Abstract
This study investigated whether women’s interest in visual sexual stimuli varied with their hormonal state. Viewing times of 30 women, 15 normal cycling (NC) and 15 oral contracepting (OC), to sexually explicit photos were measured at three different times. NC Women were tested during their menstrual, periovulatory, and luteal phases, and OC Women were tested at equivalent temporal intervals. Subjects viewed stimuli as long as desired, thus viewing time measured subject interest. Subjective ratings of stimulus sexual attractiveness were obtained on each test. There was no overall relationship between menstrual cycle phase and viewing time. However the participant’s menstrual cycle phase during first exposure to sexual stimuli predicted subsequent interest in sexual stimuli during the next two tests. NC Women who first viewed stimuli during their periovulatory phase looked longer at the sexual stimuli across all sessions than did women first tested in their luteal phase. OC Women first exposed to the sexual stimuli during menstruation looked longer at the stimuli across all sessions than did OC Women first exposed at other test phases. Neither current test phase nor initial cycle phase influenced subjective ratings. Women had increased interest in sexual stimuli across all sessions if first exposed to sexual stimuli when endogenous estrogens were most likely highest. These data suggest that women’s interest in visual sexual stimuli is modulated by hormones such that the hormonal condition at first exposure possibly determines the stimuli’s emotional valence, markedly affecting subsequent interest in sexual stimuli.
Keywords: menstrual cycle, oral contraceptives, visual sexual stimuli, sexual arousal, hormones, viewing time
Sexual desire, masturbation, and sexual initiation in women fluctuate over their menstrual cycle, with highest levels during the periovulatory period (Harvey, 1987; Tarin & Gomez-Piquer, 2002; reviewed in Wallen, 2001). Similarly, women report a greater desire to meet men when they are about to ovulate (Haselton & Gangestad, 2006) and engage in more self-grooming and ornamentation at this time (Haselton et al., 2007). During this phase, women are also more likely to engage in extra-pair copulations (Bellis & Baker, 1990) as well as have sexual fantasies about men other than their primary partners (Gangestad et al., 2002). However, sexual arousal per se, as measured either subjectively or as genital change in the laboratory, has not been found to vary reliably across the cycle and even found to be higher outside of the ovulatory period (Schreiner-Engel et al., 1981; Slob et al., 1991, 1996). The lack of a consistent overall relationship between a woman’s hormonal status and her sexual arousal to sexual stimuli may reflect that a woman’s hormonal state when first exposed to sexual stimuli influences both her initial and subsequent arousal to such stimuli (Slob et al., 1991; 1996).
Previous studies of cyclic variation in arousal to sexual stimuli have used within subjects designs in which the starting cycle phase, follicular or luteal, was randomly assigned by cycle phase and counterbalanced between subjects (Slob et al., 1991, 1996). Under these conditions, Slob and co-authors discovered that genital arousal in response to visual sexual stimuli did not depend on the woman’s hormonal state at the time of testing, but surprisingly on her hormonal state during her first laboratory exposure to sexual stimuli. Women first exposed to visual sexual stimuli during their follicular phase had higher levels of genital arousal than women first exposed to sexual stimuli in their luteal phase (Slob et al., 1991, 1996). When follicular women were subsequently tested during their luteal phase they continued to show higher arousal then did women who had initially been tested in their luteal phase, even though those women were not now in their follicular phase (Slob et al., 1991, 1996). Thus initial hormonal state had a prolonged effect on a woman’s genital response to subsequent sexual stimuli even though her hormonal state had changed. Whether this effect was specific to genital response or would be evident in explicitly cognitive processes, such as attention and interest, is unknown and was the impetus for the present study investigating hormonal influences on women’s interest in sexual stimuli.
The time that subjects choose to view sexual stimuli is a reliable measure of sexual interest (Harris et al., 1996; Israel & Strassberg, 2009; Laws & Gress, 2004; Quinsey et al., 1996; Rupp & Wallen, 2009). Subjects look longer at slides that they find more attractive, as validated by subjective reports (Rupp et al., 2009). Male and female subjects have also been shown to look longer at pornographic slides that they rate more highly arousing (Brown, 1979). Additionally, women with lower sexual arousal have shorter viewing times when evaluating sexual stimuli than women do who report higher levels of sexual arousal (Conaglen & Evans, 2006). In men, higher testosterone is correlated with longer viewing times (Rupp & Wallen, 2007). However it is not known whether hormones have similar effects on viewing times in women.
