Abstract
Advanced stage cancers acquire anoikis resistance which provides metastatic potential to invade and form tumors at distant sites. Suppression of anoikis resistance by novel molecular therapies would greatly benefit treatment strategies for metastatic cancers. Recently, digitoxin and several of its novel synthetic derivatives, such as α-l-rhamnose monosaccharide derivative (D6-MA), have been synthesized and studied for their profound anticancer activity in various cancer cell lines. In this study, we investigated the anoikis sensitizing effect of D6-MA compared with digitoxin to identify their anti-metastatic mechanism of action. D6-MA sensitized NSCLC H460 cells to detachment-induced apoptosis with significantly greater cytotoxicity (IC50 = 11.9 nM) than digitoxin (IC50 = 90.7 nM) by activating caspase-9. Screening of the Bcl-2 protein family revealed that degradation of anti-apoptotic Mcl-1 protein is a favorable target. Mcl-1 over-expression and knockdown studies in D6-MA and digitoxin exposed cells resulted in rescue and enhancement, respectively, indicating a facilitative role for decreased Mcl-1 expression in NSCLC anoikis. Transfection with mutant Mcl-1S159 attenuated detachment-induced cell death and correlated with a remaining of Mcl-1 level. Furthermore, D6-MA suppressed Mcl-1 expression via ubiquitin proteasomal degradation that is dependent on activation of glycogen synthase kinase (GSK)-3β signaling. In addition, D6-MA also targeted Mcl-1 degradation causing an increased anoikis in A549 lung cancer cells. Anoikis sensitizing effect on normal small airway epithelial cells was not observed indicating the specificity of D6-MA and digitoxin for NSCLC. These results identify a novel cardiac glycoside (CG) sensitizing anoikis mechanism and provide a promising anti-metastatic target for lung cancer therapy.
Keywords: Monosaccharide digitoxin derivative, Anoikis, Mcl-1, Glycogen synthase kinase-3β, Metastasis, NSCLC
1. Introduction
Late stage aggressive cancers undergo the phenomenon of metastasis, usually preceded by invasion of neighboring tissues. Metastasis involves cancer cells breaking free from the initial tumor mass, circulatory system transportation, reattachment at a target organ and prolonged growth, resulting in drastically elevated mortality rates [1–3]. Normal cells that detach from their extracellular matrix (ECM) quickly undergo anoikis, a programmed cell death via the mitochondrial apoptosis pathway [4,5]. Metastatic cancers, such as late stage III and stage IV lung cancer, resist anoikis via several different mechanisms including altered adhesion and growth factor signaling, epithelial to mesenchymal transition, and dysregulation of pro-apoptotic Bcl-2 proteins [6]. Metastatic lung tumors greatly reduce lung cancer survival rates by more than 40% compared to early stage tumors and cause death rates 2–4 times higher than all other cancers [7–9]. Development of novel, late stage cancer therapeutic strategies is clearly warranted. Given that 80–85% of lung cancers are non-small cell lung cancers (NSCLC), development of therapeutic strategies to sensitize aggressive NSCLC to anoikis would greatly improve malignant chemotherapy strategies and lung cancer patient prognosis.
Epidemiologic evidence suggests that congestive heart failure patients on cardiac glycoside (CG) therapy (i.e. digitalis 10–40 nM) exhibit reduced breast and leukemia cancer diagnoses and breast cancer reoccurrence [10–12]. Initial consensus on CG's anti-cancer mechanism of action (MOA) was that CGs act via direct binding to the a subunit of the Na+/K+ ATPase causing inotropic inhibition, internal Ca+ ion release, thereby resulting in apoptosis initiation. More recent evidence suggests that Na+/K+ ATPase with other membrane-associated signaling proteins act as a ‘signalosome’ complex [13,14] that activates several signaling pathways resulting in in vitro anti-neoplastic activity [15,16]. Although cancer cells exhibit greater CG sensitivity than non-malignant cells, safety concerns with their clinical use remain due to cardiotoxicity, narrow therapeutic window and protein synthesis inhibition [17–19]. Nevertheless, low reoccurrence rate and in vitro cancer cell apoptotic sensitivity to CGs suggests that cancer cells may exhibit anoikis sensitivity to CG therapy. Surprisingly, little research effort has focused on CG-induced anoikis in cancer cells given the large volume of literature on apoptotic effect. By investigating the ability of CG's to induce both apoptosis and anoikis, researchers can identify whether CGs can therapeutically target primary tumors and/or their metastatic ability. Oleandrin, ouabain and UNBS1450 exhibit ability to sensitize cells to detachment-associated cell death via programmed cell death, autophagy and cellular swelling [20,21]. Mcl-1 expression, an anti-apoptotic Bcl-2 family protein, was recently described as a key anoikis resistance mediator and may serve as a CG target for cancer therapy [22–24].
Concerns with CG cardiotoxicity and a clear MOA have restricted their clinical use and anti-neoplastic research efforts. Recent advances, however, in synthetic carbohydrate chemistry and testing low dose effects (<50 nM) in vitro have further advanced CGs for anti-neoplastic therapy. Novel digitoxin derivative syntheses via Pd-catalyzed glycosylation [25,26] and in vitro cytotoxic screening using NCI's multiple attached cancer cell lines, including NSCLC cells [27,28], identified several monosaccharide digitoxin analogs displaying greater cytotoxic potency than digitoxin. The corresponding anti-neoplastic effects were identified as apoptotic cell death via caspase-9 intrinsic apoptotic pathway and G2/M arrest in attached NSCLC cells [27,29].
To further expand and identify digitoxin monosaccharide derivatives’ anti-cancer modes of action, this study's primary objectives were to (a) compare α-l-rhamnose monosaccharide derivative (D6-MA) to digitoxin for their ability to sensitize NSCLC cells to anoikis and (b) determine the underlying anoikis sensitization mechanism. Our hypotheses stated that D6-MA would exhibit greater potency than digitoxin in initiating NSCLC anoikis via caspase-9 activation due to altered Mcl-1 expression. Identification of a NSCLC anoikis sensitization signaling mechanism for digitoxin-based derivatives will enhance understanding of CG apoptotic MOA and lend insight into whether CG reduce rate of cancer reoccurrence by inhibiting metastasis.
2. Methods
2.1. Cell culture and reagents
Human non-small cell lung cancer NCI-H460 and lung carcinoma A549 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Non-tumorigenic, human small airway epithelial cells immortalized with human telomerase (SAEC) were a kind gift from Dr. Tom Hei (Columbia University Medical Center). H460 cells were cultured in RPMI 1640 medium while A549 cells were cultured in DMEM medium (Sigma, St. Louis MO). All culture media contained 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/mL penicillin/streptomycin. SAECs were cultured in SABM medium supplemented with SAGM growth factor BulletKit (Lonza, Allendale, NJ) following manufacturer's instructions. Cells were cultured in a humid, 5% CO2 environment at 37 8C. Hoechst 33342, cisplatin, cycloheximide, and poly(2-hydroxyethyl-methacrylate; poly-HEMA) were obtained from Sigma. MG132 was obtained from Calbiochem (San Diego, CA). Antibodies for poly-ADP-ribose polymerase (PARP), caspase-8, caspase-9, Mcl-1, phosphorylate Mcl-1, Bcl-2, Bax, Bim, Bid, β-actin, and peroxidase-labeled secondary antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Ubiquitin antibody and protein-G agarose were obtained from Abcam Inc. (Cambridge, MA). Caspase-8 inhibitor (z-IETD-FMK), caspase-9 inhibitor (z-LEHD-FMK), pan-caspase inhibitor (z-VAD-FMK), and FasL were obtained from Alexis Biochemicals (San Diego, CA). Caspase-3 activity kit was purchased from Cell Signaling Technology, Inc. Caspase-8 and caspase 9 assay kits were obtained from BioVision (Milpitas, CA).
Digitoxin (>99% pure) was acquired from Sigma Chemicals (St. Louis, MO). D6-MA, and α-l-rhamnose monosaccharide digitoxin derivative (>98% pure) was synthesized using a previously described de novo Pd-catalyzed glycosylation method for unique carbohydrate synthesis [25,26]. Briefly, acetyl furan underwent asymmetric reduction, oxidative rearrangement and tert-butoxycarbonyl protection to acquire the correct glycosyl donor. The resulting α-l-pyranose was coupled to digitoxigenin, the acid-cleaved aglycone moiety of digitoxin, via Pd-catalyzed glycosylation followed by Luche reduction to acquire D6-MA. All D6-MA was purified using SiO2 gel flash chromatography, recrystallized in either methyl chloride or ethyl chloride in hexane and dried under vacuum. Sample purity was determined via 1H 13C NMR as well as melting point and optical rotation. The chemical structure was shown in Fig. 1A. Both compounds were diluted in sterile DMSO to 2 mM stock and serially diluted in DMSO to reach exposure doses.
