasmaltophilia L1 enzyme of subclass B3 (pdb ID: 1SML)27. Zinc ions are labeled and shown as spheres.
Metallo-β-lactamase substrate binding and catalysis
Subclass B1 contains the largest number of known MBLs and includes the extensively studied Bacillus cereus BcII enzyme and the clinically important and transferable IMP-, VIM-, and NDM-type enzymes. It is known that the Zn metal ions play an important role in β-lactam binding in that a properly folded BcIIapo-enzyme is not capable of binding substrate59. X-ray structures of the B. cereus BcII enzyme have been solved with the mono-form obtained at low pH and containing the zinc ion at the Zn1 (3H) site 28. A wide range of binding constants have been reported for zinc binding to each site in the BcII enzyme with values ranging from low nM to 120 µM for K1 and 1.5 µM to 24 mM for K2 where K1 and K2 are the equilibrium dissociation constants for Zn1 and Zn2, respectively60–62.More recently, nuclear magnetic resonance, mass spectrometry, and catalytic activity measurements were used to show that zinc ions bind cooperatively to the zinc sites of BcII, that is, binding of zinc at one site increases affinity for binding the other site. Because of this positive cooperativity, catalysis is proposed to be associated only with the di-zinc enzyme63. Other studies on the BcII MBL also indicate that only the di-zinc form of the enzyme is catalytically active, which is consistent with positive cooperativity of zinc binding64; 65. Also consistent with these findings are experiments with zinc chelator treatment and enzyme kinetic studies of the B1 enzyme CcrAwhich revealed positive cooperativity for zinc binding in that the monozinc enzyme was not detected66. In addition, the subclass B1 IMP-1 enzyme exhibits positive cooperativity for zinc binding 67. In contrast, the subclass B1 Bla2 enzyme from Bacillus anthracis binds zinc sequentially and the single zinc enzyme can be isolated and has enzymatic activity while the dizinc enzyme was found to be unstable68.
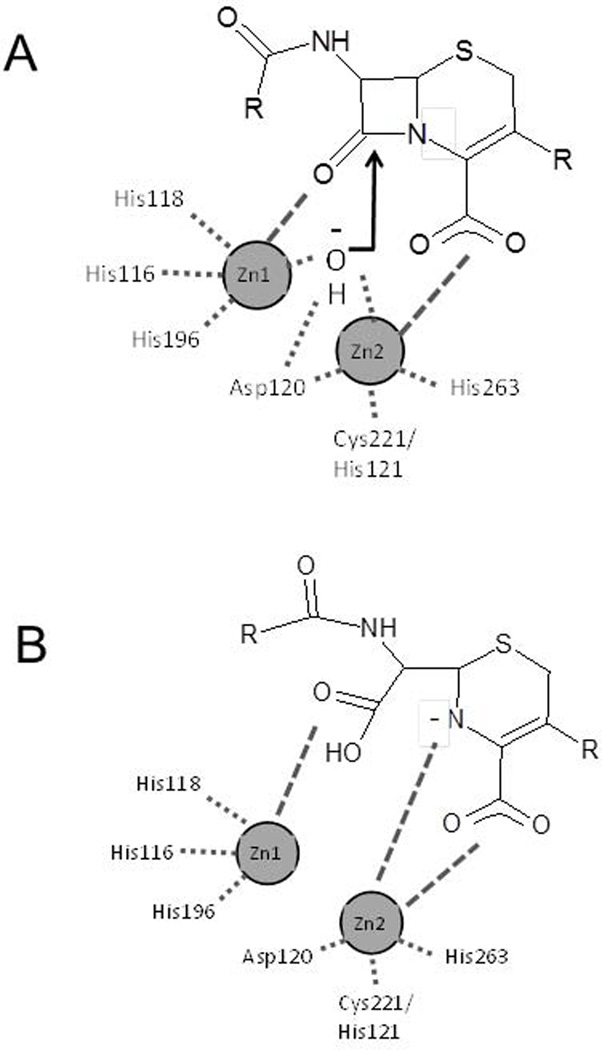
A detailed understanding of the mechanism of catalysis by metallo-β-lactamases, including knowledge of the rate limiting steps and the chemical nature of reaction intermediates for the various enzyme subclasses will facilitate the rational design of inhibitors. For the di-zinc MBLs, which includes the subclass B1 and B3 enzymes, hydrolysis is believed to occur by cleavage of the amide bond of the β-lactam ring via attack of a hydroxide ion on the carbonyl carbon23; 69; 70 (Fig. 4). Enzyme catalysis initiates with the binding of the β-lactam at the metal center with the carbonyl oxygen interacting with Zn1 and the carboxyl group on the 5- or 6-membered fused ring bound to Zn2. The hydroxide ion is stabilized by Zn1 and Zn2 and resides between the metal ionsin a position to attack the carbonyl carbon. Nucleophilic attack of the hydroxide on the carbonyl carbon leads to formation of a tetrahedralintermediate69; 71. Two possibilities exist for breakdown of the tetrahedral intermediate and cleavage of the C-N bond: 1) bond breaking could be coincident with protonation of nitrogen or 2) cleavage could occur without nitrogen protonation leading to the accumulation of an anionic nitrogen intermediate (Fig. 4)23; 70.
Figure 4.
Schematic illustration of cephalosporin binding to dizincmetallo-β-lactamase active site (subclasses B1 and B3). The zinc ions are labeled and interactions are shown with dashed lines. A. Cephalosporin substrate bound to active sites with interactions to both Zn1 and Zn2 via the carbonyl oxygen and carboxyl groups, respectively. The bound hydroxide is positioned to attack the carbonyl carbon of the substrate. B. Anionic intermediate bound in the active site via stabilizing interactions provided by Zn2.