The current study investigated whether hormonal state mediates women’s interest in visual sexual stimuli using viewing time as an objective implicit measure of interest. We hypothesized that normal cycling women’s interest in visual sexual stimuli would be highest during the periovulatory phase of their menstrual cycle and lower during the luteal phase. Although women using oral contraceptives (OC) do not experience ovulation and an actual menstrual cycle, hormone levels vary such that endogenous ovarian hormones are higher during the pill-free portion of their OC regimen when the pill’s exogenous hormones no longer inhibit hormone production (van Heusden & Fauser, 1999; Wuttke, et al., 1975). Therefore, we expected to see increased interest in sexual stimuli in OC using women during their pill-free interval when their estrogen levels (van Heusden & Fauser, 1999; Wuttke, et al., 1975), and presumably sexual desire, are highest. In addition to looking for effects of the current test cycle phase on women’s interest in visual sexual stimuli, we also investigated the influence of the hormonal state in which women entered the study on her subsequent interest in sexual stimuli under a different hormonal context.
Similar to previous studies of genital arousal (Slob et al., 1991, 1996), we present evidence of a hormonal effect on women’s interest in visual sexual stimuli such that her hormonal condition at the first of three test sessions predicts her later interest across all subsequent test sessions.
METHODS
Participants
Participants were 30 heterosexual women aged 23–28. Half were normal cycling (NC) women and half were taking oral contraceptives (OC Women). Subjects were recruited from Atlanta area graduate and professional schools by email. Interested volunteers completed a packet that included a questionnaire asking about contraceptive use, sexuality, and their previous experience viewing visual sexual stimuli (0–6, none-very extensive). Responses were used for screening purposes to select appropriate subjects with a heterosexual preference and some experience with pornography.
Study Design
Participants attended three testing sessions. NC Women attended one session during the menstrual, periovulatory, and luteal phase of their menstrual cycle, determined using a counting method from the first day of menses. The actual day of menstrual onset was based on the self-report of the participant. The menstrual phase was defined as days 1–5, the periovulatory phase was days 9–13, and the luteal phase days 20–25 following menstruation. These days were chosen to approximate the menstrual phases based on a 28-day cycle. Because self-report measures of menstrual cycle can be inaccurate (Small et al., 2007), all NC Women’s periovulatory phase was confirmed by progesterone (P) blood spot assay taken before each test session. OC Women, although not actually experiencing a menstrual cycle, were scheduled to attend sessions at the same time of the month as NC Women based on the same day count from their menstruation.
The cycle phase, or the temporal equivalent in OC women, of women’s first test sessions were balanced equally across women and continued in succession. Subjects did not choose what phase they entered the study and entry was determined by continuously balancing starting times across subjects. Therefore, all test sessions included an equal number of women in each phase of their menstrual cycle and the continuous balancing of test times across all temporal phases controlled for accidental subject bias by temporal phase. For ease of terminology, the three test times will be referred to as menstrual, periovulatory, and luteal phases for both NC and OC Women, although this is simply meant to indicate timing of testing and the authors emphasize that OC Women do not have the typical hormone profiles of NC Women at these times.
Stimuli
Subjects viewed a total of 72 pictures during each of three test sessions. Stimuli were sexually explicit photos of heterosexual couples engaged in oral sex or intercourse. We presented equal numbers of photos depicting the following heterosexual activities; oral sex to male, oral sex to female, female dominant intercourse facing male partner, female dominant intercourse away from male partner, male dominant intercourse from front of female partner, and male dominant intercourse from behind female partner. We used a distribution of sexual activities to ensure that the pictures were of equal interest to all of the participants who may have preferences for different kinds of sexual stimuli (Rupp & Wallen, 2008, 2009). To obtain the test stimuli, a total of 364 pictures were downloaded from free sites on the Internet. Due to the large number of stimuli needed, and the desired sexual activity distribution, it was necessary to collect our own stimulus set rather than use the validated stimuli from the International Affective Picture System (IAPS, Lang et al., 2005). Before the sessions began, seven women not involved in the study independently rated these photos for levels of sexual attractiveness to produce a set of pictures that women would be interested in (-1-least attractive to 4 – most attractive). The final stimulus set of 216 pictures for the viewing time experiment was those stimuli rated most sexually attractive by the pilot raters within the sexual activity categories (mean ± st. dev. = 1.33 ± .78). Each test session’s stimulus set contained stimuli with statistically equal pilot ratings of sexual attractiveness (F2,21 = .32, p = .73). Subjects viewed the same stimuli as each other during each of the three sessions. Specifically, all women saw the same stimuli during their first, second, and third test sessions. However, due to the counterbalanced menstrual cycle start phases, women within each menstrual phase did not necessarily see the same stimulus set. The photo presentation was randomized uniquely for each participant within each session so that no subject saw the same order of stimulus presentation as another subject within each session.