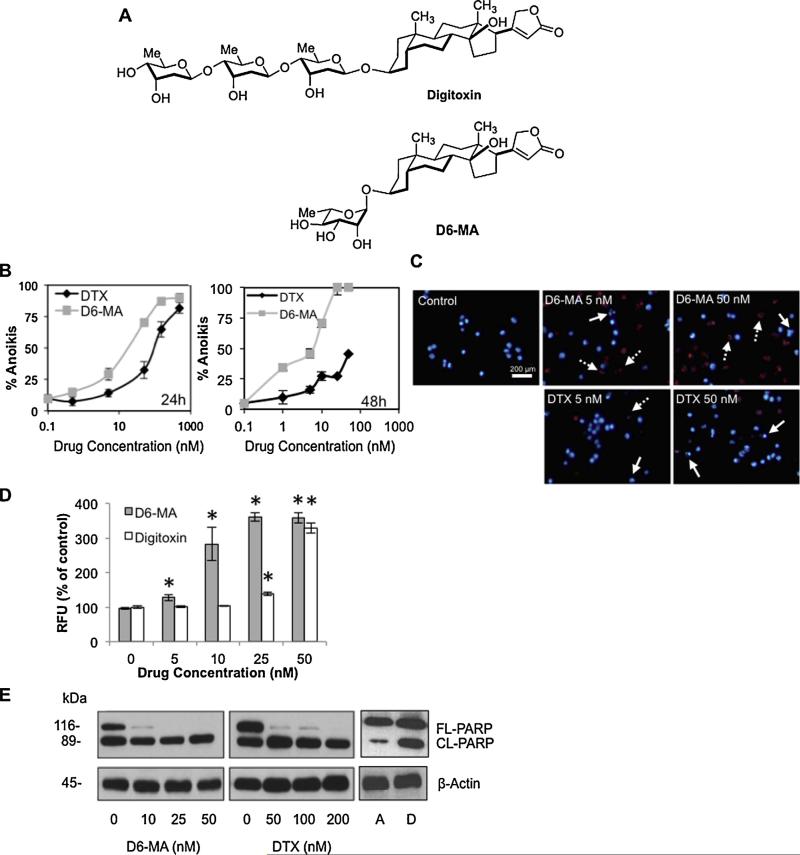
Fig. 1.
D6-MA exhibits greater efficacy than digitoxin in sensitizing non-small cell lung cancer cell anoikis. (A) Structures of digitoxin and its α-l-rhamnose monosaccharide derivative D6-MA. (B) Mean detachment-induced cell death (anoikis) was determined by Hoechst 33342/propidium iodide staining assay. H460 cells were cultured on poly-HEMA-coated plate and treated with varying concentrations of D6-MA and digitoxin (0, 1, 5, 50, 250, 500 nM for 24 h, left; 0, 1, 5, 10, 25, 50 nM for 48 h, right). (C) Anoikis sensitizing effects of D6-MA and digitoxin on unfixed cells were determined by Hoechst 33342 and propidium iodide (PI) fluorescence staining. Examples of early (blue) and late (red) apoptotic cells with fragmented nuclei are indicated by solid and dashed arrows, respectively. (D) Caspase-3 activity in suspended cells following 12 h treatment of D6-MA and digitoxin (0–50 nM). Caspase-3 activity was determined by Ac-DEVD-AMC fluorescence assay. Data represent mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells. (E) Effects of D6-MA and digitoxin on poly-ADP-ribose polymerase (PARP). H460 cells were detached and treated with D6-MA (0–50 nM) and digitoxin (0–200 nM) for 12 h. Attached (A) and detached cells (D) without treatment were also collected to clarify the spontaneously apoptosis after 12 h-detachment (right panel). Cleaved length PARP (CLPARP) and full length PARP (FL-PARP) were examined by Western blotting. PARP cleavage is out of linear range.
2.2. Anoikis sensitivity determination
Anoikis sensitization assays on H460 cells were performed in 12-well plates coated with poly-HEMA to keep cells from attaching to well bottom. A 6 mg/mL poly-HEMA solution was prepared with warm 95% ethanol, pipetted into each well and allowed to evaporate overnight. Sub-confluent H460 cells were then PBS washed, 0.05% trypsinized, suspended in 1% FBS and diluted to 1 × 105 cells/mL in microfuge tubes. Cells were exposed to 0–500 nM by pipetting diluted compound (DMSO < 0.1% v/v) to each tube, triturated and seeded to each well. Following a 24 and 48 h exposure, 10 mM Hoechst 33342 and 5 μg/mL propidium iodide (PI) dissolved in PBS were added to each well and incubated for 30 min. Stained cells were immediately photographed using a Leica DFC 490 digital camera mounted on a Leica DMIL inverse compound microscope at 400× magnification. At least three replicates per dose per compound were run in each experiment which 3–5 experiments were performed. Percentage of cells displaying condensed chromatin and/or fragmented apoptotic nuclei and necrotic cell death were determined from 5 replicate photos of each experimental replicate. Cells displaying PI-stained fragmented nuclei were considered late-stage apoptotic nuclei. A minimum of 1000 cells were counted per treatment. IC50 analyses were conducted in GraphPad Prism 5 (La Jolla, CA).
2.3. Caspase activity determination
Caspase 3 activity was measured using Ac-DEVD-AMC caspase 3 activity assay kit (Cell Signaling Technology, Inc., Beverly, MA), and caspase 8 and 9 activations were using IETD-AFC caspase 8 and LEHD-AFC caspase 9 assay kits (BioVision, Milpitas, CA) following manufacturer's instructions. Briefly, 5 × 105 suspended cells were plated in triplicate to low attachment 6-well plates (Corning, Lowell, MA) and exposed for 12 h to each compound. Cells were collected, pelleted via centrifugation, lysed and frozen at –20 °C until needed. Next, treatment medium was aspirated and cells lysed. Suspended cell lysates were incubated with Ac-DEVD-AMC (caspase-3 activity) for 3 h and fluorescent intensity determined at 380 nm excitation and 420 nm emission. Likewise, cell lysates were incubated with either IETD-AFC (caspase 8 activity) or LEHD-AFC (caspase-9 activity) for 3 h and fluorescent intensity determined at 400 nm excitation and 505 nm emission.
2.4. Generation of stable and transient transfectants
Mcl-1 plasmid (pcDNA3.1-hMcl-1), phosphorylate Mcl-1 (pcDNA3.1-hMcl-1 S159) and control plasmid (pcDNA3.1) were obtained from Addgene (Cambridge, MA) [30]. H460 cells were stably transfected with these plasmids using Amaxa Nucleofection system (Walkersville, MD), according to manufacturer's instruction. Approximately 36 h after the initiating transfection, cells were trypsinized, plated onto 75 mL culture flasks, and cultured for 28 days with neomycin containing medium (700 mg/mL). Pooled stable transfectants were identified by Western blotting of Mcl-1, and phosphorylate Mcl-1, and cultured in neomycin-free RPMI medium for at least two passages before each experiment.
Mcl-1 and control knockdown cells were generated by transient transfection with esiRNA and negative control siRNA-tagged GFP obtained from Sigma (St. Louis, MO). H460 cells were plated into 6-well plates and allowed to reach 50% confluence in 10% FBS medium without antibiotics. 900 ng/mL of either negative control or esiRNA Mcl-1 were added to their respective wells in triplicate in the presence of Lipofectamine 2000 (Invitrogen, Grand Island, NY). Cells were incubated for 6 h followed by removal of esiRNA containing medium and addition of fresh medium. After 40 h of culturing in normal conditions, cells were assessed for loss of anoikis resistance and lysed for Mcl-1 knockdown assessment.
2.5. Western blot
After CG exposure, cells were incubated with lysis buffer containing 2% Triton X-100, 1% sodium dodecyl sulfate (SDS), 100 mM NaCl, 10 mM Tris–HCl (pH 7.5), 1 mM EDTA, and a Complete Mini cocktail protease inhibitors (Roche Molecular Biochemicals, Basel, Switzerland) for 30 min on ice. SDS-PAGE and Western blot analyses were performed as previously described [31]. Mean protein band densitometry data from independent experiments were normalized with β-actin protein. Data were represented as the mean ± SD and analyzed by Student's t-test.
2.6. Immunoprecipitation
After CG exposure, cells were washed with PBS and lysed in lysis buffer at 4 °C for 20 min. Cell lysates were collected and determined for protein content using the BCA assay. Lysate proteins (60 μg) were incubated with Mcl-1 antibody for 14 h at 4 °C, followed by 4 h incubation with protein G-conjugated agarose at 4 °C. The immune complexes were washed 6 times with cold lysis buffer, and resuspended in 2× Laemmli sample buffer. The immune complexes were separated by 10% SDS-PAGE and analyzed by Western blotting as described [30].
2.7. Quantitative real time PCR
After specific treatment for 12 h, total RNA was extracted by Trizol (Invitrogen), and 1 μg was reverse transcribed in a 100 μL reaction mixture containing 500 mM dNTP, 125 units of Multi-Scribe Reverse Transcript (Applied Biosystems, Foster City, CA), 40 units of RNase inhibitor, 2.5 M olido(dT), 1× TaqMan reverse transcriptase buffer, and 5 mM MgCl2 at 48 °C for 40 min. The primers used in this study were obtained from Applied Biosystems, Mcl-1 (Hs03043899_ml*) and 18S rRNA (Hs99999901_s1). Amplification was performed at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. A SYBR Green PCR Master Mix (Applied Biosystems) was used with 1 ng of cDNA and 100–400 nM primers. A negative control without cDNA template was run with each assay. All PCR reactions were performed using ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Relative mRNA levels were determined by using the comparative CT (threshold cycle) method, where the Mcl-1 target is normalized to the control and compared with a reference sample (assigned a relative value of 1) by the equation: 2–ΔΔCT.
