Abstract
FancD2 plays a central role in the human Fanconi anemia DNA damage response (DDR) pathway. Fancd2−/− mice exhibit many features of human Fanconi anemia including cellular DNA repair defects. Whether the DNA repair defect in Fancd2−/− mice results in radiologic changes in all cell lineages is unknown. We measured stress of hematopoiesis in long-term marrow cultures and radiosensitivity in clonogenic survival curves, as well as comet tail intensity, total antioxidant stores and radiation-induced gene expression in hematopoietic progenitor compared to bone marrow stromal cell lines. We further evaluated radioprotection by a mitochondrial-targeted antioxidant GS-nitroxide, JP4-039. Hematopoiesis longevity in Fancd2−/− mouse long-term marrow cultures was diminished and bone marrow stromal cell lines were radiosensitive compared to Fancd2+/+ stromal cells (Fancd2−/− D0 = 1.4 ± 0.1 Gy, ñ = 5.0 ± 0.6 vs. Fancd2+/+ D0 = 1.6 ± 0.1 Gy, ñ = 6.7 ± 1.6), P = 0.0124 for D0 and P = 0.0023 for ñ, respectively). In contrast, Fancd2−/− IL-3-dependent hematopoietic progenitor cells were radioresistant (D0 = 1.71 ± 0.04 Gy and ñ = 5.07 ± 0.52) compared to Fancd2+/+ (D0 = 1.39 ± 0.09 Gy and ñ = 2.31 ± 0.85, P = 0.001 for D0). CFU-GM from freshly explanted Fancd2−/− marrow was also radioresistant. Consistent with radiosensitivity, irradiated Fancd2−/− stromal cells had higher DNA damage by comet tail intensity assay compared to Fancd2+/+ cells (P < 0.0001), slower DNA damage recovery, lower baseline total antioxidant capacity, enhanced radiation-induced depletion of antioxidants, and increased CDKN1A-p21 gene transcripts and protein. Consistent with radioresistance, Fancd2−/− IL-3-dependent hematopoietic cells had higher baseline and post irradiation total antioxidant capacity. While, there was no detectable alteration of radiation-induced cell cycle arrest with Fancd2−/− stromal cells, hematopoietic progenitor cells showed reduced G2/M cell cycle arrest. The absence of the mouse Fancd2 gene product confers radiosensitivity to bone marrow stromal but not hematopoietic progenitor cells.
INTRODUCTION
Fanconi anemia (FA) is an autosomal recessive syndrome associated with a biallelic mutation in one or more of the 15 FA pathway gene products leading to bone marrow failure, defective DNA damage response and predisposition to cancer (1). Fanconi anemia consists of defects in one or more of 15 complementation groups (A, B, C, D1, D2, E, F and G). FancA, FancC, FancF and FancG, proteins interact to form a nuclear complex, which is required for the downstream activation of the human FancD1 (BRCA2) protein. Activation of human FancD2 results in the assembly of FancD2/BRCA1 foci, which participate in DNA double-strand break repair (1). A review of the FA pathway and interaction of the multiple gene products with other elements of the DNA repair process has recently been published (2). It is unknown whether inactivation or absence of any one FA pathway gene alters ionizing radiation sensitivity uniformly in all cell lineages.
The defect in DNA damage response in some FA patients makes them sensitive to ionizing radiation, limiting effective radiotherapy for solid tumors as well as total-body irradiation for marrow transplantation (3). The radiosensitivity of human FancD2 cell lines has been shown to be greater than that of cell lines from patients with the FancG−/−, FancA−/− or the FancB−/− genotype (4). The radiosensitivity of Fancd2−/− mice is consistent with the radiosensitivity of FancD2−/− patient cell lines (5, 6). Radiosensitivity is not a universal feature of FA patient-derived cells (7, 8).
In the studies presented here, we evaluated the longevity of hematopoiesis in Fancd2−/− mouse long-term marrow cultures. We also compared the radiosensitivity of hematopoietic progenitor cell lines to stromal cells (mesenchymal stem cells) and analyzed stromal cells and hematopoietic progenitor cell lines for radiation induced alteration in cell cycle distribution. Furthermore, we investigated DNA damage response by comet tail intensity, induction of pro-inflammatory, oxidative stress and cell cycle regulating gene products, irradiation effects on total antioxidant stores and the effect on radiosensitivity of the mitochondrial-targeted reactive oxygen species (ROS) scavenger JP4-039 (9).
METHODS
Mice
Fancd2−/−, Fancd2+/− and Fancd2+/+ (C57BL/6J background) (10) were generously provided by the Dana Farber Cancer Institute. Mice were housed 4/cage according to Institutional IACUC regulations and fed standard Purina laboratory chow.
Long-Term Bone Marrow Cultures
Long-term bone marrow cultures (LTBMC) were established from the femur and tibia marrow of Fancd2+/+, Fancd2+/− and Fancd2−/− mice as described previously (11–13). The contents of a femur and tibia (N = 6/genotype) were flushed into McCoy’s 5A medium (Gibco, Gaithersburg, MD) supplemented with 25% horse serum (Cambrex, Rockland, ME) and 10−5 M hydrocortisone sodium hemisuccinate. Cultures were incubated at 33°C in 7% CO2. After 4 weeks, the horse serum was replaced with 25% FBS (Gibco, Gaithersburg, MD) (14). The cultures were observed weekly for hematopoietic cell production and cobblestone island formation. Cobblestone islands of greater than or equal to 50 cells were scored weekly in each flask 12–14). A two-sided two-sample t test was used to compare the number of cobblestone islands between Fancd2+/+, Fancd2+/− and Fancd2−/− cultures each week. P values less than 0.05 were regarded as significant.
Establishment of Interleukin-3-Dependent Hematopoietic Progenitor Cell Lines and Clonal Cell Sublines
Non-adherent cells were harvested from Fancd2+/+, Fancd2+/− and Fancd2−/− mouse LTBMC at week 4 and cultured in six-well tissue culture plates in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 20% fetal calf serum (FCS) and 1.0 ng/mL Interleukin-3 (IL-3) (Peprotech, Rocky Hill, NJ). The Fancd2+/+, Fancd2+/− and Fancd2−/− cell lines were passaged weekly for 10 weeks to establish primary IL-3-dependent cell lines using published methods (14, 15).