Pre-steady state kinetic studies on the subclass B1 CcrA enzyme with the chromogenic substrate nitrocefin provided evidence for the rate limiting step being protonation of a negatively charged nitrogen intermediate absorbing at 665 nm, which is stabilized by Zn270. Subsequent stopped flow kinetics studies on nitrocefin hydrolysis provided evidence for the intermediate in the subclass B1 NDM-1 and B3 L1 enzymes but not in the BcII or Bla2 enzymes 68; 72–75. In addition, one group has reported accumulation of the intermediate during nitrocefin hydrolysis by IMP-1 while two other groups have failed to detect the intermediate during IMP-1 catalyzed nitrocefinhydrolysis 67; 76; 77. Although an anionic nitrogen intermediate is not detected during BcII hydrolysis of nitrocefin, there is evidence from quench-flow studies that an intermediate does accumulate during impenem hydrolysis 78. Taken together, the results suggest that relatively small differences in the active sites of di-zinc MBLs can influence catalysis such that either C-N bond cleavage or subsequent protonation of the anionic nitrogen are rate-limiting, depending on the enzyme and substrate.
Recent structural work on the NDM-1 enzymes of subclass B1 with bound hydrolysis product has revealed details of substrate binding and hydrolysis by B1 enzymes. Zhang and Hao obtained a structure of NDM-1 with the hydrolyzed ampicillin hydrolysis product present in the active site 52. The structure revealed the C-N bond cleaved and N-1 in a coordinate bond with Zn2 (see Fig. 2A for penicillin atom numbering). In addition, the oxygens of the C-3 carboxylate group were bound by Lys224 and Zn2 respectively. The carboxylate at C-7 formed by hydrolysis of the C-N bond was found to interact with Zn1 and the side chain of Asn233 52. The structure is consistent with previously proposed mechanisms where a hydroxide stabilized by Zn1 and Zn2 for attack on the carbonyl carbon with subsequent C-N cleavage and protonation of the nitrogen. Previously, it was suggested that the source of the proton could be from a water molecule bound to Zn2 in the apoenzyme or from a bulk water entering the active site 29; 79. Based on the NDM-1 ampicillin structure, the authors propose a proton from the carboxylate (originally from the attacking hydroxide) serves to protonate the nitrogen either coincident with or after C-N bond cleavage 52.
Additional information on substrate binding and hydrolysis by subclass B1 enzymes has been provided by the structures of NDM-1 bound with hydrolyzed product for methicillin, benzylpenicillin, oxacillin, and meropenem80. The structures reveal the penicillin core consisting of the β-lactam ring and the fused thiazolidine ring coordinates precisely and rigidly to the active site with the C-7 carboxylate that is formed by hydrolysis bound to Zn1 and the N-4 atom as well as the C-3 carboxylate group bound by Zn2 80. However, the R functional groups of the penicillins displayed variable conformations and higher crystallographic temperature factors than the core region. It was also observed that the NDM-1 active site is able to accommodate the bulky R groups of methicillin and oxacillin and thus the idea of increasing bulk at the R position to reduce β-lactam action may not be valid for subclass B1 enzymes 80. In addition, this study found that hydrolyzed meropenem is bound in a somewhat different position than the penicillins. The N-4 and C-3 carboxylate interact with Zn2 in a similar fashion as penicillins but the C-7 carboxylate resulting from hydrolysis intercalates between Zn1 and Zn2 resulting in tetracoordination of Zn1 and hexacoordination of Zn2 in contrast to the penicillins where the carboxylate is shifted way from Zn2 and interacts with Zn180. In addition, the side chain of Asn233 (class B numbering41), which is conserved in B1 and B2 enzymes, exhibits a tight hydrogen bond to oxygen from the C-7 carboxylate of hydrolyzed meropenem supporting a role for this residue in carbapenem binding and catalysis 80.
A recent computational substrate docking study of various β-lactam antibiotics into the active site of the NDM-1 enzyme has also produced interesting results81. The poses of the docked substrates revealed two major modes of binding which were named “S” and “I” conformers. The “S” mode has the carbonyl oxygen of the amide of β-lactam ring coordinated to Zn1 and the carboxylate of the fused ring coordinated with Zn2 and the bridging water/hydroxide positioned for nucleophilic attack and therefore this conformer is optimally positioned for catalysis81. The “I” mode contains the carboxylic acid of the fused ring coordinating with Zn1 and Zn2 and displacing the water/hydroxide and moves the β-lactam amide group away from the metal ions thus creating an inhibitory binding mode. Interestingly, the “S” conformer dominates over the “I” in the set of docked structures for good substrates while for poor substrates, such as the monobactamaztreonam, virtually all docking structures are in the “I” mode displacing the catalytic water/hydroxide81. This result suggests that monobactams are not hydrolyzed by metallo-β-lactamases because they bind with the sulfonate group bridged between Zn1 and Zn2 and displace the catalytic water81.
The subclass B2 β-lactamases differ from B1 and B3 enzymes in that they contain one zinc in the active site and they display a narrow substrate spectrum focused almost exclusively on carbapenem hydrolysis.82 In addition, the B2 MBLs are active only in the one zinc form; with the zinc bound at the Zn2 site.47, 83 A structure has been solved of the B2 CphA and Sfh-I enzymesreveal the single zinc at the Zn2 site.47, 83 The structure of the inhibited form of CphA with two zincs bound has also been solved and indicates the inhibitory zinc is present in the Zn1, histidine site with His118 and His196 serving as metal ligands.84 In addition, the CphA structure has been solved with hydrolyzed biapenem antibiotic in the active site. Enzyme reaction mechanisms for subclass B2 MBLs for biapenem have been proposed based on the structures (Fig.5). The proposed mechanisms have some differences but common features are an interaction between Zn2 and the conserved Lys224 residue with the carbapenem β-lactam C-3 carboxyl group (see Fig. 2D for carbapenem atom numbering).47, 85, 86 In addition, a water molecule is held by interactions with His 118 and Asp120 residues and one of these could act as a general base to activate water for attack on the C-7 carbonyl carbon of the substrate (Fig. 5). It has been proposed based on the Sfh-I structure, which reveals waters in the active site, that His118 serves as the general base.83 The carbonyl group is polarized by interaction of the carbonyl oxygen with His196 in the CphA enzyme. The cleavage of the C-N bond has been proposed to proceed with accumulation of an anionic nitrogen stabilized by Zn2 (Refs. 47; 85; 86). Finally, protonation of the nitrogen has been proposed to occur via a water molecule bound by His118 and Asp120 or via a water bound to Zn2 (Refs. 47; 85; 86).
Figure 5.