2.8. Statistical analyses
Anoikis dose response curves and IC50 values for each compound were determined using non-linear regression analysis in Prism 5.0 (GraphPad Software, Inc.). Regression curves were plotted assuming a sigmoidal dose response and setting the unexposed cell response to 0. Statistical differences among treatment means for anoikis assays and densitometry data were determined using two-way ANOVA and t-test methodology, respectively (α = 0.05) in JMP 8.0 (SAS Institute).
3. Results
3.1. Digitoxin derivative D6-MA sensitizes human lung carcinoma H460 cells to detachment-induced cell death
To investigate anoikis sensitization, suspended H460 cells were treated with various concentrations of D6-MA and digitoxin (Fig. 1B; 0–500 nM), and evaluated by using Hoechst 33342/PI assays. Both D6-MA and digitoxin exposure caused a significant, dose-dependent increase in anoikis compared to non-treated cells (Fig. 1B, left). Co-staining unfixed cells with Hoechst/PI also revealed that both D6-MA and digitoxin induced a gradual increase in intense nuclear fluorescence condensation and fragmentation in H460 cells, whereas a dose-dependent increase in PI exclusion of necrotic (non-apoptotic) nuclei (<5% at all doses) was not observed (Fig. 1C). D6-MA exhibited significantly greater anoikis potency (IC50 = 11.9 nM) than digitoxin (IC50 = 90.7 nM) at 24 h. This fold potency difference was maintained at 48 h (Fig. 1B, right) indicating that bio-availability differences could not explain different potencies. To validate observed anoikis sensitizing effect, detached cells were treated with D6-MA and digitoxin, and assessed for caspase-3 activation and poly(ADP-ribose) polymerase (PARP) cleavage using Ac-DEVD-AMC fluorescence and Western blotting, respectively. Both D6-MA and digitoxin showed significant caspase-3 activation at 12 h in a dose-dependent manner (Fig. 1D). Treatment with D6-MA also caused a complete loss in PARP expression, whereas reduction of PARP was observed in response to higher concentration of digitoxin (50 nM/L; Fig. 1E). An increase of activated PARP in non-treated cells was spontaneous in response to detachment for 12 h compared to attached cells (Fig. 1E, right). These results suggested that D6-MA possessed a potent anoikis sensitizing effect.
3.2. D6-MA sensitizes NSCLC cell anoikis primarily through mitochondria pathway
It has been indicated that the defected regulation in mitochondrial and/or death receptor partway plays a significant role on anoikis resistance [23,31–33]. To further identify which pathways were stimulated during D6-MA's and digitoxin's anoikis sensitization of H460 cells, caspase-8 and caspase-9 expressions were determined by Western blotting following treatment. Exposed H460 cells displayed cleaved caspase-8 and -9 following treatment with D6-MA and digitoxin (Fig. 2A). A low amount of cleaved caspase-9 in unexposed cells was due to use of serum-free medium during exposure. Unexposed cells in serum containing medium (1% FBS) had no detectable caspase-9 cleavage (data not shown). To determine whether one or both pathways controlled anoikis-related death, detached cells were treated with either D6-MA or digitoxin in the presence or absence of pan-caspase inhibitor (z-VAD-FMK), caspase-8 inhibitor (z-IETD-FMK), and caspase-9 inhibitor (z-LEHD-FMK). Anoikis was determined by Hoechst/PI assay. Although cleaved caspase-8 was observed in treated cells, the inhibitory study demonstrated that pan-caspase and caspase-9 inhibitor significantly rescued anoikis mediated by D6-MA and digitoxin, whereas the caspase-8 inhibitor did not (Fig. 2B). A parallel study also tested the role of caspase inhibitors using known inducer of caspase-9 dependent pathway, cisplatin [34,35], and caspase-8 dependent pathway, FasL [36,37] as positive controls (Fig. 2C). To confirm that caspase-9 activity dominates and preceeds potential caspase-8 activity, both D6-MA and digitoxin treatment (0–50 nM) resulted in significant dose-dependent increase in caspase-9 activity while no significant effect was detected in caspase-8 activation (Fig. 2D) except at 50 nM D6-MA. Reactive oxygen species detection assays using dichlorofluorescene acetate for increased ROS levels associated with mitochondrial apoptotic pathway activation did not differ from unexposed cells (data not shown). These results suggested that D6-MA and digitoxin sensitize cell-detachment induced apoptosis primarily through the mitochondrial apoptotic pathway.
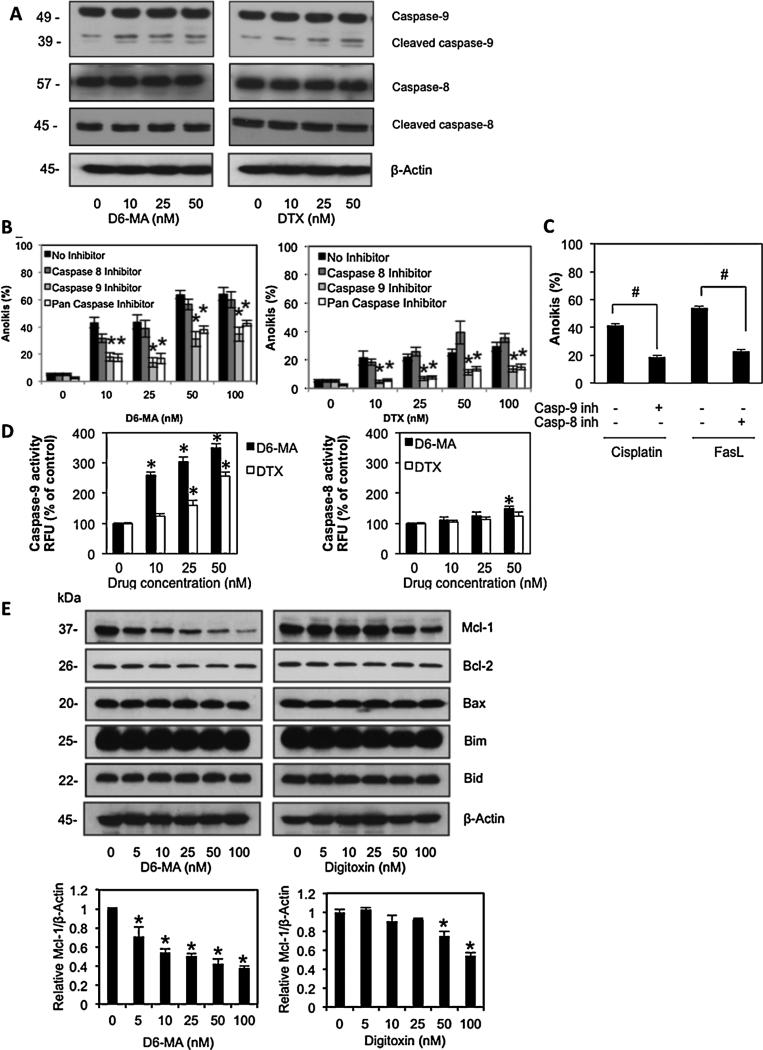
Fig. 2.
D6-MA and digitoxin mediated anoikis through mitochondria pathway. (A) Effects of D6-MA and digitoxin on caspase-9 and caspase-8 expression. Detached H460 cells in serum-free medium were treated with various doses of D6-MA and digitoxin (0–50 nM) for 12 h. Caspase-9, caspase-8 and their cleaved products were analyzed by Western blotting. (B) Detached cells were treated with either D6-MA (0–100 nM) or digitoxin (0–100 nM) in the presence or absence of 10 μM pan-caspase inhibitor (zVAD-FMK), 10 μM specific caspase-8 inhibitor (zIETD-FMK), or 10 μM specific caspase-9 inhibitor (zLEHD-FMK) for 12 h in 1% FBS medium and evaluated for anoikis using Hoechst 33342/propidium iodide staining assay. (C) H460 cells were treated with cisplatin (50 μM) in the presence or absence of 10 μM of caspase-9 inhibitor for 12 h. Under the same condition, H460 cells were treated with FasL (50 ng/mL) in the presence or absence of 10 μM of caspase-8 inhibitor for 12 h. Apoptosis cells were determined by Hoechst 33342 assay. (D) Caspase-8 and -9 activities in suspended cells following 12 h treatment of D6-MA and digitoxin (0–50 nM) were determined by LETD-AFC and LEHD-AFC fluorescence assay, respectively. (E) Effects of D6-MA and digitoxin on Bcl-2 family protein expression were analyzed by Western blotting. Immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to non-treated cells. All data represent mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells. #p < 0.05 versus control treated group.