Clonal cell sublines were established from each of the Fancd2+/+, Fancd2+/− and Fancd2−/− parent lines by expansion of single colonies. Cells from primary IL-3-dependent cell lines were plated in 0.8% methylcellulose supplemented with 10% IMDM, 30% fetal bovine serum (FBS), 1% bovine serum albumin, 2 ng/mL IL-3 (Stemcell Technologies, Vancouver, Canada) at variable cell densities. At day 14, individual colonies were harvested and each cultured in a well of a 96-well plate in 0.2 mL of IMDM supplemented with 30% FBS and 1 ng/mL of IL-3. Cells were then replated in methylcellulose, colonies selected at 14 days and cultured as above, to establish subcloned lines. Confirmation of genotype after repeated subcloning was carried out for each cell line.
Hematopoietic Cell Colony-Forming Assays
A total of 5 × 104 non-adherent cells from the Fancd2+/+, Fancd2+/− and Fancd2−/− long-term bone marrow cultures were removed from the LTBMC flasks and 1.67 × 103 cells/dish were plated in triplicate in semi-solid medium consisting of methylcellulose in IMDM, FBS, 10% bovine serum albumin (BSA), WEHI-3 cell line conditioned medium (15) (as a source of IL-3), L-glutamine, 3 U/mL erythropoietin and 2-mercaptoethanol. Colony forming unit granulocyte-macrophage (CFU-GM) of 50 cells or greater were counted on days 7 and 14 after plating. A two-sided two-sample t test was used as described above. Fresh marrow colonies were subdivided for GFU-GM, burst forming unit erythroid (BFU-E) and granulocyte-erythroid-megakaryocyte-macrophage. (CFU-GEMM)
Bone Marrow Stromal Cell Lines and Clonal Cell Sublines
Adherent cell layers from one 4-week-old LTBMC from Fancd2+/+, Fancd2+/− and Fancd2−/− mice were trypsinized and expanded by passage into DMEM + 10% FBS to establish bone marrow stromal cell lines according to published methods (12). Cells were passaged for 10 weeks to establish cell lines. Cultures were incubated at 37°C in 5% CO2.
Clonogenic Irradiation Survival Curves for Fancd2−/− Mouse Bone Marrow Stromal Cell Lines
Fancd2+/+, Fancd2+/− and Fancd2−/− bone marrow stromal cells were irradiated in suspension with doses between 0–8 Gy at 70 cGy/min using a Shepherd Mark 1 137Cs γ-ray source (J. L. Shepherd, San Fernando, CA). Cells were plated in quadruplicate in Linbro plates (Fisher Scientific, Pittsburgh, PA) and incubated at 37°C and 5% CO2 for 9–11 days, stained with crystal violet and colonies of ≥50 cells were counted using a GelCount colony counter (Oxford Optronix, Oxford, UK). Data were analyzed with single-hit multitarget models according to published methods (16, 17).
Irradiation Survival Curves for Fancd2−/− Mouse IL-3-Dependent Cell Lines and Fancd2−/− Mouse Whole Bone Marrow
Fancd2 IL-3-dependent non-adherent cells or whole bone marrow from Fancd2+/+, Fancd2+/− and Fancd2−/− mice were irradiated in suspension with doses between 0–8 Gy as described above. Cells were plated in triplicate in methylcellulose medium containing recombinant mouse stem cell factor (SCF), IL-3, IL-6 and recombinant human erythropoietin (EPO) (Stem Cell Technologies, Vancouver, BC). CFU-GM were scored on day 7–9 for the Fancd2 IL-3-dependent cell lines and CFU-GM, BFU-E and CFU-GEMM were scored between days 10–13 for the Fancd2+/+, Fancd2+/− or Fancd2−/− whole bone marrow. In some experiments, colonies growing in plates containing cells that were irradiated with 6 or 8 Gy were removed and expanded. Data were analyzed with single-hit multitarget models according to published methods (16, 17).
Reverse Transcription Polymerase Chain Reaction (RT–PCR) Analysis
Unirradiated cells from each stromal and hematopoietic progenitor cell lines, Fancd2+/+, Fancd2+/− and Fancd2−/− as well as cells exposed to 5 Gy of irradiation, were harvested at 24 h after exposure. Real time polymerase chain reaction (RT-PCR) was used to analyze radiation inducible transcripts for transcription factors Nfkb and Nrf2, and cytokines including TGFB1 for the oxidative stress response enzyme MnSOD (Sod2), and for irradiation response related transcripts p21 and p53, as well as for cell cycle checkpoint kinase Chk1 using published methods (11). The results were presented as fold increase in gene expression above baseline level, which was adjusted to that for nonirradiated C57BL/6NHsd wild-type mouse bone marrow stromal or hematopoietic progenitor cells. The magnitude of elevation in RNA attributable to irradiation was quantitated for each cell line.
RT-PCR Reaction Conditions
Total RNA was extracted from cell pellets using Trizol reagent (Invitrogen). A total of 2 μg of RNA was used to synthesize cDNA in a 20 μL reaction system, according to the instructions of High Capacity cDNA Reverse Transcription Kit (A&B Applied Systems, no. 4368814) with the reaction conditions of 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf RealPlex2 Mastercycler®. For RT-PCR, reaction conditions were as follows: 96-well plates were prepared with 10 μl of Taqman Gene Expression Master mix; 5 μl of RNase-free water; 1 μL of the corresponding Taqman Gene Expression probe; and 4 μL of cDNA (totaling 2 μg of cDNA) using the Eppendorf epMotion® 5070 automated pipetting system (Eppendorf, Westbury, NY). PCR amplification of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as the housekeeping gene (Gen-Bank: NM_008084). Expression of our RNAs of interest included: Nrf2 (Gen-bank: NM_010902.3), Sod2 (Gen-Bank: NM_013671.3), Nfkb (Gen-Bank: NM_008689.2), TGFB1 (Gen-Bank: NM_011577.1), Chk1(Gen-Bank: NM_007691), p21(Gen-Bank:NM_001111099) and p53(Gen-Bank:NM_001168250).