Procedure
At the start of each session, subjects filled out a trait Anxiety questionnaire (State Trait Anxiety Inventory, Mind Garden Inc.). Next, subjects provided a small blood spot sample obtained by finger prick. The blood spot measurement technique was minimally invasive and not uncomfortable for the subjects while allowing a convenient and accurate sampling of gonadal steroid hormone levels (Worthman & Stallings, 1997). A lancet device (BD Consumer Healthcare) was used to pierce the index finger of the participant’s left hand to produce a few small drops of blood. The subject’s finger was held above a piece of filter paper (Fisher Scientific, 903 Filter paper) with two preprinted circles and a few drops of blood were placed to fill each circle without the finger touching the paper. The sample paper was dried overnight then put in a zip lock bag and stored in a sub-zero freezer (−20 C) until later hormone assay. Progesterone (P) was assayed by the Yerkes National Primate Center Endocrine Core Lab using commercially prepared kits by Diagnostic Systems Laboratories (Webster, TX; P average intra-assay coefficient of variation, 5.44%).
Following blood spot collection, participants viewed 72 pictures depicting heterosexual oral sex or intercourse presented on a laptop (Dell Inspiron with 1024 × 768 pixel screen resolution) with Gazetracker software (Eye Response Technologies, Charlottesville, VA). Eye movements of subjects were measured using an Applied Science Laboratories Model 501 eye tracker (ASL, Bedford MA) as part of a larger study and the eye tracking data are presented elsewhere (Rupp & Wallen, 2007). Participants were instructed to view photos as long as they liked and were not told that we would be measuring how long they looked at the pictures. They ended presentation of each picture by pressing the space bar on the laptop. Between each sexual stimulus a one second fixation slide was presented. The experimenter could not see the participant or what image they were looking at throughout testing. Immediately following the viewing time paradigm, participants again privately viewed all stimuli in a new randomized order and rated them on a nine-point scale of sexual attractiveness (1 = extremely sexually unattractive, 2 = highly sexually unattractive, 3 = moderately sexually unattractive, 4 = slightly sexually unattractive, 5 = neutral, neither sexually attractive or unattractive, 6 = slightly sexually attractive, 7 = moderately sexually attractive, 8 = highly sexually attractive, 9 = extremely sexually attractive). We did not have participants rate the photos during their first viewing during which we measured their undisturbed viewing time, in order to decrease cognitive demand and promote sexual interest. Following subjective ratings, participants were compensated for their time and scheduled for their next session. Subsequent test sessions were scheduled for the same time of day as the first session for each participant. For the first session subjects received $15, $20 for the second, and $25 for the third session. All procedures were conducted with approval from the Institutional Review Board and subjects gave informed consent.
Data Analysis
In order to investigate the influence of hormonal state at the time of testing, a 2 (pill use: NCW, OCW) × 3 (cycle test phase: menstrual, periovulatory, luteal) × 3 (Session: 1,2,3) multivariate ANOVA was performed with viewing time and subjective ratings as the dependent variables across all three sessions. To examine whether there was an effect of the cycle phase during which participants entered the study, a 2 (pill use: NCW, OCW) × 3 (initial phase: menstrual, periovulatory, luteal) multivariate ANOVA was also performed on the same dependent variables.
RESULTS
Participants
Of the 30 participants enrolled, 14 normal cycling (NC) women and 14 oral contraceptive using (OC) women completed all three sessions (Table 1). Of the total 30 enrolled participants, one NC Woman only completed one session and one OC Woman was unable to begin the testing at all, both due to difficulty scheduling. We used the P levels to verify that NC Women had been correctly classified in the periovulatory phase, which is characterized by lower P than luteally. Only NC Women with progesterone levels less than three ng/ml during their periovulatory session were included in the analysis (Israel et al., 1972). This led to the exclusion of one NC Woman whose P levels were approximately 10 ng/ml. Overall, NC Women’s mean progesterone (P) levels during the periovulatory and luteal phases were 1.01 ± 2.53 ng/ml and 4.20 ± 3.70 ng/ml, respectively. OC Women had mean P levels during the “periovulatory” phase of 1.34 ± 2.81 ng/ml and during the “luteal” phase .75 ± .70 ng/ml. Hormone levels are unavailable for menstrual cycle phase test sessions due to a laboratory error.