3.3. D6-MA sensitized detachment-induced apoptosis by decreased Mcl-1 expression
Since expression of pro- and anti-apoptotic proteins in the Bcl-2 family play an important role in the mitochondrial apoptotic pathway [38], we further investigated which apoptotic regulatory protein(s) were required for D6-MA and digitoxin induced anoikis. Detached cells were incubated with D6-MA and digitoxin (0–100 nM) in attachment resistant plates for 12 h and determined expression for several key pro- and anti-apoptotic Bcl-2 family proteins by Western blotting. Among the Bcl-2 family proteins, only anti-apoptotic Mcl-1 was substantially decreased in dose-dependent manner by both compounds. D6-MA showed approximately 10-fold greater potency than digitoxin, while all other proteins displayed negligible change (Fig. 2E). Similarly, decreased Mcl-1 expression was also observed in attached H460 cells at doses that result in apoptosis (data not shown). This result suggested that Mcl-1 is a target of digitoxin-based compound induced anoikis and attached cell apoptosis in NSCLC.
Since reduced Mcl-1 expression correlated with D6-MA's and digitoxin's anoikis sensitization, we investigated whether Mcl-1 expression protects NSCLC from anoikis by stably transfecting H460 cells with Mcl-1. These transfected cells and control plasmid cells (pcDNA) were then evaluated for detachment-induced cell death following D6-MA and digitoxin exposure. Western blot analysis of Mcl-1 expression resulted in elevated Mcl-1 expression in Mcl-1-transfected cells compared with pcDNA cells (Fig. 3A). Both transfected cell lines were detached, treated with either D6-MA or digitoxin and analyzed for anoikis by Hoechst/PI assay. Mcl-1-transfected cells exhibited resistance to D6-MA and digitoxin (0–100 nM) compared to pcDNA-transfected cells (Fig. 3B). A parallel Western blot study demonstrated that Mcl-1 level was slightly decreased in Mcl-1 over-expressing cells treated with 100 nM D6-MA and 200 nM digitoxin, whereas it was extensively down-regulated in pcDNA cells (Fig. 3C). These results confirmed that Mcl-1 expression is a major target for D6-MA and digitoxin-induced anoikis.
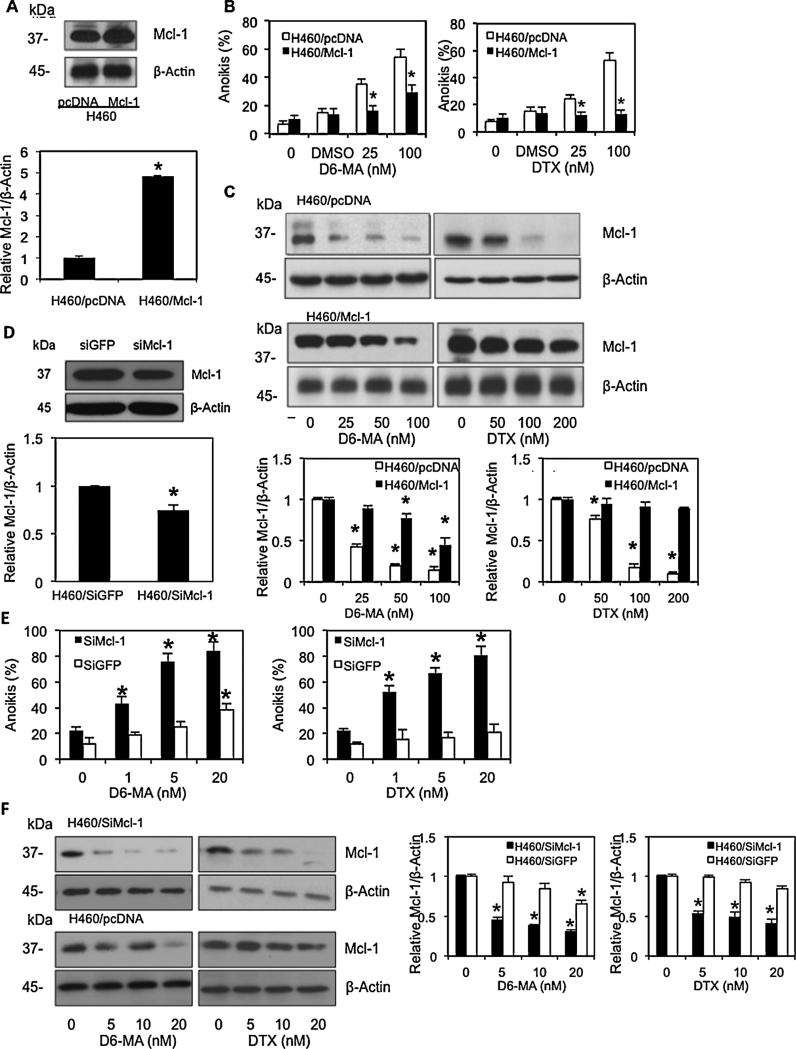
Fig. 3.
Mcl-1 over-expression rescues D6-MA and digitoxin-mediated anoikis. (A) Western blot analysis of Mcl-1 expression in control (H460/pcDNA) and Mcl-1-transfected (H460/Mcl-1) cells. Attached H460 cells were stably transfected with Mcl-1 or pcDNA control plasmid as described under Section 2. Mcl-1 expression level was evaluated by Western blotting. (B) Effects of D6-MA and digitoxin on Mcl-1 over-expressing cells (H460/Mcl-1). Suspended H460/Mcl-1 and H460/pcDNA cells were treated with various doses of D6-MA and digitoxin (0–100 nM), and DMSO as control diluent for 24 h. Anoikis cells were evaluated by Hoechst 33342/propidium iodide staining assay. Data are the mean ± S.D. (n = 4). *p < 0.05 versus control transfected cells (H460/pcDNA). (C) Effects of D6-MA and digitoxin on Mcl-1 expression in H460/Mcl-1 and H460/pcDNA cells. Detached cells were treated with indicated concentration of D6-MA and digitoxin for 24 h, and analyzed for Mcl-1 expression by Western blotting. (D) Attached H460 cells were transfected with either esiMcl-1 or SiGFP control, and Mcl-1 expression was determined by Western blotting. Data are the mean ± S.D. (n = 4). *p < 0.05 versus control transfected cells (H460/siGFP). (E) Effects of D6-MA and digitoxin treatment on siMcl-1 H460 knockdown and negative siRNA GFP control. Anoikis were assayed by Hoechst 33342/propidium iodide staining assay after treatment for 24 h. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells. (F) Effects of D6-MA and digitoxin on Mcl-1 expression in H460/SiMcl-1 and H460/SiGFP cells. Detached cells were treated with indicated concentrations of D6-MA and digitoxin for 24 h, and analyzed for Mcl-1 expression by Western blotting. All blots were reprobed with β-actin to confirm equal loading. Immunoblot signals were quantified by densitometry and mean data from independent experiment were normalized to the results. Bars are the means SD (n = 4). *P < 0.05 versus non-treated cells.
Furthermore we assessed whether decreased Mcl-1 expression facilitates or induces anoikis by performing anoikis assays on siRNA Mcl-1 knockdown compared to normal H460 cells. Negative control and siRNA Mcl-1 knockdowns were assessed for anoikis following D6-MA and digitoxin treatment. Western blot analysis of Mcl-1 expression resulted in decreased Mcl-1 levels in attached, siRNA Mcl-1 transfected H460 cells compared to attached negative control siRNA-GFP tagged cells (Fig. 3D). Both transfected cell lines were suspended and exposed to D6-MA and digitoxin. Unexposed Mcl-1 knockdown cells showed a significant increase in anoikis compared to negative controls, supporting the positive role of Mcl-1 on anoikis resistance. Furthermore, CG-exposed Mcl-1 knockdown cells displayed significantly decreased Mcl-1 level which correlated with increasing anoikis in response to D6-MA and digitoxin treatment at known therapeutic digitoxin concentrations (Fig. 3E and F). Collectively, these results suggest that decreased Mcl-1 expression facilitates anoikis and that both D6-MA and digitoxin stimulate other key protein(s) to drive anoikis-related signaling.
3.4. D6-MA mediated Mcl-1 degradation through proteasomal degradation pathway
Recent studies demonstrated that Mcl-1 expression is regulated at both transcriptional and post-translational levels [40–42]. To investigate whether D6-MA and digitoxin down-regulated Mcl-1 via suppression of protein synthesis or induction of protein degradation, Mcl-1 expression was determined in detached cells treated with D6-MA (25 nM) and digitoxin (100 nM) in the presence or absence of proteasome inhibitor MG132 or protein translation inhibitor cycloheximide (CHX). Both D6-MA and digitoxin suppressed Mcl-1 levels, which were significantly turned over by pretreatment of the cells with MG132, but not CHX (Fig. 4A).