Flow Cytometric Apoptosis Analysis
Fancd2+/+, Fancd2+/− and Fancd2−/− mouse stromal cell lines and IL-3-dependent hematopoietic progenitor cell lines were harvested at baseline (unirradiated, control) and at 4 or 24 h after irradiation with 3 or 5 Gy. Cells were collected, washed with ice-cold phosphate buffered saline (PBS) and re-suspended in binding buffer at a cell density of 1 × 106/mL. Apoptosis analysis was performed using an Annexin V-FITC apoptosis detection kit (Abcam, Cambridge, MA) according to manufacturer’s instructions. Cells were stained with 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI) (20 μg/mL), then incubated in the dark at 25°C for 15 min. Samples were acquired on an Accuri™ C6 flow cytometer (BD sciences, Franklin Lakes, NJ) and 30,000 cells/experimental group were analyzed using Cellquest software. Details of methods for cell cycle distributions are as follows: Cells from Fancd2+/+, Fancd2+/− and Fancd2−/− mouse stromal and IL-3-dependent cell lines were exposure to 0, 3 or 5 Gy of irradiation. At 4 and 24 h after irradiation, the cells were washed three times in PBS, fixed in 70% ethanol and stored for 24 h at −20°C. The cells were then stained with 0.1 μg/ml of propidium iodide and cell cycle analysis was performed by flow cytometry on 1 × 107 cells from each cell line. Experiments were carried out in triplicate.
Measurement of Irradiation Induced DNA Damage by Comet Tail Intensity Assay
Measurement of DNA damage after irradiation was performed as described previously (18). Cells from each mouse Fancd2+/+, Fancd2+/− and Fancd2−/− cell line were irradiated with either 0 or 5 Gy and incubated at 37°C for 10 min, 1, 6 and 24 h, at which time the cells were rapidly chilled to 4°C to stop DNA repair. The zero time point samples used as reference are taken from unirradiated cells. The cells were mixed in low melt agarose, and 500 cells were placed on the sample area of a CometSlide (CometAssay® 4250-050-K, Trevigen, Inc., Gaithersburg, MD). The slides were rapidly chilled to 4°C and kept in the dark to prevent DNA repair. The slides were then placed in prechilled lysis solution and kept at 4°C for 60 min followed by washing in neutral electrophoresis buffer for 30 min at 4°C and electrophoresis at 21 V for 1 h at 4°C, then immersed in DNA precipitation solution for 30 min at room temperature followed by 70% ethanol for 30 min, and dried at 45°C for 15 min. The cells were then stained with SYBR Green 1 and examined with a fluorescence microscope. The comet tails for each of 150 cells were quantified using the Comet Assay IV software (Trevigen, Inc.).
Assay for Intracellular Antioxidant Levels
Fancd2+/+, Fancd2+/− and Fancd2−/− mouse stromal and IL-3-dependent hematopoietic progenitor cell lines were irradiated with 0, 5 or 10 Gy. Cells were harvested at 24 h after irradiation and snap-frozen in liquid nitrogen. Cell pellets were then thawed and mechanically homogenized in cold phosphate buffer solution. Protein concentrations were standardized by Bradford assay to 1 mg/mL of protein sample and antioxidant reductive capacity (antioxidant status) was measured using a commercial kit (Northwest Life Science Specialties, Vancouver, WA). This assay measures the antioxidant capacity of cells based on the ability of cellular antioxidants to reduce Cu++ to Cu+, which reacts with bathocuproine to form a color complex absorbing at 480–490 nm. The antioxidant activity was compared to a standard curve generated using trolox units and all data was, therefore, expressed as millimolar equivalents of trolox units.
Western Analysis for p21, p53 and MnSOD Protein Expression
Cells from each Fancd2+/+, Fancd2+/− and Fancd2−/− mouse stromal and IL-3-dependent hematopoietic progenitor cell line were pelleted and lysed in NP-400 buffer [50 mM Tris, pH 7.8, 10 mM ethylenediaminetetaacetic acid (EDTA), 150 mM NaC1, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% NP-40 and a protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN)]. Protein samples were separated in 15% polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes. Primary anti-MnSOD antibody (1:2000 dilution, no. ab13533,) and anti p53 antibody (1:10 dilution, mAB PAB421, no. OP03L) were obtained from Abcam. Primary antibody against p21 was obtained from Santa Cruz Chemical Laboratories, Santa Cruz, CA (1:200 dilution, no. SC-397). As a control gene product, GAPDH (Sigma Aldrich, St. Louis, MO) antibody was used. Subsequently, horseradish peroxidase anti-rabbit or anti-mouse secondary antibody (Promega, Pittsburgh, PA) was applied and membranes developed with SuperSignal West Dura ECL (Thermo Scientific, Rockford, IL). Western blots were carried out in triplicate and total protein density tabulated using LabWorks Image Acquisition and Analysis Software (UVP Imaging System, Upland, CA).
Statistical Methods
The in vitro radiation survival curves were analyzed with the single-hit multi-target model and were compared using D0 (final slope representing multiple-event killing) and ñ (extrapolation number measuring width of the shoulder on the radiation survival curve) (16,17). Results for D0 and ñ were presented as the mean ± standard error (SD) from multiple measurements and compared with the two-sided two-sample t test. For the other continuous endpoints, comparisons were also performed using a t test if they were normally distributed or with Wilcoxon rank sum test otherwise. For the comet assay, data were log transformed so that their distribution was close to normal, and a linear regression model was built on the log transformed data in all 5 Gy irradiated groups, using cell line and time of measurement and their interaction term as explanatory variables. The F test in this model was used to compare slopes between any two of the 3 cell lines and a significant P value indicated a significant difference in the change of tail length with time after irradiation. In all these tests, a P value of less than 0.05 was regarded as significant. As an exploratory analysis, we did not adjust P values for multiple comparisons.