Carbapenem substrate and anionic intermediate binding to monozincmetallo-β-lactamase active site (subclass B2). A. Carbapenem binding to monozinc active site. An active site water bonds with Asp120 and His118 and is activated for attack on the carbonyl carbon of the carbapenem. B. Anionic intermediate shown stabilized in the active site by interactions with the Zn2 ion.
Role of active site flexible loop in substrate binding and catalysis
Conformational changes are known to play an important role in enzyme catalysis. An important type of conformational change is closure of a flexible loop over enzyme-bound substrates 87. Numerous enzymes, including triosphosphateisomerase (TIM), orotate monophosphate decarboxylase, and glycosyltransferases, to name a few, undergo conformational changes upon substrate binding whereby a flexible loop closes over the bound substrate 87, 88. Loop closure over substrate has been shown in several systems to sequester substrate from water and decrease the effective active site dielectric constant which can result in an increase in transition state stabilization from enhanced electrostatic interactions with active site amino acids 87.
Subclass B1 and B3 MBLs also contain a loop structure near the active site (loop L3) that is thought to undergo a conformational change and close over bound substrate based on structures of MBLs with and without inhibitor in the active site (Fig. 6).89, 90 In addition, NMR studies on the class B1 Bacteroides fragilis CcrA enzyme revealed significant chemical shifts of L3 loop residues upon inhibitor binding consistent with a role for the loop for substrate/inhibitor binding.91 In particular, a tryptophan residue (Trp49 in CcrA-Trp64 in class B numbering) at the tip of the L3 loop displays decreased motion when inhibitor is bound suggesting it plays a role in recruiting and stabilizing ligands in the active site.91 A tryptophan and phenylalanine is found at a similar position at the tip of the loop for the IMP-1 and NDM-1 enzymes, respectively, although other residue types are found at this position among various B1 subclass β-lactamases 58. The results of site-directed mutagenesis studies of Trp64 in CcrA and IMP-1 are consistent with a role for the residue in substrate binding in that KM values are invariably increased for hydrolysis of β-lactam substrates for Trp64 substituted enzymes of both CcrA and IMP-1 (Refs. 76 and 92). The effects of Trp64 substitutions on kcat were variable depending the substrate but, interestingly, for the IMP-1 enzyme, mutants displayed increased kcat values for some substrates 76. A suggested explanation for this result is that the loop in the wild type enzyme facilitates tight binding of substrate but forces the substrate into a position that is not optimal for nucleophilic attack and mutation of Trp64 leads to weaker binding but improved positioning for catalysis 76.
Figure 6.
Illustration showing the closure of loop L3 over bound inhibitor in the subclass B1 CcrAβ-lactamase. A. Active site structure with L3 loop shown in cartoon of the CcrA enzyme without inhibitor bound. Zn2 is closest to the loop to the left and Zn1 is on the right side of the figure (PDB ID: 2BMI). B. CcrA active site with L159,061 inhibitor bound (PDB ID: 1A8T)90. The bound inhibitor molecule is colored white. The Trp64 (class B numbering system) is shown on the tip of the L3 loop.
Studies on the S. maltophilia L1 enzyme of subclass B3 also support a role for the flexible loop in substrate binding and catalysis. Spencer et al. showed that binding of substrate to L1 results in rapid fluorescence quenching and that the initial quenching was related to the rate of substrate binding and that the return of fluorescence to the resting enzyme was correlated with kcat93. Crowder and colleagues subsequently showed that the fluorescence changes were associated with a particular tryptophan residue (Trp39).94 Mutation of Trp39 along with introduction of a tryptophan to the flexible loop allowed monitoring of the movement of the loop by fluorescence. These studies showed that the kinetics of loop movement correlated with the non-rate limiting formation of the anionic intermediate during nitrocefin hydrolysis94. Taken together, the results for the various MBLs support an important role for the flexible loop in substrate binding and catalysis.
Mutagenesis studies on metallo-β-lactamases
A large number of site directed mutagenesis experiments have been performed on MBLs to examine the importance of zinc chelating residues as well as other residues closely associated with the active site region including the L3 loop as described above76. For example, the zinc-binding Cys221 residue has been substituted in the subclass B1 and B2 enzymes including B. cereus BcII, CcrA, CphA, and IMP-1 and loss of the cysteine side chain results in a large decrease in rates of hydrolysis95–99. It was also noted in these studies for the IMP-1 enzyme that addition of exogenous zinc could restore activity of the Cys221 mutant suggesting the mutant has reduced zinc binding affinity that is compensated for by excess zinc.96; 99 Similarly, substitution of Asp120 in all three subclasses results in reduced catalytic activity pointing to a critical role for this residue for hydrolysis of substrates.77; 100; 101 In the case of the subclass B1 IMP-1 β-lactamase, characterization of site directed mutant enzymes indicated that Asp120 does not contribute to a decrease in the pKa for the catalytic water between Zn1 and Zn2 and is not a proton donor for the anionic intermediate during catalysis 77. In addition, as indicated above, several active site histidine residues are strongly conserved and serve as zinc chelating residues in each of the subclasses (Fig. 3) 41; 58. Site directed mutagenesis studies of the active site histidines in MBLs have been performed and the results are consistent with an important role for these residues in zinc binding and hydrolytic activity.96; 100; 102; 103
Saturation mutagenesis and directed evolution studies of MBLs have also yielded insights into the sequence requirements for enzyme function. Materon et al. analyzed the residues in and near the active site of the subclass B1 IMP-1 enzyme using a randomization and genetic selection strategy102–104. For these studies, the codons for 29 residue positions in IMP-1 were individually randomized by oligonucleotide mutagenesis to create 29 random libraries. Each random library was then introduced into E. coli and clones expressing functional β-lactamase mutants were identified by selection for growth on agar plates containing β-lactam antibiotic. The resulting functional IMP-1 mutants were sequenced for each library to generate information on the sequence requirements at each position 103. This experiment was subsequently expanded to include selection of functional mutants from the random libraries for several different β-lactam antibiotics. A comparison of the sequencing results from each antibiotic selection allowed the identification of residue positions that are critical for hydrolysis of all antibiotics tested. These residues included all of the IMP-1 zinc chelating residues as well as the Gly65 residue at the tip of the flexible L3 loop 102. At several residue positions, however, it was found that the sequence requirements for hydrolysis were dependent on the antibiotic used for selection, that is, the sequence requirements were context dependent. These residues included Gly65, Lys69, Asp84, Leu95, Lys224, Pro225, Gly232, Asn233, Asp236, Ser262 (class B numbering scheme) and the results suggest these positions influence the substrate specificity of the enzyme 102. Subsequent studies on substitutions at the context-specific residue Asn233 showed with enzyme kinetic studies on hydrolysis that substitutions at this position have varying effects based on the substrate chosen for study which is consistent with the randomization and selection results 104.