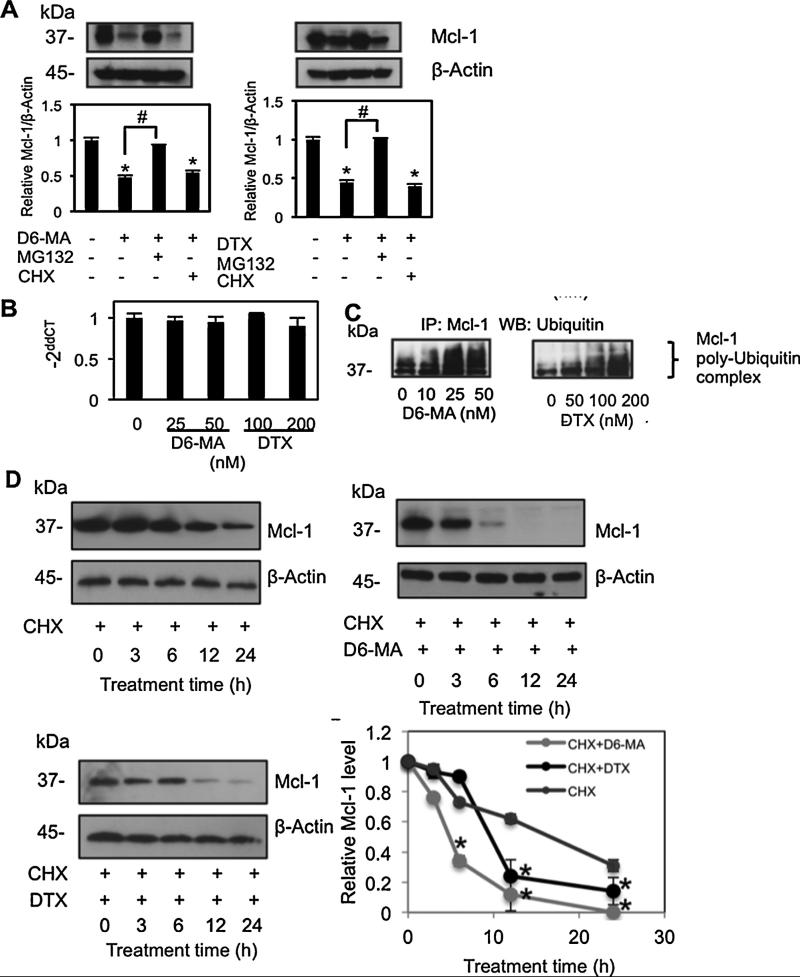
Fig. 4.
D6-MA and digitoxin mediate Mcl-1 degradation through ubiquitin-proteasomal pathway. (A) Suspended H460 cells were treated with D6-MA (25 nM) or digitoxin (100 nM) in the presence or absence of proteasome inhibitor MG132 (10 μM), or protein translation inhibitor cycloheximide (CHX, 10 μg/mL) for 12 h to determine if protein degradation or synthesis regulated Mcl-1 expression. Mcl-1 expression was determined by Western blot analysis. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells; #p < 0.05 versus D6-MA or digitoxin treated cells. (B) Detached H460 cells were treated with D6-MA (0–50 nM) and digitoxin (0–200 nM) for 12 h, and mRNA was separated and determined by quantitative real-time PCR. The relative Mcl-1 mRNA expression was determined by using the comparative CT method as described under Section 2. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells. (C) Detached H460 cells were treated with various concentrations of D6-MA (0–50 nM) or digitoxin (0–200 nM) for 2 h post-treatment where ubiquitination was found to be maximal. Cell lysates were immunoprecipitated with anti-Mcl-1 antibody and the immune complexes were analyzed for ubiquitin by Western blots using anti-ubiquitin antibody. (D) Detached H460 cells were pretreated with 10 μg/mL of protein translation inhibitor, cycloheximide (CHX) for 1 h in the presence or absence of either 25 nM of D6-MA or 200 nM of digitoxin for various times. Mcl-1 level was analyzed by Western blotting. Immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to sample at time = 0 h. All data represent mean ± S.D. (n = 4). *p < 0.05 versus CHX-treated cells at each time point.
Quantitative-real time PCR analysis also showed that no significant change in Mcl-1 mRNA expression was observed in cells treated with both D6-MA and digitoxin (Fig. 4B), whereas protein level was substantial decreased (Fig. 2C). Thus, reduction of Mcl-1 protein was regulated through a transcription-independent mechanism. Our results suggested that proteasomal degradation is a pivotal mechanism in Mcl-1 down-regulation mediated by D6-MA and digitoxin. Since proteins targeted by the proteasome require ubiquitination and subsequent degradation [42], Mcl-1 ubiquitination was confirmed by co-immunoprecipitation. Both D6-MA and digitoxin induced Mcl-1 ubiquitination in a dose-dependent manner (Fig. 4C). Furthermore, a cycloheximide half-life study demonstrated that D6-MA treatment accelerated constitutive Mcl-1 down-regulation (Fig. 4D). Mcl-1 half-life in CGs-untreated, detached H460 cells was approximately 17 h, whereas it was dramatically decreased to 4.5 h and 10 h following exposure to equipotent doses of D6-MA and digitoxin, respectively. Together, these results supported the mechanism of D6-MA and digitoxin sensitized anoikis of NSCLC via Mcl-1 proteasomal degradation.
3.5. Proteasomal degradation of Mcl-1 mediated by D6-MA is dependent on GSK-3β pathway
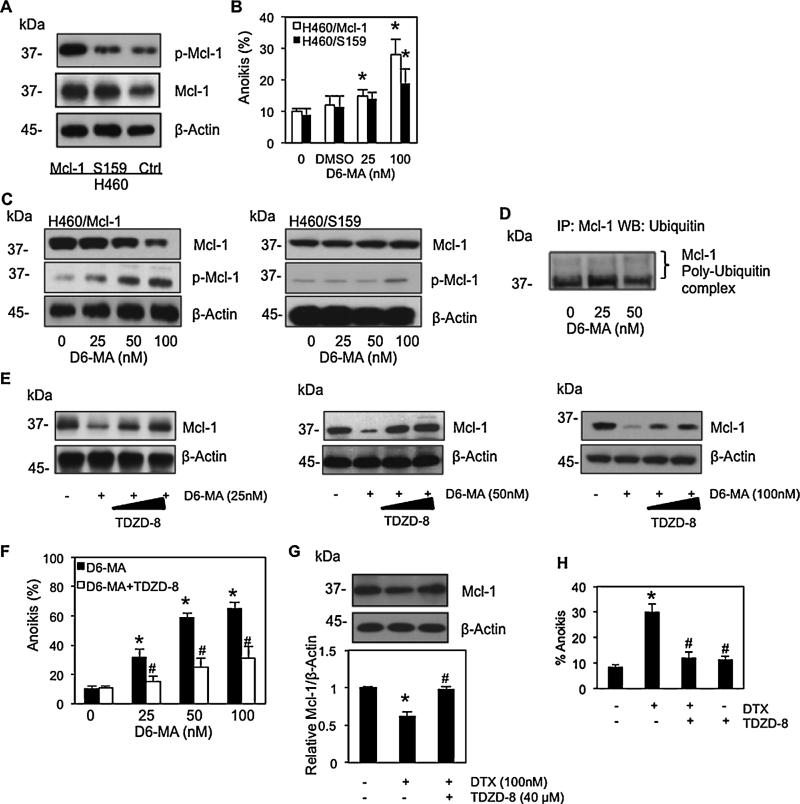
Since D6-MA showed more potent anoikis sensitizing, we focused deeper on the molecular mechanism of D6-MA. Recently, it was reported that glycogen synthase kinase (GSK)-3β plays a central role in negatively regulating Mcl-1 stability [41,43] by phosphorylating serine 159 which targets it for ubiquitin degradation. To investigate whether D6-MA sensitizes H460 cells to anoikis through GSK-3β-mediated Mcl-1 degradation, cells were stably transfected with mutant Mcl-1 at S159 and wild-type (WT) Mcl-1 plasmids. Transfected cells were suspended, exposed to increasing concentrations of D6-MA (0–50 nM), and evaluated for anoikis by Hoechst/PI assay. Fig. 5A showed that phosphory-late Mcl-1 level was substantially suppressed in cells transfected with mutant Mcl-1S159 compared to WT Mcl-1-transfected cells. The presence of phosphorylate Mcl-1 in mutant transfected cells (H460/S159) was observed due to endogeneous Mcl-1 which was also found in control cells (H460/Ctrl). Treatment with 100 nM D6-MA significantly increased anoikis in WT Mcl-1 transfected cells compared to Mcl-1S159-transfected cells (Fig. 5B). D6-MA was unable to significantly increase anoikis in Mcl-1S159-transfected cells compared to unexposed controls (p < 0.05). Similarly, D6-MA exhibited reduced anoikis induction ability in WT Mcl-1-transfected cells compared to pcDNA transfected-cells (Fig. 3B). This suggested that Mcl-1S159 over-expressing cells were more resistant to anoikis mediated by D6-MA (Fig. 5B). Western blot analysis with similar treatment also confirmed the correlation of Mcl-1 level and anoikis cells. There was no detectable change in Mcl-1 level in cells transfected with mutant Mcl-1S159 plasmid as compared to control cells (Fig. 5C). Phosphorylated Mcl-1 in H460/S159 cells was slightly increased in response to high dose of D6-MA (100 nM) compared to its gradual dose-dependent increase in H460/Mcl-1 cells (Fig. 5C). Co-immuno-precipitation of Mcl-1 and ubiquitin in Mcl-1S159-transfected cells showed that Mcl-1 ubiquitination was not significantly altered by D6-MA compared with non-treated control cells (Fig. 5D). These results implied that inhibition of Mcl-1 phosphorylation at S159 was able to prevent D6-MA activated GSK-3β designation of Mcl-1 for degradation.