For LTBMC data, weekly cobblestone island numbers; non-adherent cell numbers (×105); percentage confluence of adherent cells; day 7 colony forming cells at each week 14 harvest; and day 14 colony forming cell count at each weekly harvest were counted as described (11–13). Data are summarized as mean ± standard deviation, n is the number of mice used and P values were calculated with the two-sided two-sample t test. Significant P values are shown in red. A two-sided two-sample t test was used to analyze gene expression.
For Western blots data were analyzed by densitometry and summarized with mean ± standard deviation in each group. P values for the comparison between any two groups were calculated with the two-sided two-sample t test. n is the sample size. P1 is for the comparison with the corresponding 0 Gy group in the same column; P2 is for the comparison with the corresponding Fancd2+/+ mouse group in the same row.
RESULTS
Continuous Bone Marrow Cultures from Fancd2−/− Mice have Reduced Longevity of Hematopoiesis
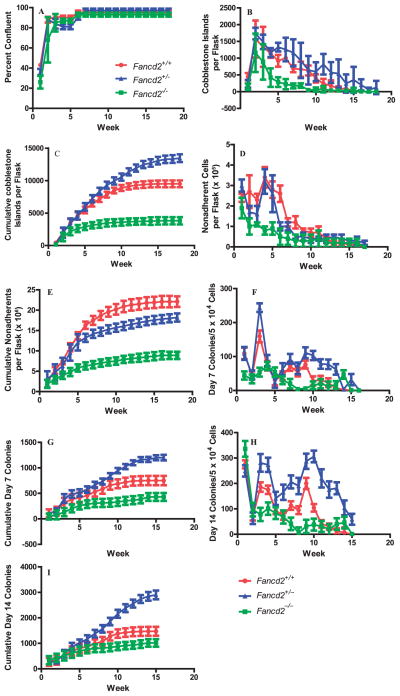
To determine if the longevity of hematopoiesis in long term culture was diminished in the bone marrow of Fancd2−/− mice, several parameters of long-term hematopoiesis in continuous bone marrow cultures were quantitated comparing Fancd2−/− with Fancd2+/+ and Fancd2+/− explanted mouse bone marrow. As shown in Fig. 1A, cultures reached confluence of the adherent layer at different times, indicating slower proliferation of stromal cells from Fancd2−/− marrow. Cobblestone island formation, representative of hematopoietic cell generating foci on the adherent layer, was detected in all marrow culture groups at around four weeks (Fig. 1B), however, increased numbers of these cobblestone islands were seen in Fancd2+/+ compared to Fancd2+/− and Fancd2−/− long-term bone marrow cultures. Cumulative production of cobblestone islands was higher in Fancd2+/+ and Fancd2+/− marrow cultures compared to Fancd2−/− marrow cultures, reflecting diminished hematopoietic activity in Fancd2−/− cultures in vitro (Fig. 1C).
FIG. 1.
Reduced hematopoietic longevity in Fancd2−/− mouse long-term bone marrow cultures. Eight marrow cultures per group were established as described in the Methods section and cultured as described. Groups were tested weekly for (panel A) percentage confluence of adherent layer; (panel B) cobblestone islands per flasks scored weekly; (panel C) cumulative cobblestone islands; (panel D) non-adherent cells/flask; (panel E) cumulative non-adherent cells/flask; (panel F) weekly day 7 colony forming progenitor cell; (panel G) cumulative day 7 non-adherent colony forming cells; (panel H) weekly non-adherent day 14 colony forming cells; and (panel I) cumulative day 14 colony forming cells. (●) Fancd2+/+; (▲) Fancd2+/−; and (■) Fancd2−/−. (Statistical analysis is shown in Supplementary Table S1; http://dx.doi.org/10.1667/RR13405.1.S1).
The production of non-adherent cells followed weekly cell cycling parameters as previously published for long-term bone marrow cultures (12, 13) and was observed in all culture groups. Figure 1D demonstrates the continuous robust production of hematopoietic cells in Fancd2+/+ and Fancd2+/− long-term bone marrow cultures compared to the earlier decrease in production of hematopoietic cells in Fancd2−/− long-term bone marrow cultures. Cumulative production of non-adherent cells reached a plateau earlier in Fancd2−/− cultures compared to other groups (Fig. 1E).
The diminished production of non-adherent cells in Fancd2−/− cultures correlated with decreased weekly production (Fig. 1F) and cumulative production (Fig. 1G) of cells forming colonies at day 7 in semi-solid media. The diminished production of hematopoietic progenitors by Fancd2−/− cultures were even more dramatic with the more primitive day 14 hematopoietic progenitor cell colonies that diminished in numbers sooner than in either Fancd2+/− or Fancd2+/+ cultures (Fig. 1H). Cumulative day 14 colony forming progenitor production was reduced in Fancd2−/− cultures compared to either Fancd2+/− or Fancd2+/+ cultures (Fig. 1I). Results were analyzed as described in the statistical analysis of the Supplementary Table S1; http://dx.doi.org/10.1667/RR13405.1.S1. These data were consistent with decreased capacity of Fancd2−/− long-term bone marrow cultures to withstand the oxidative stress of continuous culture in high humidity and high oxygen atmosphere (11, 12). These data confirm and extend other reports indicating that long-term bone marrow cultures from mice with genetically deficient capacity to handle oxidative stress show a more rapid decrease in hematopoiesis in vitro (11, 12). Several parameters of increased hematopoiesis were observed in marrow cultures from heterozygous Fancd2+/− mouse compared to Fancd2+/+ mouse marrow, but not in cumulative non-adherent cell production (Fig. 1E), which showed the highest levels with Fancd2+/+ mouse marrow.
Radiosensitivity of Fancd2−/− Mouse Marrow Stromal Cell Lines
We isolated and cloned adherent marrow stromal cell lines from Fancd2−/−, Fancd2+/− and Fancd2+/+ mouse marrow cultures as described in the Method section. In survival curve assays, Fancd2−/− marrow stromal cells were radiosensitive (D0 = 1.4 ± 0.1 Gy, ñ = 5.0 ± 0.6) compared to Fancd2+/+ (D0 = 1.6 ± 0.1Gy, ñ = 6.7 ± 1.6), P = 0.0124 and P = 0.0023, respectively. Fancd2+/− stromal cells were also radiosensitive, by a smaller shoulder on the survival curve, ñ compared to Fancd2+/+ cells (D0 = 1.7 ± 0.1 Gy and ñ = 4.2 ± 0.7), P = 0.0004 for ñ, respectively (Table 1, Fig. 2A).