Vila and colleagues have performed directed evolution studies on the subclass B1 BcII enzyme using DNA shuffling 105. In these experiments, DNA shuffling, which is a combination of PCR mutagenesis and in vitro recombination, was used with selection for increased resistance to cephalexin to evolve the enzyme towards increased hydrolysis of this cephalosporin 105. The experiment resulted in the generation of a variant enzyme (M5) with four mutations that exhibited an expanded substrate spectrum and also retained catalytic efficiency towards normally good substrates. One of the mutations discovered was Gly262Ser, which is also found in natural variant MBLs and is a second shell zinc ligand 105. The results of this study indicate second shell mutations can influence zinc binding and thereby influence catalytic characteristics. Structural characterization of the evolved variant from this experiment revealed that, although none of the substitutions are directly in the active site, the zinc ions are closer together than in BcII106. In addition, in contrast to the wild type BcII enzyme, nitrocefin hydrolysis by the Gly262Ser mutant is associated with accumulation of the anionic intermediate with the negatively charged nitrogen arising after amide bond cleavage as described above for the CcrA enzyme. Therefore, the mutation identified by directed evolution acts by stabilizing a reaction intermediate and decreasing the rate-limiting step for hydrolysis 106. Naturally evolved mutations that alter catalysis could act by a similar mechanism.
Conclusion
As summarized in this review, MBLs are a diverse group of metallo-enzymes that are capable of catalyzing the hydrolysis of a wide range of β-lactam antibiotics. The dissemination of the genes encoding these enzymes on genetic structures such as integrons and plasmids makes MBLs an important potential source of resistance and a cause for concern. A great deal of work has been done on MBL structure and function and mechanisms have been proposed for both di-zinc and monozinc enzymes. Although not the subject of this review, there are currently no clinically available inhibitors for MBLs and this represents an active area of research (for reviews, see Refs. 30 and 31). The detailed understanding of MBL structure and function that is emerging from these studies should facilitate the design of new inhibitors.
Acknowledgments
The author’s work on β-lactamase structure and function is funded by NIH Grant AI32956.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Livermore DM. The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006;14:413–420. doi: 10.1016/j.tim.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Livermore DM. Fourteen years in resistance. IntJAntimicrob. Chemother. 2012;39:283–294. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and roled in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Ann. Rev. Microbiol. 2011;65:455–478. doi: 10.1146/annurev-micro-090110-102911. [DOI] [PubMed] [Google Scholar]
- 5.Babic M, Hujer AM, Bonomo RA. What's new in antibiotic resistance? Focus on beta-lactamases. Drug Resistance Updates. 2006;9:142–156. doi: 10.1016/j.drup.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee W, McDonough MA, Kotra L, Li ZH, Silvaggi NR, Takeda Y, Kelly JA, Mobashery S. A 1.2-A snapshot of the final step of bacterial cell wall biosynthesis. Proc. Nat. Acad. Sci. USA. 2001;98:1427–1431. doi: 10.1073/pnas.98.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buynak JD. Cutting and stitching: The cross-linking of peptidoglycan in the assembly of the bacterial cell wall. ACS Chem. Biol. 2007;2:602–605. doi: 10.1021/cb700182u. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Meroueh SO, Fisher JF, Mobashery S. A computational evaluation of the mechanism of penicillin-binding protein catalyzed cross-linking of the bacterial cell wall. J. Amer. Chem. Soc. 2011;133:5274–5283. doi: 10.1021/ja1074739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K. The coming age of antibiotics: discovery and therapeutic value. Ann. N.Y. Acad. Sci. 2010;1213:1–4. doi: 10.1111/j.1749-6632.2010.05872.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan GB, Balachandran L. Antibacterial agents from actinomycetes- a review. Front. Biosci. 2012;4:240–253. doi: 10.2741/373. [DOI] [PubMed] [Google Scholar]
- 11.Neu HC. Aztreonam activity, pharmacology, and clinical uses. Amer. J. Med. 1990;88:2S–5S. doi: 10.1016/0002-9343(90)90079-s. [DOI] [PubMed] [Google Scholar]
- 12.Sykes RB, Bonner DP. Aztreonam: The first monobactam. Amer. J. Med. 1985;78:2–10. doi: 10.1016/0002-9343(85)90196-2. [DOI] [PubMed] [Google Scholar]
- 13.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob. Agents Chemother. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maveyraud L, Mourey L, Kotra LP, Pedelacq J, Guillet V, Mobashery S, Samama J. Structural basis for clinical longevity of carbapenem antibiotics in the face of challenge by the common class A β-lactamases from the antibiotic resistant bacteria. J. Amer. Chem. Soc. 1998;120:9748–9752. [Google Scholar]
- 15.Nukaga M, Bethel CR, Thomson JM, Hujer AM, Distler A, Anderson VE, Knox JR, Bonomo RA. Inhibition of class A β-lactamases by carbapenems: Crystallographic observation of two conformations of meropenem in SHV-1. J. Amer. Chem. Soc. 2008;130:12656–12662. doi: 10.1021/ja7111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay LW, Fan F, Blanchard JS. Biochemical and structural characterization of Mycobacterium tuberculosis β-lactamase with the carbapenems ertapenem and doripenem. Biochemistry. 2010;49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 2010;13:558–564. doi: 10.1016/j.mib.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Hakenbeck R, Bruckner R, Denapaite D, Maurer P. Molecular mechanisms of β-lactam resistance in Streptococcus pneumoniae. Future Microbiol. 2012;7:395–410. doi: 10.2217/fmb.12.2. [DOI] [PubMed] [Google Scholar]