TABLE 1.
Participant number by menstrual phase for each session.
| Session 1 | Session 2 | Session 3 | |
|---|---|---|---|
| OC | |||
| Menstrual | 5 | 4 | 5 |
| Periovulatory | 5 | 5 | 4 |
| Luteal | 4 | 5 | 5 |
| Total | 14 | 14 | 14 |
| NC | |||
| Menstrual | 5 | 4 | 5 |
| Periovulatory | 5 | 5 | 4 |
| Luteal | 5 | 5 | 5 |
| Total | 15 | 14 | 14 |
| TOTAL | 29 | 28 | 28 |
Women’s self-reported measures of Anxiety were low overall (Mean ± st. dev., 29.62 ± 7.26). Differences in reported Anxiety were assessed using a 2 (pill use: NCW, OCW) × 3 (initial phase: menstrual, periovulatory, luteal) × 3 (Session: 1,2,3) univariate ANOVA. Self reports of anxiety at did not differ by test session number, but did differ by start phase for NC and OC Women (NC Women, Mean ± st. dev., 31.28 ± 7.36; F2,42 = 4.94, p = .01); OC Women, Mean ± st. dev., 27.91 ± 6.81; F2,41 = 3.94, p = .03). Specifically, NC Women who started in their menstrual phase (Mean ± st. dev = 35.73 ± 4.77) reported significantly more anxiety than those women who started during their periovulatory (p = .006; Mean ± st. dev = 28.33 ± 6.85) or luteal phases (p = .02; Mean ± st. dev = 29.54 ± 8.34). OC Women who started in their periovulatory phase (Mean ± st. dev = 31.33 ± 9.72) reported significantly more anxiety than those women who started during their menstrual phase (p = .009; Mean ± st. dev = 24.93 ± 2.96), but no difference compared to OC Women who started in their luteal phase (Mean ± st. dev = 27.33 ± 3.60). There was no relationship between anxiety and women’s viewing times or subjective ratings of stimuli (Pearson two-tailed correlations).
Test Cycle Phase
Across all sessions combined, we did not see an effect of contraceptive use (viewing time, partial η2 = .02; subjective ratings, partial η2 = .04), session number (viewing time, partial η2 = .003; subjective ratings, partial η2 = .003), or test session cycle phase (viewing time, partial η2 = .001; subjective ratings, partial η2 = .02), nor was there an interaction between the factors on viewing time (NC Women, Mean ± st. dev., 5.25 ± 4.58 secs.; OC Women, 6.23 ± 3.88 secs.; F2,42 = 2.90, p = .06, Figure 1) or subjective ratings (NC Women, Mean ± st. dev., 6.05 ± 0.80 secs.; OC Women, 5.75 ± 0.88; viewing time, contraceptive use X session, partial η2 = .002; contraceptive use X test cycle phase, partial η2 = .000; session X test cycle phase, partial η2 = . 12; contraceptive use X session X test cycle phase, partial η2 = .09; subjective ratings, contraceptive use X session, partial η2 = .01; contraceptive use X test cycle phase, partial η2 = . 000; session X test cycle phase, partial η2 = .10; contraceptive use X session X test cycle phase, partial η2 = .07),).
Figure 1.