TABLE 1.
Radiosensitivity of Fancd2−/− Bone Marrow Stromal Cells
| Bone marrow stromal cell lines | D0 (Gy) | ñ | Plating efficiency |
|---|---|---|---|
| Fancd2−/− | 1.4 ± 0.1 P = 0.0124 |
5.0 ± 0.6 P = 0.0023 |
26.5 ± 2.3 |
| Fancd2+/− | 1.7 ± 0.1 P = 0.0906 |
4.2 ± 0.7 P = 0.0004 |
38.2 ± 14.3 |
| Fancd2+/+ | 1.6 ± 0.1 | 6.7 ± 1.6 | 40.4 ± 7.6 |
Notes. Cells from Fancd2+/+, Fancd2+/− and Fancd2−/− bone marrow stromal cell lines were irradiated and assayed for colony formation as described in the Methods section. Plating densities were 100–400 cells/well. Results are the mean ± SEM of at least 3 experiments. Significant P values compared to Fancd2+/+ stromal cells are bold.
FIG. 2.

Radiosensitivity of Fancd2−/− bone marrow stromal cells and radioresistance of Fancd2−/− IL-3-dependent hematopoietic progenitor cells. Cells from each bone marrow stromal (panel A) or hematopoietic progenitor (panel B) cell line were irradiated and assayed for colony formation as described in the Methods section (plating efficiencies were 40–70% and plating densities were 100–400 cells/well. Results are the mean ± SEM of at least 3 experiments comparing cell lines of each phenotype plotted, as single-hit, multi-target models (16, 17).
Relative comet tail intensity was next analyzed in 5 Gy irradiated Fancd2−/− stromal cell lines by comet assay. Comet tail intensity as a measure of maximal double-strand DNA break level was higher in Fancd2−/− stromal cells 15.8 ± 0.5 compared to 8.7 ± 0.3 in Fancd2+/+ stromal cells (P < 0.0001) or Fancd2+/− stromal cells (12.6 ± 0.4, P < 0.0001) (Fig. 3). There was recovery of comet tail length indicative of partial repair of damaged DNA in Fancd2+/+ and Fancd2+/− cells within 1 h and nearly complete repair by 6 h (P =0.0002 and P < 0.0001, respectively). In contrast, the Fancd2−/− stromal cells were slower to show recovered comet tail intensity as an indication of repair of DNA, with no detectable change at 10 min and1 h after irradiation (P = 0.0001 at each time point). By 6 h, Fancd2−/− bone marrow stromal cells detectably recovered comet tail intensity (P = 0.0014); however, this induction of DNA damage did not return to baseline levels by 24 h after irradiation (P = 0.0001). Relative cell cycle distributions were next analyzed and correlated with the radiation-induced changes in comet tail intensity.
FIG. 3.
Different rates of repair of ionizing-radiation-induced DNA strand breaks in Fancd2−/− bone marrow stromal compared to IL-3-dependent hematopoietic progenitor cell lines. Comet assays were carried out as described in the Methods section comparing: (panel A) Bone marrow stromal cell lines and (panel B) IL-3-dependent hematopoietic progenitor cell lines from each genotype. Fancd2−/− stromal cells had higher DNA damage post-irradiation (*P < 0.0001) and had delayed recovery compared to Fancd2+/+ (*P < 0.0001) or Fancd2+/− (*P < 0.0001) stromal cells. *Indicates a significant difference compared to Fancd2+/+ cells.
Cell Cycle Distribution of Fancd2−/− Mouse Bone Marrow Stromal Cells
We found no significant differences in G2/M, S phase or G1 arrest between irradiated Fancd2−/− bone marrow stromal cells compared to Fancd2+/+ or Fancd2+/− stromal cells (Supplementary Table S2; http://dx.doi.org/10.1667/RR13405.1.S1).
Antioxidant Capacity of Fancd2−/− Bone Marrow Stromal Cell Lines
Fancd2−/− marrow stromal cells had decreased baseline antioxidant levels (0.34 ± 0.04 trolox units) compared to Fancd2+/+ stromal cells (0.45 ± 0.11 trolox units) P = 0.0363. Fancd2−/− stromal cells showed depletion of antioxidant stores as did Fancd2+/− and Fancd2+/+ stromal cells at 24 h after 10 Gy irradiation (0.23 ± 0.01 trolox units compared to preirradiation level, P = 0.0407) (Fig. 4).
FIG. 4.
Different levels of baseline and radiation-induced antioxidant capacity between Fancd2−/− bone marrow stromal and IL-3-dependent hematopoietic progenitor cell lines. Cell lines from Fancd2−/− mouse marrow stromal (panel A) and hematopoietic progenitor (panel B) cells were exposed to 5 or 10 Gy irradiation. Cells were harvested at 24 h after irradiation. Protein concentrations were standardized to 1 mg/mL and total antioxidant reductive capacity (antioxidant status) was measured as described in the Methods section. The antioxidant activity was compared to a standard curve generated using trolox units and all data was, therefore, expressed as millimolar (mM) equivalents of trolox units. *Significant difference compared to Fancd2+/+ cell lines.
Radiation Resistance of Fancd2−/− Mouse IL-3-Dependent Hematopoietic Progenitor Cell Lines and Fresh Marrow CFU-GM
In contrast to the data with Fancd2−/− marrow stromal cells, Fancd2−/− IL-3-dependent hematopoietic progenitor cells were radioresistant compared to Fancd2+/+ hematopoietic progenitor cells (Table 2). We confirmed this unexpected radioresistance of Fancd2−/− IL-3-dependent hematopoietic progenitor cell lines with 8 different clonal sublines and all were radioresistant (Table 2), for clone 5 (D0 = 1.38 ± 0.06 Gy and ñ = 5.75 ± 0.87) and for clone 1 (D0 = 1.90 ± 0.06 Gy and ñ = 2.85 ± 0.98), all P ≤ 0.043) (Fig. 2B, Table 2).