- 19.Fuda C, Surorov M, Vakulenko SB, Mobashery S. The basis for resistance to β-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004;279:40802–40806. doi: 10.1074/jbc.M403589200. [DOI] [PubMed] [Google Scholar]
- 20.Ambler RP, Coulson FW, Frere J-M, Ghuysen J-M, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. A standard numbering scheme for the class A β-lactamases. Biochem. J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghuysen J-M. Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 22.Crowder MW, Spencer J, Vila AJ. Metallo-β-lactmases: novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Fast W, Valentine AM, Benkovic SJ. Metallo-β-lactamase: structure and mechanism. Curr. Opion. Chem. Biol. 1999;3:614–622. doi: 10.1016/s1367-5931(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 24.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to b-lactam antibiotics: compelling opportusism, compelling opportunity. Chem. Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 25.Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob. Agents Chemother. 1998;42:1–17. doi: 10.1128/aac.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilke MS, Lovering AL, Strynadka NCJ. Beta-lactam antibiotic resistance: a current structural perspective. Curr. Opin. Microbiol. 2005;8:525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Ullah JH, Walsh TR, Taylor IA, Emery DC, Verma CS, Gamblin SJ, Spencer J. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Åresolution. J. Mol. Biol. 1998;284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
- 28.Carfi A, Pares S, Duee E, Galleni M, Duez C, Frere JM, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concha NO, Rasmussen BA, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 30.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Llarena FJ, Bou G. β-lactamase inhibitors: The story so far. Curr. Med. Chem. 2009;16:3740–3765. doi: 10.2174/092986709789104957. [DOI] [PubMed] [Google Scholar]
- 32.Stachyra T, Pechereau M-R, Bruneau J-M, Claudon M, Frere JM, Miossec C, Coleman K, Black MT. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob. Agents Chemother. 2010;54:5132–5138. doi: 10.1128/AAC.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim HM, Pene JJ, Shaw RW. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase structural gene. J. Bacteriol. 1988;170:2873–2878. doi: 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh TR, Hall L, Assinder SJ, Nichols WW, Cartwright SJ, MacGowan AP, Bennett PM. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 35.Laraki N, Galleni M, Thamm I, Riccio ML, Amicosante G, Frere J-M, Rossolini GM. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. Cloning and characterization of bla VIM, a new integron-borne metallo- β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–90. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis. 2011;11:381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 38.Bennett PM. Plasmid encoded antibiotic resistance: acquistion and transfer of antibiotic resistance genes in bacteria. British J. Pharm. 2008;153:S347–S357. doi: 10.1038/sj.bjp.0707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-β-lactamase gene, bla(NDM-1) , and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol. 2011;19:588–595. doi: 10.1016/j.tim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Garau G, Garcia-Saez I, Bebrone C, Anne C, Mercuri P, Galleni M, Frere JM, Dideberg O. Update of the standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 2004;48:2347–2349. doi: 10.1128/AAC.48.7.2347-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borra PS, Leiros HKRA, Spencer J, Leiros I, Walsh TR, Sundsfjord A, Samuelsen O. Structural and computational investigations of VIM-7: insights into the substrate specificity of vim metallo-β-lactamases. J. Mol. Biol. 2011;411:174–189. doi: 10.1016/j.jmb.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Carfi A, Duee E, Galleni M, Frere JM, Dideberg O. 1.85 A resolution structure of the zinc (II) β-lactamase from Bacillus cereus. Acta Cryst. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 44.Concha NO, Janson CA, Rowling P, Pearson S, Cheever CA, Clarke BP, Lewis C, Galleni M, Frere J-M, Payne DJ, Bateson JH, Abdel-Meguid SS. Crystal structure of the IMP-1 metallo β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 45.Docquier JD, Benvenuti M, Calderone V, Stoczko M, Menciassi N, Rossolini GM, Mangani S. High-resolution crystal structure of the subclass B3 metallo-β-lactamase BJP-1: rational basis for substrate specificity and interaction with sulfonamides. Antimicrob. Agents Chemother. 2010;54:4343–4351. doi: 10.1128/AAC.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabiane SM, Sohi MK, Wan T, Payne DJ, Bateson JH, Mitchell T, Sutton BJ. Crystal structure of the zinc-dependent beta-lactamase from Bacillus cereus at 1.9 A resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–11. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 47.Garau G, Bebrone C, Anne C, Galleni M, Frere JM, Dideberg O. A metallo-β-lactamase enzyme in action: Crystal structures of the monozinc carbapenemase CphA and its complex with biapenem. J. Mol. Biol. 2005;345:785–795. doi: 10.1016/j.jmb.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Saez I, Docquier JD, Rossolini GM, Dideberg O. The three-dimensional structure of VIM-2, a Zn-β-lactamase from Pseudomonas aeruginosa in its reduced and oxidised form. J. Mol. Biol. 2008;375:604–611. doi: 10.1016/j.jmb.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Saez I, Hopkins J, Papamicael C, Franceschini N, Amicosante G, Rossolini GM, Galleni M, Frere JM, Dideberg O. The 1.5-A structure of Chryseobacterium meninosepticum zinc β-lactamase in complex with the inhibitor, D-capropril. J. Biol. Chem. 2003;278:23868–23873. doi: 10.1074/jbc.M301062200. [DOI] [PubMed] [Google Scholar]