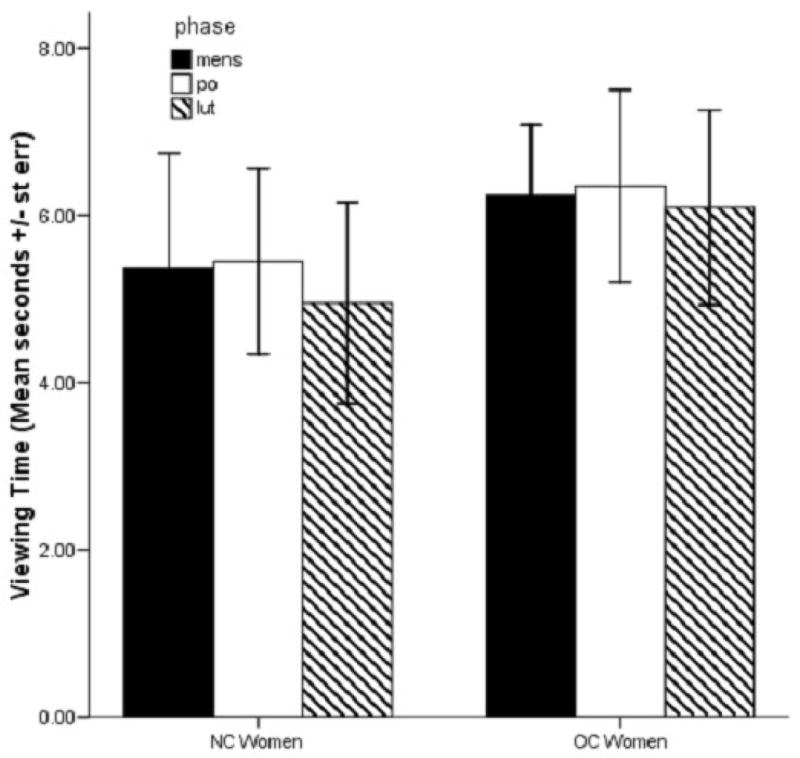
Average Viewing Time across all test sessions by menstrual test phase depending on contraceptive use. Viewing time did not differ overall by menstrual test cycle phase.
Viewing time was positively correlated with participants’ subjective ratings (Pearson two-tailed bivariate correlation, r(84) = .30, p = .006), supporting viewing time as an indicator of increased interest and/or preference.
Initial Cycle Phase
Table two presents the average viewing times (mean ± st. dev.) for each group of women at all starting menstrual cycle phases, illustrating a similar range of viewing times within each test session. Tracking subsequent viewing times by the women’s initial menstrual cycle phase (indicated by the same superscript) across test sessions shows that subsequent viewing times were very similar to the initial viewing time for that group of women.
There was an interaction between initial cycle phase and contraceptive use (NC vs OC) (F2,78 = 3.74, p = .028, partial η2 = .09; Figure 2). In NC Women, viewing time (F2,42 = 2.90, p = .06, partial η2 = .13) for participants who began testing in their periovulatory phase was greater overall than was overall viewing time for women who started the study in their luteal phase (p = .02). OC Women who started testing during their “menstrual” phase looked longer across test sessions than did women who started at any other phase (F2,40 = 6.73, p = .003, partial η2 = .26; vs. “periovulatory”, p = .002; vs.” luteal” p = .004). There was not an overall interaction effect of initial phase and contraceptive use on subjective ratings (partial η2 = .06, data not shown).
Figure 2.
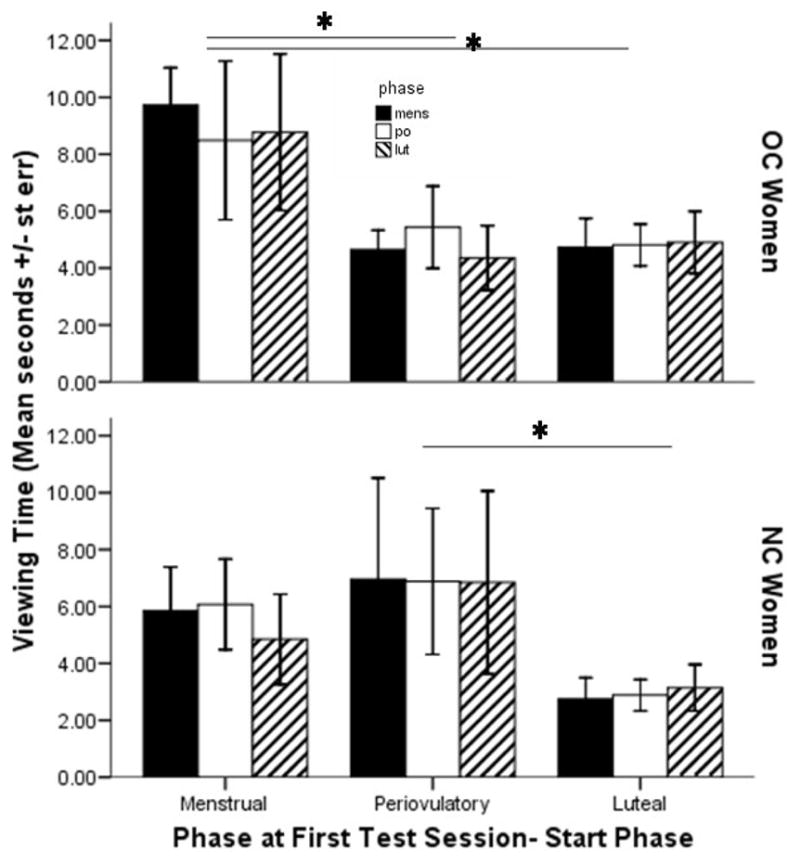
Average Viewing Time across at each test session by menstrual start phase depending on contraceptive use. Viewing time was longest for NC Women tested initially during their periovulatory versus luteal phase (p = .03), and longest for OC Women tested first during their menstrual phase (F2,40 = 6.73, p = .003) compared to both other cycle phases (vs. periovulatory, p =.002; vs. luteal p = .004).