TABLE 2.
Radioresistance of Fancd2−/− Hematopoietic Progenitor Cell Lines
| Cell line | D0 | ñ | Plating efficiency |
|---|---|---|---|
| Fancd2−/− | 1.71 ± 0.04 P = 0.001 |
5.07 ± 0.52 | 0.27 ± 0.02 |
| Fancd2+/− | 1.64 ± 0.08 P = 0.0004 |
4.40 ± 0.99 P = 0.002 |
0.21 ± 0.09 |
| Fancd2+/+ | 1.39 ± 0.09 | 2.31 ± 0.85 | 0.81 ± 0.18 |
| Fancd2−/− clones | |||
| clone 1 | 1.90 ± 0.06 P = 0.004 |
2.85 ± 0.98 | 0.24 ± 0.15 |
| clone 2 | 1.63 ± 0.08 P = 0.007 |
5.34 ± 2.56 | 0.18 ± 0.006 |
| clone 3 | 1.56 ± 0.02 | 6.06 ± 0.30 P = 0.006 |
0.25 ± 0.0 |
| clone 4 | 1.65 ± 0.09 P = 0.043 |
2.47 ± 0.37 | 0.17 ± 0.006 |
| clone 5 | 1.38 ± 0.06 | 5.75 ± 0.87 P = 0.013 |
0.15 ± 0.006 |
| clone 6 | 1.44 ± 0.0 | 3.74 ± 0.11 P = 0.025 |
0.14 ± 0.04 |
| clone 7 | 1.55 ± 0.04 | 3.84 ± 0.20 P = 0.018 |
0.12 ± 0.006 |
| clone 8 | 1.72 ± 0.04 P = 0.016 |
2.04 ± 0.18 | 0.12 ± 0.0 |
Notes. Cells from Fancd2+/+ IL-3-dependent cell line (32D cl3), Fancd2+/− and Fancd2−/− hematopoietic progenitor cell lines were irradiated and assayed for colony formation as described in the Methods section. Clones 7 and 8 were cell lines expanded from colonies growing in plates containing cells exposed to 6 and 8 Gy irradiation, respectively. Plating densities were 100–400 cells/well. Results are the mean ± SEM of at least 3 experiments. P values compared to Fancd2+/+ IL-3-dependent cell line, (32D cl 3) are bold.
Because the radioresistance of Fancd2−/− IL-3-dependent hematopoietic progenitor cells was unexpected, we tested for reversion mosaicism, which has been observed in peripheral blood lymphocytes (PBLs) and hematopoietic precursor cells of hematopoiesis in some FA patients, and which can reverse hematopoietic failure and normalize mitomycin resistance (17, 18). We performed copy number PCR analysis of the FancD2 gene in all our clonal cell lines (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13405.1.S1). All the Fancd2−/− stromal and hematopoietic progenitor subclones tested showed a biallelic loss of the FancD2 gene while the heterozygote Fancd2+/− stromal and hematopoietic progenitor cells had one Fancd2 allele. The data confirm that Fancd2+/+ (wild-type), Fancd2+/− and Fancd2−/− stromal as well as hematopoietic progenitor cell lines retained appropriate copies of the mutant or wild type FancD2 allele relative to the starting original mouse genotype (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13405.1.S1).
We measured radiation-induced DNA damage in Fancd2−/− IL-3-dependent hematopoietic progenitor cells using the comet assay; double-strand DNA break level by comet tail intensity was 13.5 ± 0.4 in Fancd2−/− after irradiation compared to 12.8 ± 0.4 in Fancd2+/+ or Fancd2+/− (10.1 ± 0.3) hematopoietic progenitor cells. As shown in Fig. 3B, Fancd2−/− hematopoietic progenitor cells were faster at repairing DNA double-strand breaks by 1 h. This result was clearly different from that of Fancd2−/− stromal cells, which had delayed repair detected at 1 h after irradiation (Fig. 3A).
Radioresistance of Fresh Marrow CFU-GM from Fancd2−/− Mice
To confirm that clonogenic Fancd2−/− mouse IL-3-dependent hematopoietic progenitor cells were radioresistant, we next performed CFU-GM assays using Fancd2+/+, Fancd2+/− and Fancd2−/− fresh whole bone marrow. CFU-GM, BFU-E and CFU-GEMM were scored between days 10 and 13. Radioresistance was detected with Fancd2−/− marrow CFU-GM measured by the shoulder on the survival curve (ñ = 3.02 ± 0.10, P = 0.001 compared to Fancd2+/+) and (P = 0.0001 compared to Fancd2+/−). Radioresistance was not detected with heterozygote Fancd2+/− relative to Fancd2+/+ marrow CFU-GM (ñ = 1.24 ± 0.18 compared to ñ = 1.35 ± 0.18, respectively). There was no significant difference observed in the BFU-E or CFU-GEMM among the three genotypes (Fancd2+/+, Fancd2+/− and Fancd2−/−) (Table 3). Therefore, as with Fancd2−/− IL-3-dependent cell lines, fresh marrow CFU-GM from Fancd2−/− mice was radioresistant.
TABLE 3.
Radioresistance of Hematopoietic Colony Forming Progenitor Cells from Fresh Fancd2 −/− Mouse Bone Marrow
| Fancd2 | CFU-GM | BFU-E | CFU-GEMM | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| bone marrow | D0 | ñ | D0 | ñ | D0 | ñ |
| Fancd2−/− | 1.62 ± 0.09 P1 = 0.049a P2 = 0.007b |
3.02 ± 0.10 P1 = 0.001a P2 = 0.0001b |
1.67 ± 0.18 | 1.4 ± 0.2 | 2.46 ± 0.25 | 1.02 ± 0.02 |
| Fancd2+/− | 2.23 ± 0.07 | 1.24 ± 0.03 | 2.15 ± 0.11 | 1.64 ± 0.36 | 2.35 ± 0.18 | 1.00 ± 0.00 |
| Fancd2+/+ | 2.44 ± 0.27 | 1.35 ± 0.18 | 2.11 ± 0.43 | 1.66 ± 0.33 | 2.02 ± 0.11 | 2.11 ± 0.60 |
Notes. Whole-femur bone marrow from Fancd2+/+, Fancd2+/− and Fancd2−/− mice were irradiated in single cell suspension with doses between 0–8 Gy as described in the Methods section. CFU-GM, BFU-E and CFU-GEMM were scored when colonies reached ≥50 cells in days 10–13. Results are the mean ± SEM of three separate experiments.