Fig. 5.
D6-MA mediated Mcl-1 degradation via GSK-3β-dependent pathway. (A) Western b lot analysis of Mcl-1 expression in wild-type (WT), Mcl-1S159 and control (Ctrl)-transfected cells. H460 cells were stably transfected with WT Mcl-1, mutant Mcl-1S159, or control plasmid as described under Section 2. Phosphorylate Mcl-1 and Mcl-1 were examined by Western blotting. (B) Effects of D6-MA on Mcl-1S159 over-expressing (H460/S159) cells. Suspended H460/S159 and H460/Mcl-1 cells were treated with various doses of D6-MA (0–100 nM), and DMSO as control diluent for 24 h. Anoikis cells were evaluated by Hoechst 33342/propidium iodide staining assay. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated transfected cells. (C) Effects of D6-MA on Mcl-1 expression in H460/S159 and H460/Mcl-1 cells. Detached cells were treated with indicated concentration of D6-MA for 24 h and Mcl-1 and phosphorylate Mcl-1 expressions were examined by Western blotting. (D) Effect of Mcl-1S159 plasmid on Mcl-1 ubiquitination-mediated by D6-MA. Suspended Mcl-1S159-transfected cells were treated with D6-MA (0–50 nM) for 2 h post-treatment where ubiquitination was found to be maximal. Cell lysates were immunoprecipitated with anti-Mcl-1 antibody and the immune complexes were analyzed for ubiquitin by Western blots using anti-ubiquitin antibody. (E) Mcl-1 expression in suspended H460 treated with 25, 50, or 100 nM of D6-MA for 12 h under increasing doses of GSK-3β inhibitor TDZD-8 (40–60 mM) was analyzed by Western blotting. (F) Anoikis cells were evaluated by Hoechst assay in suspended H460 cells treated with D6-MA (0–100 nM) in the presence or absences of GSK-3β inhibitor TDZD-8 (40 mM) for 12 h. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated control cells; #p < 0.05 versus D6-MA treated cells. (G) GSK-3β inhibitor rescued H460 cells from digitoxin induced anoikis. Mcl-1 expression in suspended H460 treated with digitoxin (100 nM) under GSK-3β inhibitor TDZD-8 (40 μM) for 12 h was analyzed by Western blotting. Immunoblot signals were quantified by densitometry. All data represent mean ± S.D. (n = 4). *p < 0.05 versus non-treated control cells; #p < 0.05 versus digitoxin treated cells. (H) Anoikis cells were evaluated by Hoechst 33342/propidium iodide staining assay in suspended H460 cells treated with digitoxin (100 nM) in the presence or absences of GSK-3β inhibitor TDZD-8 (40 mM) for 12 h. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated control cells; #p < 0.05 versus digitoxin treated cells.
To evaluate GSK-3β activity on Mcl-1 expression, detached cells were incubated with D6-MA (0–100 nM) in the presence or absence of GSK-3β inhibitor TDZD-8, and probed for Mcl-1 expression by Western blot. TDZD-8 is a well-established inhibitor of GSK-3β and shows no inhibitory activity against several kinases involved in signal transduction pathways [44,45]. Western blot analysis revealed that cells pretreated with various concentrations of TDZD-8 caused a dose-dependent Mcl-1 stabilization as compared to cells treated with D6-MA alone (Fig. 5E). The relationship between Mcl-1 expression and cell anoikis regulated by GSK-3β in response to D6-MA was also examined. Hoechst/PI assay demonstrated that TDZD-8 was able to rescue H460 cell anoikis mediated by D6-MA, whereas TDZD-8 alone did not significantly increase anoikis compared to non-treated cells (Fig. 5F). TDZD treatment also rescued H460 cells from digitoxin induced anoikis (Fig. 5G and H). Together, these findings indicated that GSK-3β plays an important regulatory role in suppressing Mcl-1 expression during D6-MA induced anoikis.
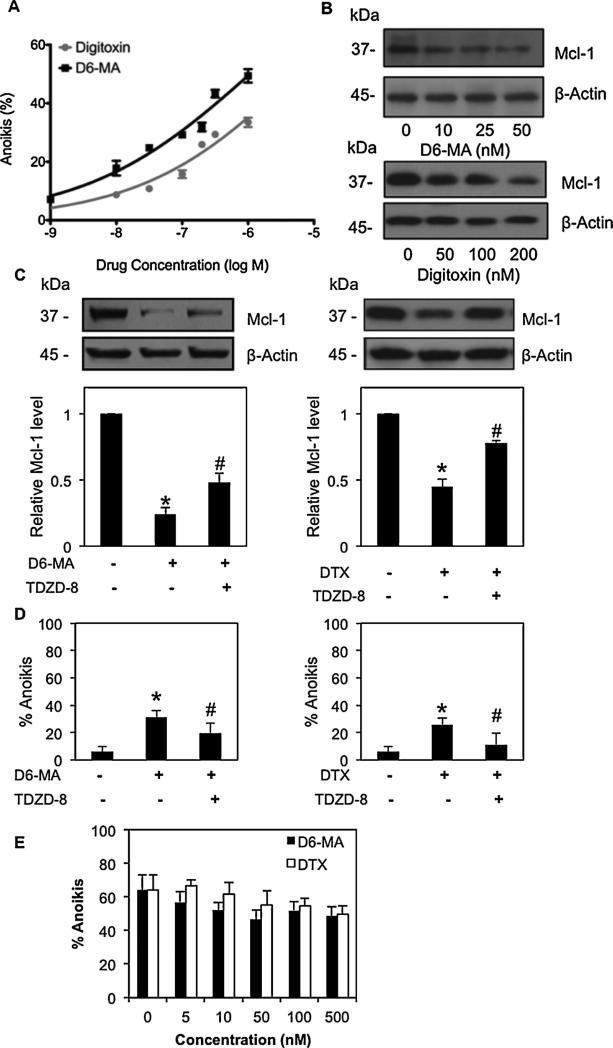
3.6. Effect of digitoxin and its derivative D6-MA on A549 and normal lung epithelial cell anoikis
To substantiate the effect of D6-MA and digitoxin on anoikis sensitization, we broadened our study to include A549 lung cancer and non-tumorigenic small airway epithelial cells (SAEC). A549 cells were treated with D6-MA and digitoxin followed by assessment for anoikis induction and Mcl-1 protein expression. D6-MA and digitoxin induced anoikis in A549 cell lines which correlated with decreased Mcl-1 expression (Fig. 6A and B). Similar to H460 cells, decreased Mcl-1 expression in A549 cells was reversed by pre-treatment with GSK-3β inhibitor (Fig. 6C). Furthermore, TDZD treatmentresulted in the protection of A549 cells from D6-MA and digitoxin-sensitized A549 anoikis (Fig. 6D). Both D6-MA and digitoxin exhibited higher potency against suspended H460 cells (IC50 = 11.9 and 90.7 nM) while both compounds exhibited 50–100-fold reduction in potency against A549 cells (IC50 > 500 nM). Lastly, both compounds did not significantly affect anoikis status of suspended SAEC cells (Fig. 6E) which displayed 52% to 61% anoikis at 24 h with <8% necrosis from 0 to 500 nM. This further suggests promising NSCLC-specific, anti-metastatic activity of D6-MA and digitoxin. In summary, both D6-MA and digitoxin induced anoikis in lung cancer cells via decreased Mcl-1 expression. D6-MA and digitoxin activate GSK-3β leading to Mcl-1 degradation and NSCLC anoikis.
Fig. 6.
D6-MA and digitoxin sensitized A549, but not normal small airway epithelial cells to anoikis. (A) Detachment-induced cell death was determined by Hoechst 33342/propidium iodide staining assay. A549 cells were seeded onto poly-HEMA-coated plate and treated with varying concentrations of D6-MA and digitoxin (0–1000 nM) for 24 h. (B) Effects of D6-MA and digitoxin on Mcl-1 protein expression were analyzed by Western blotting. (C) Effects of GSK-3β on Mcl-1 expression mediated by D6-MA and digitoxin. Suspended A549 cells were pretreated with GSK-3β inhibitor TDZD-8 (40 μM), prior to D6-MA (50 nM) and digitoxin (200 nM) or left untreated as control cells. Mcl-1 expression was analyzed by Western blotting. Immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to non-treated cells. All data represent mean ± S.D. (n = 4). *p < 0.05 versus non-treated cells. #p < 0.05 versus D6-MA or digitoxin treated cells. (D) Anoikis cells were evaluated by Hoechst 33342/propidium iodide staining assay in suspended A549 cells treated with either D6-MA (50 nM) or digitoxin (100 nM) in the presence or absences of GSK-3β inhibitor TDZD-8 (40 μM) for 12 h. Data are the mean ± S.D. (n = 4). *p < 0.05 versus non-treated control cells; #p < 0.05 versus treated cells. (E) Detachment-induced anoikis in human small airway epithelial cells (SAEC) determined by Hoechst/PI staining assay. SAEC were suspended onto poly-HEMA-coated plates and treated with varying concentrations of D6-MA and digitoxin (0–500 nM) for 24 h. Anoikis cells were evaluated by Hoechst 33342/propidium iodide staining assay.