Previous Viewing Experience
It was possible that there were cohort effects unrelated to cycle phase in that the women who began testing during different phases of their cycle differed on some measure other than their start phase. An important possible variable that was addressed in their original screening questionnaire included their previous viewing experience with visual sexual stimuli. Women’s viewing times and subjective ratings were significantly correlated with their previous viewing experience (Pearson two-tailed bivariate correlation, viewing time, r(84) = .27, p = .01; subjective rating, r(84) = .51, p < .001). A One-Way ANOVA within each group of women (OC and NC) with initial cycle phase as the independent measure was therefore conducted to ensure that previous viewing experience did not differ by initial cycle phase cohort. OC and NC women did not differ in their previous experience with visual sexual stimuli overall (NC Women, Mean ± st. dev., 1.83 ± 0.86; OC Women Mean ± st. dev., 1.93±0.87) or by initial cycle phase.
DISCUSSION
The goal of this study was to investigate the role of menstrual cycle phase on women’s interest in visual sexual stimuli. Because hormones are known to have a significant effect on sexual motivation (Wallen, 2001), we thought that we would see changes in interest in the stimuli consistent with the different hormonal states that occur during the menstrual cycle. However, we did not find a relationship predicted by the women’s hormonal condition at a specific menstrual cycle phase, but instead found that her hormonal state when first exposed to sexual stimuli affected her subsequent interest and attention to visual sexual stimuli. NC women’s estradiol is highest during the midfollicular to periovulatory portion of their cycle (Sherman & Korenman, 1975). In OC women estradiol is highest during the pill free portion of their cycle (van Heusden & Fauser, 1999; Wuttke, et al., 1975). Thus, the effect of repeated testing was the same in both NC and OC Women such that they were more interested in pictures if they were first exposed to the sexual stimuli when endogenous estrogen, and sexual motivation are highest. These data suggest that exposure to visual sexual stimuli when endogenous estrogen, but not progesterone, is elevated predisposed women to find sexual stimuli more interesting and this interest continued when later exposed to sexual stimuli under markedly different hormonal conditions. This finding suggests a role for women’s hormonal state in setting the initial valence of sexual stimuli which affects later response to stimuli under different conditions.
Our results on interest in sexual stimuli parallel previous findings on menstrual cycle influences on genital arousal (Slob et al., 1991, 1996), suggesting that genital responses reflect underlying psychological processes that are influenced by hormone status when first exposed to sexually arousing stimuli. The continuing effect of the woman’s hormonal state from her first exposure to sexual stimuli to her subsequent response under different hormonal conditions was sufficiently robust that it eliminated any overall relationship between interest and menstrual cycle phase when subjects starting the study at different cycle phases were combined by cycle phase. The mechanisms producing this effect remain obscure. If a woman’s first session is one in which they experience higher interest in the pictures, then one might expect them to respond more positively to the pictures at future test sessions than women whose initial experience was not as positive. Possibly our results reflect a hormonally modulated conditioning process analogous to a conditioned place preference in animals, where the location in which a subject engaged in sexual intercourse becomes rewarding in its own right (Pfaus et al., 2001). In this view a woman’s hormonal state affects the psychological valence of sexual stimuli, presumably making them more rewarding when estrogen (or androgen) levels and sexual desire are high (Wallen, 2001).