P1 = comparison between +/+ group and −/− group.
P2 = comparison between heterozygote +/− group and −/− group.
Irradiation Alters the Cell Cycle Distribution of Fancd2−/− Mouse IL-3-Dependent Cell Lines
The cell cycle distribution of Fancd2−/− IL-3-dependent hematopoietic progenitor cells showed a significantly reduced G2/M pileup at 4 h after 5 Gy irradiation (P = 0.0079), and at 4 and 24 h after 3 Gy compared to Fancd2+/+ IL-3-dependent cells (P = 0.0432 and P = 0.0079, respectively) and this alteration was not normalized by 24 h after 3 Gy (Supplemental Table S3; http://dx.doi.org/10.1667/RR13405.1.S1). These results were in contrast to the lack of cell cycle effect of irradiation on stromal cell lines (Supplemental Table S2; http://dx.doi.org/10.1667/RR13405.1.S1).
Antioxidant Levels in IL-3-Dependent Fancd2−/− Mouse Hematopoietic Progenitor Cells
We hypothesized that the different radiation responses of stromal cells versus hematopoietic cells from Fancd2−/− mice might be attributable to cell phenotype dependent differences in the oxidative stress response. We next assayed for baseline and post-irradiation total antioxidant reduction capacity and antioxidant enzyme gene expression in stromal cells compared to hematopoietic progenitor cells from each genotype. Baseline and post-irradiation antioxidant reduction capacity, measured by millimolar trolox equivalents was higher in Fancd2−/− IL-3-dependent hematopoietic progenitor cells compared to bone marrow stromal cells (Fig. 4). Furthermore, Fancd2−/− hematopoietic progenitor cells maintained total antioxidant stores after 5 Gy and showed less depletion after 10 Gy (P = 0.031) compared to Fancd2+/+ or heterozygote Fancd2+/− hematopoietic cells (Fig. 4B). Thus, the baseline and post-irradiation antioxidant levels in irradiated Fancd2−/− hematopoietic cell were very different from those in stromal cells. Fancd2−/− marrow stromal cells showed decreased baseline and further radiation-induced depletion of antioxidant levels.
Radiation Resistance of Fancd2−/− Mouse Hematopoietic Cell Lines Correlates with Different Baseline and Radiation-induced Levels of Stress Response Related RNA Transcripts and Proteins
We next tested the hypothesis that the different irradiation response of Fancd2−/− mouse stromal compared to hematopoietic cells might be supported by differences in levels of radiation-induced RNA transcripts (Fig. 5) and proteins (Fig. 6). We analyzed levels of transcripts for representative cell cycle regulator and oxidative stress response genes.
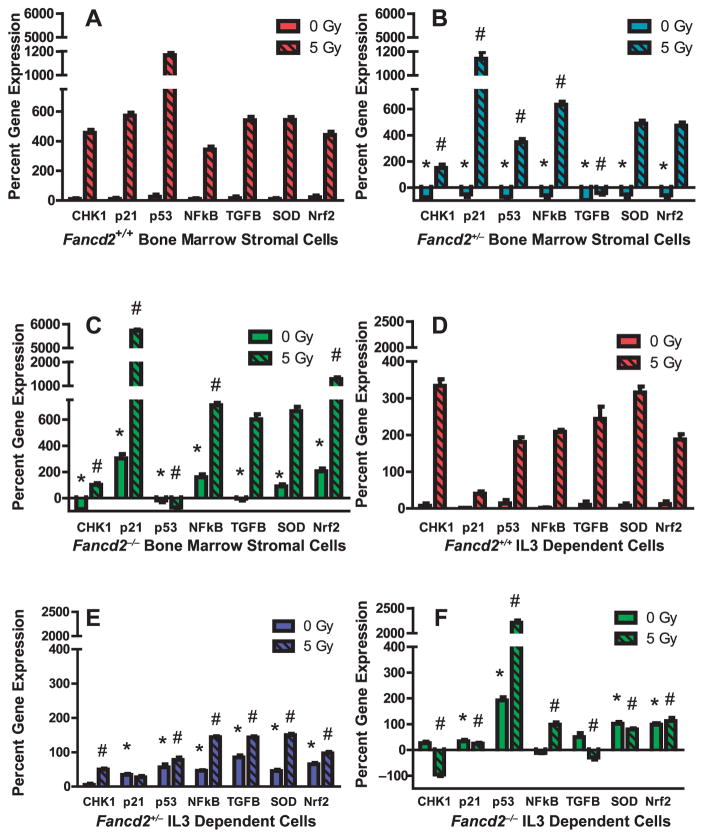
FIG. 5.
Different levels of gene transcripts in baseline and radiation-induced Fancd2−/− marrow stromal cells compared to IL-3-dependent hematopoietic progenitor cell lines. Marrow stromal cells (panels A–C) and IL-3-dependent hematopoietic progenitor cell lines (panels D–F) were compared. RNA was extracted from cell pellets from each cell line using trizol. RT-PCR was performed as described in the Methods section using primers specific for promoters associated with: p21, Chk1, p53, MnSOD, Nrf2, Nfkb and TGFB1. *Significant difference compared to Fancd2+/+ cell lines. *P < 0.05 compared to Fancd2+/+ at 0 Gy; #P < 0.05 compared to Fancd2+/+ at 5 Gy.
FIG. 6.