- 50.García-Sáez I, Mercuri PS, Papamicael C, Kahn R, Frère JM, Galleni M, Rossolini GM, Dideberg O. Three-dimensional structure of FEZ-1, a monomeric subclass B3 metallo-β-lactamase from Fluoribacter gormanii in native form and in complex with D-captopril. J. Mol. Biol. 2003;325:651–660. doi: 10.1016/s0022-2836(02)01271-8. [DOI] [PubMed] [Google Scholar]
- 51.Murphy TA, Catto LE, Halford SE, Hadfield AT, Minor W, Walsh TR, Spencer J. Crystal structure of Pseudomonas aeruginosa SPM-1 provides insights into variable zinc affinity of metallo-beta-lactamases. J. Mol. Biol. 2006;357:890–903. doi: 10.1016/j.jmb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang HM, Hao Q. Crystal structure of NDM-1 reveals a common β-lactam hydrolysis mechanism. FASEB J. 2011;25:2574–2582. doi: 10.1096/fj.11-184036. [DOI] [PubMed] [Google Scholar]
- 53.Daiyasu H, Osaka K, Ishino Y, Toh H. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 2001;503:1–6. doi: 10.1016/s0014-5793(01)02686-2. [DOI] [PubMed] [Google Scholar]
- 54.Campos-Bermudez VA, Gonzalez JM, Tierney DL, Vila AJ. Spectroscopic signature of a ubiquitous metal binding site in the metallo-β-lactamase superfamily. J. Biol. Inorg. Chem. 2010;15:1209–1218. doi: 10.1007/s00775-010-0678-2. [DOI] [PubMed] [Google Scholar]
- 55.Galleni M, Lamotte-Brasseur J, Rossolini GM, Spencer J, Dideberg O, Frere JM group, M.-β-I.w. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents and Chemother. 2001;45:660–663. doi: 10.1128/AAC.45.3.660-663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King DT, Strynadka N. Crystal structure of New Delhi metallo-β-lactamase reveals molecular basis for antibiotic resistance. Protein Sci. 2011;20:1484–1491. doi: 10.1002/pro.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Tesar C, Mire J, Jedrzejczak R, Binkowski A, Babnigg G, Sacchettini J, Joachimiak A. Structure of apo- and monometalated forms fo NDM-1 - a highly potent carbapenem-hydrolyzing metallo-β-lactamase. PLoS One. 2011;6:e24621. doi: 10.1371/journal.pone.0024621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cadag E, Vitalis E, Lennox KP, Zhou CLE, Zemla AT. Computational analysis of pathogen-borne metallo-β-lactamases reveals discriminating structural features between B1 types. BMC Res. Notes. 2012;6:96. doi: 10.1186/1756-0500-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasia RM, Vila AJ. Structural determinants of substrate binding to Bacillus cereus metallo-β-lactamase. J. Biol. Chem. 2004;279:26046–26051. doi: 10.1074/jbc.M311373200. [DOI] [PubMed] [Google Scholar]
- 60.de Seny D, Heinz U, Wommer S, Kiefer M, Meyer-Klaucke W, Galleni M, Frere JM, Bauer R, Adolph HW. Metal ion binding and coordination geometry for wild type and mutants of metallo-β-lactamase from Bacillus cereus 569/H/9 (BcII): a combined thermodynamic, kinetic, and spectroscopic approach. J. Biol. Chem. 2001;276:45065–45078. doi: 10.1074/jbc.M106447200. [DOI] [PubMed] [Google Scholar]
- 61.Paul-Soto R, Bauer R, Frere JM, Galleni M, Meyer-Klaucke W, Nolting H, Rossolini GM, de Seny D, Hernandez-Valladares M, Zeppezauer M, Adolph HW. Mono- and binuclear Zn2+-β-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 1999;274:13242–13249. doi: 10.1074/jbc.274.19.13242. [DOI] [PubMed] [Google Scholar]
- 62.Rasia RM, Vila AJ. Exploring the role and the binding affinity of the second zinc equivalent in B. cereus metallo-β-lactamase. Biochemistry. 2002;41:1853–1860. doi: 10.1021/bi010933n. [DOI] [PubMed] [Google Scholar]
- 63.Jacquin O, Balbeur D, Damblon C, Marchot P, De Pauw E, Roberts GC, Frère JM, Matagne A. Positively cooperative binding of zinc ions to Bacillus cereus 569/H/9 β-lactamase II suggests that the binuclear enzyme is the only relevant form for catalysis. J. Mol. Biol. 2009;392:1278–1291. doi: 10.1016/j.jmb.2009.07.092. [DOI] [PubMed] [Google Scholar]
- 64.Badarau A, Damblon C, Page MI. The activity of the dinuclear cobalt-β-lactamase from Bacillus cereus in catalyzing the hydrolysis of β-lactams. Biochem. J. 2007;401:197–203. doi: 10.1042/BJ20061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badarau A, Page MI. Loss of enzyme activity during turnover of the Bacillus cereus β-lactamase catalzyed hydrolysis of β-lactams due to loss of zinc ions. J. Biol. Inorg. Chem. 2008;13:919–928. doi: 10.1007/s00775-008-0379-2. [DOI] [PubMed] [Google Scholar]
- 66.Fast W, Wang Z, Benkovic SJ. Familial mutations and zinc stoichiometry determine the rate-limiting step of nitrocefin hydrolysis by metallo-β-lactamase from Bacteroides fragilis. Biochemistry. 2001;40:1640–1650. doi: 10.1021/bi001860v. [DOI] [PubMed] [Google Scholar]
- 67.Griffin DH, Richmond TK, Sanchez C, Jon Molller A, Breece RM, Tierney DL, Bennett B, Crowder MW. Structural and kinetic studies on metallo-β-lactamase IMP-1. Biochemistry. 2011;50:9125–9134. doi: 10.1021/bi200839h. [DOI] [PubMed] [Google Scholar]
- 68.Hawk MJ, Breece RM, Hajdin CE, Bender KM, Hu Z, Costello AL, Bennett B, Tierney DL, Crowder MW. Differential binding of Co(II) and Zn(II) to metallo-β-lactamase Bla2 from Bacillus anthracis. J. Amer. Chem. Soc. 2009;131:10753–10762. doi: 10.1021/ja900296u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park H, Brothers EN, Merz KM. Hybrid QM/MM and DFT investigations of the catalytic mechanism and inhibition of the dinuclear zinc metallo-β-lactamase CcrA from Bacteroides fragilis. J. Amer. Chem. Soc. 2005;127:4232–4241. doi: 10.1021/ja042607b. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Fast W, Benkovic SJ. On the mechanism of metallo-β-lactamase from Bacteroides fragilis. Biochemistry. 1999;38:10013–10023. doi: 10.1021/bi990356r. [DOI] [PubMed] [Google Scholar]