4. Discussion
Anoikis, detachment-induced apoptosis, plays an important role in the prevention of cancer cell migration and establishment of secondary tumors. Since anoikis resistance provides a survival mechanism for advanced metastatic cancers, sensitizing cancer cells to anoikis will provide benefit for cancer therapy [4]. Our study has reported for the first time on the potent anoikis sensitizing effect of the digitoxin derivative D6-MA. Its underlying MOA in NSCLCs was shown to involve Mcl-1 degradation associated with GSK-3β-mediated signaling. Along with epidemiological evidence, digitoxin possesses anticancer properties in various cancers including breast, melanoma, and NSCLC [10–12,16,19,27,28]. Apoptotic induction by digitoxin is believed to occur through downstream signaling cascades activated by the conformational change of Na+/K+ ATPase leading to diverse cellular responses [14,46]. Although prior studies reported digitoxin's ability to initiate cancer cell anoikis, a clear MOA had not been established.
Recently, α-l-rhamnose monosaccharide digitoxin derivative, D6-MA, has shown greater potency in apoptotic induction of attached NSCLC than digitoxin through a caspase-9 dependent pathway [27,28] and that this potency difference correlated with inhibition of isolated Na+/K+ ATPase [29]. Here, we found that D6-MA exhibits a promising anoikis sensitizing effect by showing 7.5-fold greater potency than digitoxin (Fig. 1B), and that this activity was maintained over time, similar to our previous attached cell findings [28,29]. Simpson et al. screened the anoikis sensitizing effect of several CGs on prostate cancer cells including ouabain, peruvoside, digoxin, digitoxin, and strophanthidin, and showed anoikis induction was mediated through inhibition of Na+/K+ ATPase causing hypoosmotic stress and caspase activation [21]. By comparing the anoikis IC50 to attached cell apoptosis IC50 values previously reported by our group [28,29], we concluded that D6-MA exhibited equal ability to induce mitochondrial apoptotic cell death in attached or suspended NSCLC cells. Digitoxin, however, was more effective at inducing apoptotic death in attached NSCLC cells compared to suspended cells. This suggests that D6-MA is equally effective at targeting primary NSCLC tumors and decreasing metastasis risk at low doses. Conversely, digitoxin exhibits ability to target primary NSCLC tumors near therapeutic doses. Administration of D6-MA may target both primary tumors and suspended metastatic cells, thus reducing metastatic risk and improving patient prognosis. Maintenance of cell death activity over time in both unattached and attached cells suggests that potency is not a bioavailability difference, but is potentially due to differences in target binding affinity. ATPase binding studies are currently underway in a collaborator's laboratory. Our study provides additional anti-metastatic evidence and a MOA for D6-MA's and digitoxin's anticancer properties.
Because apoptotic induction by CGs was demonstrated to mediate both mitochondria and death receptor pathway [27,47], we identified the pathway through which D6-MA and digitoxin sensitize NSCLCs to anoikis. Several signaling pathways regulate anoikis including c-Jun amino-terminal kinase (JNK), phosphatidylinositol-3 kinase (PI3K)/Akt, focal adhesion kinase (FAK), and extracellular signal-regulated protein kinase (ERK) [48–50]. Furthermore, deregulation of mitochondria apoptotic pathway was suggested to be the important downstream anoikis signaling mechanism [5,32]. Our results also clearly showed that mitochondrial apoptotic pathway is the MOA for anoikis sensitization (Fig. 2). Of note, the cell death (i.e. apoptosis-like programmed cell death; 51) observed with co-treatment of pan-caspase inhibitor and D6-MA doses at 5- to 10-fold above IC50 was potentially due to Na+/K+ ATPase inhibition causing an ionotropic cascade, internal Ca2+ release and a caspase independent apoptotic cell death which has been reported for other CGs [14]. This cell death mechanism, however, may not be relevant for clinical therapeutic doses of digitoxin-based compounds (<100 nM) and for D6-MA's high potency for NSCLCs. Caspase-8 inhibitor was minimally effective in rescuing NSCLC cells from D6-MA and digitoxin induced anoikis (Fig. 2B). Frese et al. showed that CG treatment caused over-expression of death receptors 4 and 5, facilitating cells to become more susceptible to TRAIL and caspase-8 mediated apoptosis [47]. Based on our data, digitoxin and D6-MA exposure strongly cleaved caspase-9, an initiator caspase in mitochondria pathway, to induce NSCLC cell anoikis while cleaved caspase-8 was not a sufficient signal.
Increasing evidence suggests the importance of Bcl-2 family proteins in cell survival during anchorage condition [22,31,32]. In addition, sufficient Mcl-1 expression was widely implicated as a positive regulator of anoikis resistance in cancer [22,23]. This present study also shows that Mcl-1 is an appreciable target for D6-MA and digitoxin in anoikis sensitizing effect on NSCLC at clinically relevant doses (Fig. 2E, 3B and C). In addition, the failure of over-expressed Mcl-1 to completely block anoikis at high D6-MA dose (Fig. 3B) further supports an ionotropic, caspase-independent cell death [21,51] that does not rely on Mcl-1. Transcriptional regulation and post-translational modifications (i.e. ubiquitination) of Mcl-1 are principle regulators for its stability and function [39,40]. Nijhawan et al. demonstrated that proteasomal pathway is a key regulator of Mcl-1 in response to apoptotic induction-mediated by UV [52], supporting our finding. We herein found that Mcl-1 was primarily regulated by proteasomal degradation in response to D6-MA and digitoxin-mediated anoikis (Fig. 4). In addition, D6-MA exposure to Mcl-1 knockdown cells increased anoikis incidence lending further support to Mcl-1's role sensitizing suspended NSCLC to cardiac glycosides (Fig. 3E and F).
We conducted further studies with D6-MA to determine the MOA of CG-mediated Mcl-1 ubiquitination in anoikis cells. GSK-3β is a well-recognized requirement for Mcl-1 degradation since it phosphorylates Mcl-1 at serine 159, leading to the association with E3-ligase prior to ubiquitination and degradation [41,43]. In the present study, we also showed the importance of Mcl-1 serine 159 motif in regulating its proteasomal degradation in NSCLC. Furthermore, GSK-3β inhibition was able to reverse D6-MA's effect on Mcl-1 expression (Fig. 5E). Our findings coincide with previous reports that GSK-3β activation in breast cancer with high levels of inactive pGSK-3β (ser9) results in Mcl-1 degradation [45,53]. GSK-3β is increasingly seen as a potential chemotherapeutic target due to its role in mediating numerous cancer cell survival and development pathways; however, its role as a tumor ‘suppressor’ or ‘enhancer’ is tumor cell type dependent [54]. Lung and oral cancers display high levels of inactive pGSK-3β (ser9) [55]. Alternatively, our findings contradict a recent report that cinobufagin, a cardenolide, inactivates GSK-3β via serine 9 phosphorylation leading to decreased p65 NF-kB expression and apoptosis in osteosarcoma cells [56]. Cardiac glycosides and cardenolides are known to target (1) over-expressed NF-kB in cancer cells [13,14,24] and (2) upstream Bim, Bak and Bax that target Mcl-1 [21,24]. It is highly likely that endogenous GSK-3β activation state and cancer cell type will influence cardiac glycoside effects on GSK-3 β regulated cell signaling pathways regulating apoptosis. Our present study provides additional novel mechanistic insight of the requirement of activated GSK-3β in D6-MA-induced Mcl-1 degradation in NSCLCs. Since anoikis resistance attributes to the metastatic ability of cancer, our novel findings on the anoikis sensitizing effect of D6-MA and digitoxin expands the mechanistic understanding of CG anti-metastatic properties and supports their potential future use in cancer therapy.
Acknowledgements
Special thanks to Y. Lu, M. Chen, S. Talbott, D. Thapa and N. Hoffman for assistance with cell culture and initial anoikis assays. The authors thank Dr. Kathleen Brundage for her help with the flow cytometry experiments, which were performed in the West Virginia University Flow Cytometry Core Facility and supported in part by NIH grants RR016440 and RR020866. This study was partially funded by National Institutes of Health [GM088839, GM090259, HL095579]; and National Science Foundation [EPS1003907].
Abbreviations
- Bcl-2
B-cell lymphoma 2
- Casp-3
caspase-3
- CHX
cycloheximide
- CG
cardiac glycoside
- DMSO
dimethyl sulfoxide
- D6-MA
α-l-rhamnose monosaccharide derivative
- ECM
extracellular matrix
- ERK
extracellular signal-regulated protein kinase
- FAK
focal adhesion kinase
- GSK-3β
glycogen synthase kinase-3β
- JNK
c-Jun amino-terminal kinase
- MOA
mode of action
- NSCLC
non-small cell lung cancer
- PARP
poly(ADP-ribose) polymerase
- PI
propidium iodide
- PI3K
phosphatidylinositol-3 kinase
- TDZD
thiadiazolidinones
Footnotes
Competing interests
Non-financial competing interests: TAS is currently employed by NIOSH. GO consults at Protea Biosciences.
