Chromosomal Instability (CIN) is a common feature of most human neoplasms and was defined, in a seminal study by Vogelstein et al., as persistently elevated rates of whole chromosome mis-segregation [1]. Since then, it was shown that certain errors in mitosis, including defects in the spindle assembly checkpoint [2], sister chromatid cohesion [3], kinetochore-microtubule (kMT) attachments [4, 5], and centrosome number [6]can cause chromosome mis-segregation in the form of merotelically attached anaphase lagging chromosomes— chromosomes that lag behind at the spindle equator while all the other chromosomes move toward the spindle poles [7] (Figure 1A). A recent study has suggested that pre-mitotic replication stress generates partially replicated chromosomes during mitosis, and that this results in both numerical and structural chromosome abnormalities through the formation of chromosome bridges and acentric chromosome fragments during anaphase [8]. To determine whether whole chromosome instability in cancer cells is caused by defects originating in mitosis (lagging chromosomes) or from ones originating pre-mitotically (chromatin bridges and acentric fragments), we compared a variety of CIN+ to CIN− cells to determine the types of segregation defects that phenotypically distinguish CIN+ from CIN− cells and whose abrogation can rescue whole chromosomal instability.
Figure 1.
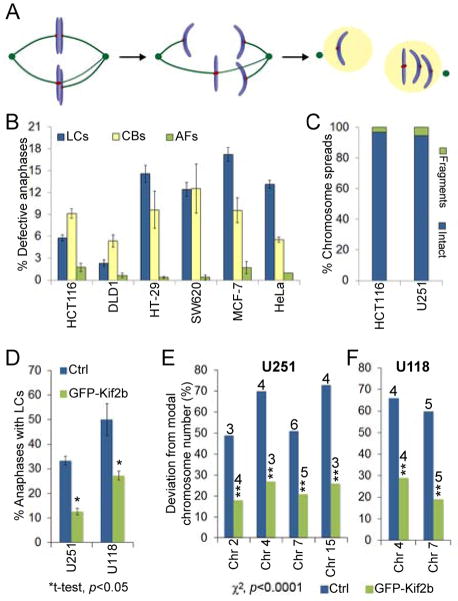
Mitotic errors cause chromosome instability(Au: OK?).
(A) Model of whole chromosome a neuploidy arising via formation of a merotelically attached (kMTs to both poles) lagging chromosome (LC) in mitosis. (B) Percent of anaphase cells with lagging chromosomes, chromatin bridges (CBs) or acentric fragments (ACs) in CIN− (HCT116 and DLD1) and CIN+ (HT-29, SW620, MCF-7, HeLa) cells. Data are represented as mean ± s.e.m., n = 448–1316 cells in 3–6 independent experiments. χ2, p<0.0001 for lagging chromosomes between CIN− and CIN+ cells.(C) Percent of chromosome spreads containing intact chromosomes or chromosome fragments in HCT116 (CIN−) and U251 (CIN+) cells, n = >300 spreads, p=0.26, χ2-test. (D) Percent of anaphase cells with lagging chromosomes in control and GFP–Kif2b over-expressing U251 and U118 cells. Data are reported as mean ± s.e.m., n = 150 cells. (E–F) Percent deviation from the modal chromosome number (noted above the bars) for the given chromosomes in a clonogenic assay (30 days) in CIN+ U251 and U118 control and GFP–Kif2b-overexpressing cells, n = 300 cells.
We used high-resolution microscopy to discriminate lagging chromosomes from acentric fragments and chromatin bridges in cells immunostained for kinetochore proteins and microtubules. We found that the only consistently significant difference between CIN− and CIN+ cells was in their rates of lagging chromosomes, with only 2–6% anaphase CIN− cells displaying lagging chromosomes compared to 12–17% anaphase CIN+ cells (Figure 1B). CIN+ cells also contained multiple lagging chromosomes per anaphase, whereas multiple lagging chromosomes were rarely observed in CIN− cells (not shown). Very few acentric fragments were observed in anaphase for both CIN− and CIN+ cells, and this result was also confirmed by analysis of chromosome spreads from a CIN− (HCT116) and a CIN+ (U251) cell line (Figure 1C). Finally, the rates of anaphase cells containing chromatin bridges, albeit higher than those with acentric fragments and in some cases similar to the rates of lagging chromosomes, were indistinguishable between CIN− and CIN+ cells (Figure 1B). These results demonstrate a paucity of acentric chromosome fragments in CIN+ cells and support the conclusion that lagging chromosomes, rather than chromosome segregation defects arising from chromosome breakage, are the primary phenotypic difference distinguishing CIN− from CIN+ cells. These results are in agreement with previous findings indicating that lagging chromosomes are the most frequent chromosome segregation error in CIN+ cancer cells [9] and represent the first report directly comparing the chromosome segregation defects, broken down by type, in CIN− vs. CIN+ cells.