- 71.Crowder MW, Spencer J, Vila AJ. Metallo-β-lactamases: Novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 72.Garrity JD, Bennett B, Crowder MW. Direct evidence that reaction intermediate in metallo-β-lactamase is metal bound. Biochemistry. 2005;44:1078–1087. doi: 10.1021/bi048385b. [DOI] [PubMed] [Google Scholar]
- 73.Llarrull LI, Tioni MF, Kowalski J, Bennett B, Vila AJ. Evidence for a dinuclear active site in the metallo-β-lactamase BcII with substoichiometric Co(II): A new model for metal uptake. J. Biol. Chem. 2007;282:30586–30595. doi: 10.1074/jbc.M704613200. [DOI] [PubMed] [Google Scholar]
- 74.Rasia RM, Vila AJ. Mechanistic study of the hydrolysis of nitrocefin mediated by B. cereus metallo-β-lactamase. ARKIVOC. 2003;3:507–516. [Google Scholar]
- 75.Yang H, Aitha M, Hetrick AM, Richmond TK, Tierney DL, Crowder MW. Mechanistic and spectroscopic studies of metallo-β-lactamase NDM-1. Biochemistry. 2012;51:3839–3847. doi: 10.1021/bi300056y. [DOI] [PubMed] [Google Scholar]
- 76.Moali C, Anne C, Lamotte-Brasseur J, Groslambert S, Devreese B, Van Beeumen J, Galleni M, Frere JM. Analysis of the importance of the metallo-β-lactamase active site loop in substrate binding and catalysis. Chem. Biol. 2003;10:319–329. doi: 10.1016/s1074-5521(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 77.Yamaguchi Y, Kuroki T, Yasuzawa H, Higashi T, Jin W, Kawanami A, Yamagata Y, Arakawa Y, Goto M, Kurosaki H. Probing the role of Asp-120(81) of metallo-β-lactamase (IMP-1) by site-directed mutagenesis, kinetic studies, and X-ray crystallography. J. Biol. Chem. 2005;280:20824–20832. doi: 10.1074/jbc.M414314200. [DOI] [PubMed] [Google Scholar]
- 78.Tioni MF, Llarrull LI, Poeylaut-Palena AA, Marti MA, Saggu M, Periyannan GR, Mata EG, Bennett B, Murgida DH, Vila AJ. Trapping and characterization of a reaction intermediate in carbapenem hydrolysis by B. cereus metallo-β-lactamase. J. Amer. Chem. Soc. 2008;130:15852–15863. doi: 10.1021/ja801169j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dal Peraro M, Vila AJ, Carloni P, Klein MI. Role of zinc content on the catalytic efficiency of B1 metallo-β-lactamases. J. Amer. Chem. Soc. 2007;129:2808–2816. doi: 10.1021/ja0657556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.King DT, Worrall LJ, Gruninger RJ, Strynadka NCJ. New Delhi metallo-β-lactamase: Structural insights into β-lactam recognition and inhibition. J. Amer. Chem. Soc. 2012;134:11362–11365. doi: 10.1021/ja303579d. [DOI] [PubMed] [Google Scholar]
- 81.Yuan Q, He L, Ke H. A potential substrate binding conformation of β-lactams and insight into the broad spectrum of NDM-1 activity. Antimicrob. Agents Chemother. 2012 doi: 10.1128/AAC.05896-11. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wommer S, Rival S, Heinz U, Galleni M, Frere JM, Franceschini N, Amicosante G, Rasmussen B, Bauer R, Adolph HW. Substrate-activated zinc binding of metallo-β-lactamases: physiological importance of mononuclear enzymes. J. Biol. Chem. 2002;277:24142–24147. doi: 10.1074/jbc.M202467200. [DOI] [PubMed] [Google Scholar]
- 83.Fonseca F, EH B, Saavedra MJ, Correia A, Spencer J. Crystal structure of Serratia fonticola Sfh-I: activation of the nucleophile in mono-zinc metallo-β-lactamases. J. Mol. Biol. 2011;411:951–959. doi: 10.1016/j.jmb.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 84.Bebrone C, Delbrück H, Kupper MB, Schlömer P, Willmann C, Frère JM, Fischer R, Galleni M, Hoffmann KM. The structure of the dizinc subclass B2 metallo-β-lactamase CphA reveals that the second inhibitory zinc ion binds in the histidine site. Antimicrob. Agents Chemother. 2009;53:4464–4471. doi: 10.1128/AAC.00288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simona F, Magistrato A, Dal Peraro M, Cavalli A, Vila AJ, Carloni P. Common mechanistic features among metallo-β-lactamases. A computational study of Aeromonas hydrolphila CphA enzyme. J. Biol. Chem. 2009;284:28164–28171. doi: 10.1074/jbc.M109.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu S, Xu D, Guo H. QM/MM studies of monozinc β-lactamase CphA suggest that the crystal structure of an enzyme-intermediate complex represents a minor pathway. J. Amer. Chem. Soc. 2010;132:17986–17988. doi: 10.1021/ja104241g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malabanan MM, Amyes TL, Richard JP. A role for flexible loops in enzyme catalysis. Curr. Opin. Struc. Biol. 2010;20:702–710. doi: 10.1016/j.sbi.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qasba PK, Ramakrishnan B, Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005;30:53. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Concha NO, Janson CA, Rowling P, Pearson S, Cheever CA, Clarke BP, Lewis C, Galleni M, Frere JM, Payne DJ, Bateson JH, Abdel-Meguid SS. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: Binding determinants of a potent, broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 90.Toney JH, Fitzgerald PMD, Grover-Sharma N, Olson SH, May WJ, Sundelof JG, Vanderwall DE, Cleary KA, Grant SK, Wu JK, Kozarich JW, Pompliano DL, Hammond GG. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem. Biol. 1998;5:185–195. doi: 10.1016/s1074-5521(98)90632-9. [DOI] [PubMed] [Google Scholar]
- 91.Scrofani SDB, Chung J, Huntley JJA, Benkovic SJ, Wright PE, Dyson HJ. NMR characterization of the metallo-β-lactamase from Bacterioides fragilis and its interaction with a tight-binding inhbitior: Role of an active-site loop. Biochemistry. 1999;38:14507–14514. doi: 10.1021/bi990986t. [DOI] [PubMed] [Google Scholar]