Disclaimer: Research findings and conclusions are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2011;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 5.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions or proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaprashantha LD, Vatsyayan R, Lelsani PC, Awasthi S, Singhal SS. The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int J Cancer. 2011;128:743–52. doi: 10.1002/ijc.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute SEER stat fact sheets: lung and bronchus. http://seer.cancer.gov/statfacts/html/lungb.html.
- 10.Stenkvist B, Bengtsson E, Eriksson O, Holmquist J, Nordin B, Westman-Naeser S. Cardiac glycosides and breast cancer. Lancet. 1979;1:563. doi: 10.1016/s0140-6736(79)90996-6. [DOI] [PubMed] [Google Scholar]
- 11.Stenkvist B, Pengtsson E, Dahlqvist B, Eriksson O, Jarkrans T, Nordin B. Cardiac glycosides and breast cancer, revisited. N Engl J Med. 1982;306:484. [PubMed] [Google Scholar]
- 12.Haux J, Klepp O, Spigset O, Tretli S. Digitoxin medication and cancer; case control and internal dose-response studies. BMC Cancer. 2001;1:11. doi: 10.1186/1471-2407-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta. 2007;1776:32–57. doi: 10.1016/j.bbcan.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 16.Newman RA, Yang P, Hittelman WN, Lu T, Ho DH, Ni D, et al. Oleandrin-mediated oxidative stress in human melanoma cells. J Exp Ther Oncol. 2006;5:167–81. [PubMed] [Google Scholar]
- 17.Bhatia SJ, Smith TW. Digitalis toxicity: mechanisms, diagnosis, and management. J Card Surg. 1987;2:453–65. doi: 10.1111/j.1540-8191.1987.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Haux J. Digitoxin is a potential anticancer agent for several types of cancer. Med Hypotheses. 1999;53:543–8. doi: 10.1054/mehy.1999.0985. [DOI] [PubMed] [Google Scholar]
- 19.Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, et al. Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One. 2009;4:e8292. doi: 10.1371/journal.pone.0008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akimova OA, Poirier M, Kotelevtsev SV, Hamet P, Orlov SN. The death of ouabain-treated renal epithelial cells: evidence against anoikis occurrence. Apoptosis. 2008;13:670–80. doi: 10.1007/s10495-008-0204-y. [DOI] [PubMed] [Google Scholar]
- 21.Simpson CD, Mawji IA, Anyiwe K, Williams MA, Wang X, Venugopal AL, et al. Inhibition of the sodium potassium adenosine triphosphatase pump sensitizes cancer cells to anoikis and prevents distant tumor formation. Cancer Res. 2009;69:2739–47. doi: 10.1158/0008-5472.CAN-08-2530. [DOI] [PubMed] [Google Scholar]
- 22.Woods NT, Yamaguchi H, Lee FY, Bhalla KN, Wang HG. Anoikis, initiated by mcl-1 degradation and bim induction, is deregulated during oncogenesis. Cancer Res. 2007;67:10744–52. doi: 10.1158/0008-5472.CAN-07-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juncker T, Cerella C, Teiten MH, Morceau F, Schumacher M, Ghelfi J, et al. UNBS1450, a steroid cardiac glycoside inducing apoptotic cell death in human leukemia cells. Biochem Pharmacol. 2011;81:13–23. doi: 10.1016/j.bcp.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Langenhan JM, Peters NR, Guzei IA, Hoffmann FM, Thorson JS. Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization. Proc Natl Acad Sci U S A. 2005;102:12305–10. doi: 10.1073/pnas.0503270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou M, O'Doherty G. The de novo synthesis of oligosaccharides: application to the medicinal chemistry SAR-study of digitoxin. Curr Top Med Chem. 2008;8:114–25. doi: 10.2174/156802608783378828. [DOI] [PubMed] [Google Scholar]
- 27.Iyer AK, Zhou M, Azad N, Elbaz H, Wang L, Rogalsky DK, et al. A direct comparison of the anticancer activities of digitoxin MeON-neoglycosides and O-glycosides: oligosaccharide chain length-dependent induction of caspase-9-mediated apoptosis. ACS Med Chem Lett. 2010;1:326–30. doi: 10.1021/ml1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang HYL, Xin W, Zhou M, Stueckle TA, Rojanasakul Y, O'Doherty GA. Stereochemical survey of digitoxin monosaccharides: new anticancer analogues with enhanced apoptotic activity and growth inhibitory effect on human non-small cell lung cancer cell. ACS Med Chem Lett. 2011;2:73–8. doi: 10.1021/ml100219d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbaz H, Stueckle TA, Wang HL, O'Doherty G, Lowry DT, Sargeant LM, et al. Digitoxin and a synthetic monosaccharide analog induce inhibit cell viability in lung cancer cells. Toxicol Appl Pharm. 2012;258:51–60. doi: 10.1016/j.taap.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel C, Carlson SM, White FM, Davis RJ. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol. 2009;29(14):3845–52. doi: 10.1128/MCB.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pongrakhananon V, Nimmannit U, Luanpitpong S, Rojanasakul Y, Chanvorachote P. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis. 2010;15:574–85. doi: 10.1007/s10495-010-0461-4. [DOI] [PubMed] [Google Scholar]
- 32.Martin SS, Vuori K. Regulation of bcl-2 proteins during anoikis and amorphosis. Biochim Biophys Acta. 2004;1692:145–57. doi: 10.1016/j.bbamcr.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–43. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Yang C, Wang X, Xu C, Li S, Zhang B, et al. Overexpression of Smac promotes Cisplatin-induced apoptosis by activating caspase-3 and caspase-9 in lung cancer A549 cells. Cancer Biother Radiopharm. 2013;28(2):177–82. doi: 10.1089/cbr.2012.1261. [DOI] [PubMed] [Google Scholar]
- 35.Gibalová L, Sereš M, Rusnák A, Ditte P, Labudová M, Uhrík B, et al. P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. Toxicol In Vitro. 2012;26(3):435–44. doi: 10.1016/j.tiv.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Morgan MJ, Kim YS, Liu ZG. Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex1. J Immun. 2009:183. doi: 10.4049/jimmunol.0803428. http://dx.doi.org/10.4049/jimmunol.0803428. [DOI] [PMC free article] [PubMed]
- 37.Bando M, Hasegawa M, Tsuboi Y, Miyake Y, Shiina M, Ito M, et al. The mycotoxin penicillic acid inhibits Fas ligand-induced apoptosis by blocking self-processing of caspase-8 in death-inducing signaling complex. J Biol Chem. 2003;278(8):5786–93. doi: 10.1074/jbc.M204178200. [DOI] [PubMed] [Google Scholar]
- 38.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–9. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 40.Nencioni A, Hua F, Dillon CP, Yokoo R, Scheiermann C, Cardone MH, et al. Evidence for a protective role of mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005;105:3255–62. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 41.Opferman JT. Unraveling MCL-1 degradation. Cell Death Differ. 2006;13:1260–2. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 42.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 43.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3b (GSK-3b) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem. 2002;45:1292–9. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 45.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3B activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–71. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 46.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frese S, Frese-Schaper M, Andres AC, Miescher D, Zumkehr B, Schmid RA. Cardiac glycosides initiate Apo2L/TRAIL-induced apoptosis in non-small cell lung cancer cells by up-regulation of death receptors 4 and 5. Cancer Res. 2006;66:5867–74. doi: 10.1158/0008-5472.CAN-05-3544. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Kuo C, Tsai Y, Yu C, Wang G, Liao H. Norcantharidin induces anoikis through Jun-N-terminal kinase activation in CT26 colorectal cancer cells. Anticancer Drugs. 2008;19:55–64. doi: 10.1097/CAD.0b013e3282f18826. [DOI] [PubMed] [Google Scholar]
- 49.Dufour G, Demers MJ, Gagne D, Dydensborg AB, Teller IC, Bouchard V, et al. Human intestinal epithelial cell survival and anoikis differentiation state-distinct regulation and roles of protein kinase B/Akt isoforms. J Biol Chem. 2004;279:44113–22. doi: 10.1074/jbc.M405323200. [DOI] [PubMed] [Google Scholar]
- 50.Collins NL, Reginato MJ, Paulus JK, Sgroi DC, Labaer J, Brugge JS. G1/S cell cycle arrest provides anoikis resistance through erk-mediated bim suppression. Mol Cell Biol. 2005;25:5282–91. doi: 10.1128/MCB.25.12.5282-5291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broker LE, Kruyt FAE, Giaccone G. Cell death independent of caspase: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 52.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–86. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–17. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo J. Glycogen synthase kinase 3B (GSK3b) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin JQ, Wen L, Wu LC, Gao ZH, Huang G, Wang J, et al. The glycogen synthase kinase-3B/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells. Toxicol Lett. 2013;218:129–36. doi: 10.1016/j.toxlet.2012.11.006. [DOI] [PubMed] [Google Scholar]