An alternate, and not mutually exclusive, view is that hormonal state alters the emotional state of the woman to produce a different emotional reaction to the sexual stimuli on the first and subsequent sessions. In this view, an emotional response, such as anxiety, is heightened by elevated progesterone and reduced by elevated estrogens (Toufexis et al., 2006) resulting in the formation of a negative or positive association with the stimuli which then endures across later test sessions. Our anxiety data do not support this view. Although anxiety levels differed by start phase, they had no significant relationship to women’s viewing times. In addition, in NC Women, measured anxiety levels were the same for women starting in their periovulatory or luteal phases, yet interest in sexual stimuli was significantly higher for women starting in the periovulatory phase than it was for women starting in the luteal. Thus it seems unlikely that our results reflect systematic differences in anxiety. Thus changes in positive association or reward value across the cycle (Pfaus, 2009; Rupp et al., 2009) seem more likely the domain in which this order effect operates. We do not think this hormonal effect is likely truly enduring, in that it permanently changes a woman’s response to sexual stimuli. It is more likely that it is context specific and of relatively short duration, owing its evident power in our testing conditions to the artificial laboratory setting which would be of particular salience to the subjects. Whether there are longer-term effects of hormones on the valence of sexual stimuli is an issue that deserves further investigation.
As is the case with most of the studies looking at menstrual cycle variation, there were limitations to our determination of each menstrual cycle. We set wide ranges of 4–5 days for each cycle phase which may have led to misclassification between NC Women’s menstrual and periovulatory phases. Additionally, we cannot confirm that estrogen levels peaked at this time in OC Women due to a laboratory assay error. Although we found significant differences using multivariate techniques, our sample was too small to ideally test the effect we report here. Because our results corroborate those of Slob et al. (1991, 1996) using completely different methodology and dependent measures, we have greater confidence in these results than we might otherwise. Still, our sample size is a concern even though the study had adequate power to test the effect of the current menstrual phase on attention, with an N of 15 women in each group using multivariate analysis techniques. Additionally, the current study would have benefited from measurements of estradiol and testosterone levels at each session. While we attributed the observed hormonal influences on interest and attention to changes in estrogen across the cycle, it is possible also that testosterone or the estrogen to progesterone ratio could be mediating interest in to visual sexual stimuli. Future investigations should look for specific influences of testosterone, estrogen and progesterone on the viewing patterns of sexually explicit stimuli in order to enhance knowledge about the role of these hormones in sexual motivation and desire.
To summarize, we found that the hormonal state of women at their first exposure to visual sexual stimuli predicted the degree of later interest in similar stimuli. That hormonal state of first exposure predicted later interest in visual sexual stimuli suggests that women form viewing patterns reflecting their initial exposure. These consistent viewing patterns appear to override possible hormonal influences on sexual desire with repeated exposure to sexual stimuli, highlighting the importance of experience affecting expectation, memory, and possibly conditioning in women’s sexual interest. Understanding the influence of hormones in the priming and shaping of sexual desire and expectation could have ramifications for the treatment of women’s sexual dysfunction and study of female sexual arousal.
TABLE 2.
Viewing time (Mean±st. dev. seconds) by menstrual cycle phase and test session for each session. There were no overall differences between cycle phases for either OC or NC subjects. (Note: the sample for a given cycle phase on each test session is a different group of women since women were tested at all three different cycle phases.