- 92.Huntley JJA, Fast W, Benkovic SJ, Wright PE, Dyson HJ. Role of a solvent-exposed tryptophan in the recognition and binding of antibiotic substrates for a metallo-β-lactamase. Protein Sci. 2003;12:1368–1375. doi: 10.1110/ps.0305303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spencer J, Clarke AR, Walsh TR. Novel mechanism of hydrolysis of therapeutic β-lactams by Stenotrophomonas maltophilia L1 metallo-β-lactamase. J. Biol. Chem. 2001;276:33638–33644. doi: 10.1074/jbc.M105550200. [DOI] [PubMed] [Google Scholar]
- 94.Garrity JD, Pauff JM, Crowder MW. Probing the dynamics of a mobile loop above the active site of L1, a metallo-β-lactamase from Stenotrophomonas maltophilia via site-directed mutagenesis and stopped-flow fluorescence spectroscopy. J. Biol. Chem. 2004;279:39663–39670. doi: 10.1074/jbc.M406826200. [DOI] [PubMed] [Google Scholar]
- 95.de Seny D, Prosperi-Meys C, Bebrone C, Rossolini GM, Page MI, Noel P, Frère JM, Galleni M. Mutational analysis of the two zinc-binding sites of the Bacillus cereus 569/H/9 metallo-β-lactamase. Biochem. J. 2002;363:687–696. doi: 10.1042/0264-6021:3630687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haruta S, Yamaguchi H, Yamamoto ET, Eriguchi Y, Nukaga M, O'Hara K, Sawai T. Functional analysis of the active site of a metallo-β-lactamase proliferating in Japan. Antimicrob. Agents and Chemother. 2000;44:2304–2309. doi: 10.1128/aac.44.9.2304-2309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanhove M, Zakhem M, Devreese B, Franceshini N, Anne C, Bebrone C, Amicosante G, Rossolini GM, Van Beeumen J, Frere JM, Galleni M. Role of Cys221 and Asn116 in the zinc-binding sites of the Aeromonas hydrophilia metallo-β-lactamase. Cell. Mol. Life Sci. 2003;60:2501–2509. doi: 10.1007/s00018-003-3092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y, Keeney D, Tang X-J, Canfield N, Rasmussen BA. Kinetic properties and metal content of the metallo-β-lactamase CcrA harboring selective amino acid substitutions. J. Biol. Chem. 1999;274:15706–15711. doi: 10.1074/jbc.274.22.15706. [DOI] [PubMed] [Google Scholar]
- 99.Horton LB, Shanker S, Mikulski R, Brown NG, Phillips K, Lykissa E, Prasad BV, Palzkill T. Mutagenesis of zinc ligand residue Cys221 reveals plasticity in the IMP-1 metallo-β-lactamase active site. Antimicrob. Agents Chemother. 2012 doi: 10.1128/AAC.01276-12. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bebrone C, Anne C, Kerff F, Garau G, De Vriendt K, Lantin R, Devreese B, Van Beeumen J, Dideberg O, Frère JM, Galleni M. Mutational analysis of the zinc- and substrate-binding sites in the CphA metallo-β-lactamase from Aeromonas hydrophila. Biochem. J. 2008;414:151–159. doi: 10.1042/BJ20080375. [DOI] [PubMed] [Google Scholar]
- 101.Garrity JD, Carenbauer AL, Herron LR, Crowder MW. Metal binding Asp-120 in metallo-β-lactamase L1 from Stenotrophomonas maltophilia plays a crucial role in catalysis. J. Biol. Chem. 279:920–927. doi: 10.1074/jbc.M309852200. (279) [DOI] [PubMed] [Google Scholar]
- 102.Materon IC, Beharry Z, Huang W, Perez C, Palzkill T. Analysis of the context dependent sequence requirements of active site residues in the metallo-β-lactamase IMP-1. J. Mol. Biol. 2004;344:653–663. doi: 10.1016/j.jmb.2004.09.074. [DOI] [PubMed] [Google Scholar]
- 103.Materon IC, Palzkill T. Identification of residues critical for metallo-β-lactamase function by codon randomization and selection. Protein Sci. 2001;10:2556–2565. doi: 10.1110/ps.40884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown NG, Horton LB, Huang W, Vongpunsawad S, Palzkill T. Analysis of the functional contributions of Asn233 in metallo-β-lactamase IMP-1. Antimicrob. Agents Chemother. 2011;55:5696–5702. doi: 10.1128/AAC.00340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomatis PE, Rasia RM, Segovia L, Vila AJ. Mimicking natural evolution in metallo-β-lactamases through second-shell ligand mutations. Proc. Natl. Acad. Sci. USA. 2005;102:13761–13766. doi: 10.1073/pnas.0503495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomatis PE, Fabiane SM, Simona F, Carloni P, Sutton BJ, Vila AJ. Adaptive protein evolution grants organismal fitness by improving catalysis and flexibility. Proc. Natl. Acad. Sci. USA. 2008;105:20605–20610. doi: 10.1073/pnas.0807989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamaguichi Y, Jin W, Matsunaga K, Ikemizu S, Yamagata Y, Wachino J, Shibata N, Arakawa Y, Kurosaki H. Crystallographic investigation of the inhibition mode of a VIM-2 metallo-β-lactamase from Pseudomonas aeruginosa by a mercaptocarboxylate inhibitor. J. Med. Chem. 2007;50:6647–6653. doi: 10.1021/jm701031n. [DOI] [PubMed] [Google Scholar]
- 108.Lassaux P, Traore DA, Loisel E, Favier A, Docquier JD, Sohier JS, Laurent C, Bebrone C, Frere JM, Ferrer JL, Galleni M. Biochemical and structural characterization of the subclass B1 metallo-β-lactamase VIM-4. Antimicrob. Agents Chemother. 2011;55:1248–1255. doi: 10.1128/AAC.01486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saradhi P, Leiros HK, Ahmad R, Spencer J, Leiros I, Walsh TR, Sundsfjord A, Samuelsen O. Structural and computational investigations of VIM-7: Insights into the substrate specificity of VIM metallo-β-lactamases. J. Mol. Biol. 2011;411:174–189. doi: 10.1016/j.jmb.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 110.Leiros HK, Borra PS, Brandsdal BO, Edvardsen KSW, Spencer J, Walsh TR, Samuelsen O. Crystal structure of the mobile metallo-β-lactamase AIM-1 from Pseudomonas aeruginosa : Insights into antibiotic binding and the role of Gln157. Antimicrob. Agents Chemother. 2012;56:4341–4353. doi: 10.1128/AAC.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]