It was previously shown that CIN+ cells have increased kinetochore-microtubule attachment stability relative to CIN− cells [4], which leads to the persistence of kinetochore-microtubule (kMT) attachment errors and subsequent chromosome mis-segregation [7]. Furthermore, destabilizing kMT attachments by overexpression of microtubule-depolymerizing kinesin-13 proteins reduced the numbers of lagging chromosomes and suppressed CIN in two separate CIN+ cell lines (U2OS and MCF7) [5]. Here, we overexpressed the kinesin-13 protein (GFP–Kif2b) in two additional glioblastoma cell lines that are known to exhibit high rates of chromosome segregation errors. GFP–Kif2b overexpression significantly reduced the frequency of anaphase lagging chromosomes in both U251 and U118 cells (Figure 1D).
CIN has been defined as persistently elevated rates of chromosome mis-segregation. As such, approaches that aim to suppress CIN should be evaluated in the setting of multiple generations showing suppression of karyotypic heterogeneity. We used a clonogenic assay and fluorescence in situ hybridization, as described previously [1], to test the long-lasting effect of GFP–Kif2b overexpression on the karyotype of clonal cell populations. GFP–Kif2b overexpressing clones exhibited significant reductions in the deviations from the modal chromosome number for each chromosome analyzed compared to the control clones in both U251 and U118 cells (Figure 1E–F). Thus, targeted destabilization of kMT attachments and suppression of lagging chromosomes can suppress CIN in four unrelated CIN+ cancer cell lines ([5] and Figure 1E–F).
Collectively, these data support several important conclusions: firstly, the most conspicuous difference between CIN− and CIN+ cells is the presence of lagging chromosomes during anaphase (Figure 1A). Secondly, acentric fragments are rare and the frequencies of both acentric fragments and chromatin bridges are not significantly different between CIN− and CIN+ cells. Thirdly, repression of the mitotic defect that causes lagging chromosomes leads to long-term significant suppression of CIN illustrating the causative relationship between these mitotic defects and CIN. Importantly, the latter conclusion derives from experiments using clonogenic assays performed over multiple cell generations which is the current gold standard for revealing an increased rate of chromosome segregation error that defines CIN [1].
These data along with other published work provide solid evidence supporting the conclusion that errors arising in mitosis (lagging chromosomes), rather than defects resulting from pre-mitotic errors (acentric fragments and chromatin bridges), are the root cause of numerical changes in chromosome number associated with CIN [1,5,9]. We acknowledge that incomplete DNA replication before mitosis would contribute to structural rearrangements of chromosomes, but contend that this is not a defining feature of CIN+ cells, and possibly affects CIN+ and CIN− cells to similar levels. However, with respect to the numerical instability, we strongly support the view that the primary mechanisms causing CIN arise from defects during mitosis. This is in agreement with spectral karyo typing analysis showing that numerical aberrations are ~60-fold more frequent in CIN+ compared to CIN− cells, whereas the differences in structural aberrations are only a few-fold [10].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Duane A. Compton, Email: duane.a.compton@dartmouth.edu.
Daniela Cimini, Email: cimini@vt.edu.
References
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 2.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 3.Zhang N, Ge G, Meyer R, Sethi S, Basu D, Pradhan S, Zhao Y-J, Li X-N, Cai W-W, El-Naggar AK, et al. Over expression of Separase induces aneuploidy and mammary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:13033–13038. doi: 10.1073/pnas.0801610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silkworth WT, Cimini D. Transient defects of mitotic spindle geometry and chromosome segregation errors. Cell Div. 2012;7:19. doi: 10.1186/1747-1028-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrell RA, McClelland SE, Endesfelder D, Groth P, Weller MC, Shaikh N, Domingo E, Kanu N, Dewhurst SM, Gronroos E, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghadimi BM, Sackett DL, Difilippantonio MJ, Schröck E, Neumann T, Jauho A, Auer G, Ried T. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer. 2000;27:183–190. [PMC free article] [PubMed] [Google Scholar]