| Session 1 | Session 2 | Session 3 | |
|---|---|---|---|
| OC | |||
| Menstrual | 9.73±2.63 a | 4.73±2.04 c | 4.65±1.53 b |
| Periovulatory | 5.43±3.23 b | 8.49±6.24 a | 4.81±1.47 c |
| Luteal | 4.90±2.18 c | 4.36±2.53 b | 8.77±6.15 a |
| NC | |||
| Menstrual | 5.85±3.46a | 2.75±1.49 c | 6.96±7.95 b |
| Periovulatory | 6.88±5.74 b | 6.07±3.56 a | 2.88±1.10 c |
| Luteal | 3.14±1.82 c | 6.85±7.19 b | 4.85±3.54 a |
The same superscripts and types face (e.g. bold, italic, and plain) indicate the response of women starting in a specific menstrual cycle phase at subsequent cycle phases. Thus a indicates the responses of women starting in the menstrual cycle phase at their periovulatory and luteal phases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellis MA, Baker RR. Do female faces promote sperm competition? Data for humans Anim Behav. 1990;40:997–999. [Google Scholar]
- Brown M. Viewing time of pornography. J Psychol. 1979;102:83–95. [Google Scholar]
- Conaglen HM, Evans IM. Pictorial cues and sexual desire: an experimental approach. Arch Sex Behav. 2006;35:197–212. doi: 10.1007/s10508-005-9000-8. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R, Garver CE. Changes in women’s sexual interests and their partners’ mate-retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc R Soc Lond. 2002;269:975–982. doi: 10.1098/rspb.2001.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GT, Rice ME, Quinsey VL, Chaplin TC. Viewing time as a measure of sexual interest among child molesters and normal heterosexual men. Behav Res Ther. 1996;34:389–394. doi: 10.1016/0005-7967(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Harvey SM. Female sexual behavior: fluctuations during the menstrual cycle. J Psychosomatic Res. 1987;31:101–110. doi: 10.1016/0022-3999(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Gangestad SW. Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Horm Behav. 2006;49:509–518. doi: 10.1016/j.yhbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Rechek A, Frederick DA. Ovulatory shifts in human female ornamentation: near ovulation, women dress to impress. Horm Behav. 2007;51:40–45. doi: 10.1016/j.yhbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Israel R, Mishell DR, Stone SC, Thorneycroft JH, Moyer DL. Single luteal phase serum progesterone assay as indicator of ovulation. Am J Obstet Gynecol. 1972;112:1053–6. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- Israel E, Strassberg DS. Viewing time as an objective measure of sexual interest in heterosexual men and women. Arch Sex Behav. 2009 doi: 10.1007/s10508-007-9246-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Laws DR, Gress CLZ. Seeing things differently: the viewing time alternative to penile plethysmography. Leg Crim Psych. 2004;9:183–196. [Google Scholar]
- Pfaus JG. What’s behind her smile? Horm Behav. 2008;55:267–271. doi: 10.1016/j.yhbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: A review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Quinsey VL, Ketsetzis M, Earls C, Karamanoukian A. Viewing time as a measure of sexual interest. Ethol Sociobiol. 1996;17:341–354. [Google Scholar]
- Rupp HA, Wallen K. Relationship Between Testosterone and Interest in Sexual Stimuli: The Effect of Experience. Horm Behav. 2007;52:581–589. doi: 10.1016/j.yhbeh.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex Differences in Response to Visual Sexual Stimuli: A Review. Arch Sex Behav. 2008;37:206–218. doi: 10.1007/s10508-007-9217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex-Specific Content Preferences for Visual Sexual Stimuli. Arch Sex Behav. 2009;38:417–426. doi: 10.1007/s10508-008-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, Librach G, Feipel N, Ketterson E, Sengelaub D, Heiman J. Partner Status Influences Women’s Interest in the Opposite-Sex. Hum Nat. 2009;20:93–104. doi: 10.1007/s12110-009-9056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural Activation in the Orbitofrontal Cortex in Response to Male Faces Increases During the Follicular Phase. Horm Behav. 2009;56:66–72. doi: 10.1016/j.yhbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner-Engel P, Schiavi RC, Smith H, White D. Sexual arousability and the menstrual cycle. Psychosomatic Med. 1981;43:199–214. doi: 10.1097/00006842-198106000-00002. [DOI] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55:699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, Ernste M, Van der Werff ten Bosch JJ. Menstrual cycle phase and sexual arousability in women. Arch Sex Behav. 1991;20:567–577. doi: 10.1007/BF01550955. [DOI] [PubMed] [Google Scholar]
- Slob AK, Bax CM, Hop WCJ, Rowland DL, van der Werff ten Bosch JJ. Sexual arousability and the menstrual cycle. Psychoneuroendocrinology. 1996;21:545–558. doi: 10.1016/0306-4530(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Small CN, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007;3:163–170. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V. Do women have a hidden heat period? Hum Reprod. 2002;17:2243–2248. doi: 10.1093/humrep/17.9.2243. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- van Heusden AM, Fauser BCJM. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception. 1999;59:237–243. doi: 10.1016/s0010-7824(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav. 2001;40:339–57. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104:1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wuttke P, Arnold P, Becker D, Creutzfeldt O, Langenstein S, Tirsch W. Circulating hormones, eeg, and performance in psychological tests of women with and without oral contraceptives. Psychoneuroendocrinology. 1975;1:141–151. doi: 10.1016/0306-4530(75)90006-2. [DOI] [PubMed] [Google Scholar]