Different levels of baseline and radiation-induced p21, p53 and MnSOD protein in Fancd2−/− bone marrow stromal and IL-3-dependent hematopoietic progenitor cell lines. Protein level in each cell lines was analyzed by Western analyses as described in the Methods section: at 0 Gy (baseline), at 24 or 48 h after 5 Gy irradiation. Panel A: p53 in stromal cells; panel B: p21 in Fancd2−/− and control Fancd2+/+ and Fancd2+/− stromal cells; panel C: MnSOD in stromal cells; panel D: p53 in IL-3-dependent cells; panel E: p21 in IL-3-dependent hematopoietic progenitor cells; and panel F:) MnSOD in IL-3-dependent hematopoietic progenitor cells. Molecular size for these proteins as follows: MnSOD is 25 kDa; GAPDH is 37 kDa; p21 is 21 kDa and p53 is 53 kDa. See Supplemental Tables 4A–C) for p53, p21 and MnSOD protein expression in Fancd2+/+, Fancd2+/− and Fancd2−/− stromal cells. (For p53, p21 and MnSOD protein expression in Fancd2+/+, Fancd2+/− and Fancd2−/− IL-3-dependent cells see Supplementary Table S4D–F; http://dx.doi.org/10.1667/RR13405.1.S1.)
Fancd2−/− stromal but not IL-3-dependent hematopoietic progenitor cells showed irradiation-increased levels of CHK1 transcripts (Fig. 5C, F).
Levels of baseline and radiation-induced transcripts for the oxidative stress induced transcription factor Nrf2 (nuclear-erythroid related factor 2) and MnSOD (manganese superoxide dismutase) were first compared. Fancd2−/− hematopoietic and stromal cells had higher baseline MnSOD and Nrf2 transcript levels compared to Fancd2+/+ and Fancd2+/− stromal and hematopoietic cells (Fig. 5C, F). After 5 Gy irradiation, Fancd2−/− stromal but not hematopoietic cells showed increases in both MnSOD and NRF2 (P = 0.0001 and P = 0.0001, respectively). However, MnSOD gene transcript levels in Fancd2−/− stromal cells did not correlate with protein levels (Fig. 6C, Supplementary Table S4C; http://dx.doi.org/10.1667/RR13405.1.S1).
There were elevated baseline and radiation-induced levels of p53 gene transcripts in Fancd2−/− IL-3-dependent hematopoietic but not stromal cells (Fig. 5C, F). As with MnSOD and Nrf2, p53 protein levels in irradiated Fancd2−/− hematopoietic or stromal cells did not correlate with the RT-PCR elevated transcripts and was not statistically significantly elevated (P = 0.466) (Fig. 6D, Supplementary Table S4A and D; http://dx.doi.org/10.1667/RR13405.1.S1).
A concordance of elevated RNA transcript levels with elevated protein was detected with p21 in irradiated Fancd2−/− as well as in Fancd2+/− and Fancd2+/+ stromal cells (Fig. 5C, Fig. 6B) (Supplementary Table S4B; http://dx.doi.org/10.1667/RR13405.1.S1). While p21 transcript levels were not elevated in irradiated Fancd2−/− IL-3-dependent cell lines (Fig. 5F), there was elevated protein (Fig. 6E, Supplementary Table S4E; http://dx.doi.org/10.1667/RR13405.1.S1).
The ROS Scavenger JP4-039 Increases Radioresistance of Fancd2−/− Marrow Stromal Cell Lines
The effect of the mitochondrial-targeted nitroxide radio-protector and radiation mitigating drug JP4-039 (9, 19) on radiosensitivity was tested with bone marrow stromal and IL-3-dependent hematopoietic cell lines derived from Fancd2−/− compared to Fancd2+/+ mice. As shown in Table 4, Fancd2−/− and Fancd2+/+ marrow stromal and IL-3-dependent hematopoietic progenitor cell lines were treated with 10 μM JP4-039 for 1 h preirradiation. Both Fancd2+/+ marrow stromal and IL-3-dependent hematopoietic progenitor cell lines were radioprotected by JP4-039 treatment (Table 4). Fancd2−/− bone marrow stromal cells were also protected by JP4-039 in the clonogenic assay. In contrast, there was no detectable protective effect of JP4-039 on Fancd2−/− and Fancd2+/− IL-3-dependent hematopoietic progenitor cells (Table 4).
TABLE 4.
Radiation Protection of Fancd2−/− Marrow Stromal Cells by ROS-Scavenging Nitroxide, JP4-039
| Stromal cell line | JP4-039 | D0 | ñ |
|---|---|---|---|
| Fancd2−/− | 0 | 1.45 ± 0.05 P1 = 0.0021 |
4.99 ± 0.65 P1 = 0.0040 |
| Fancd2−/− + JP4-039 | preirradiation | 1.73 ± 0.10 P2 = 0.0237 P3 = 0.0458 |
5.10 ± 2.00 |
| Fancd2+/+ | 0 | 1.80 ± 0.08 | 8.33 ± 0.73 |
| Fancd2 +/++ JP4-039 | preirradiation | 2.33 ± 0.23 P1 = 0.0177 |
5.37 ± 1.44 |
| Hematopoietic cell line | D0 | ñ | |
| Fancd2−/− | 0 | 1.56 ± 0.25 | 3.46 ± 1.99 |
| Fancd2−/− + JP4-039 | preirradiation | 1.44 ± 0.17 | 3.49 ± 1.10 |
| Fancd2+/− | 0 | 1.87 ± 0.21 | 3.85 ± 1.57 |
| Fancd2+/− + JP4-039 | preirradiation | 2.09 ± 0.28 | 2.65 ± 0.57 |
| Fancd2+/+ | 0 | 1.31 ± 0.10 | 1.00 ± 0.10 |
| Fancd2+/+ + JP4-039 | preirradiation | 2.3 ± 0.3 P = 0.043 |
1.00 ± 0.10 |
Notes. In vitro survival curves were performed with bone stromal and hematopoietic progenitor cells lines. Cells were incubated in 10 μM JP4-039 for 1 h before exposure as described in the literature (9, 19), then irradiated with doses ranging from 0–8 Gy, plated in 4-well tissue culture plates, incubated for 7 days at 37°C, and stained with crystal violet. Colonies >50 cells were counted and analyzed using a linear-quadratic model or single-hit, multi-target model (9, 19). D0 and ñ are expressed as mean ± SD of at least 3 experiments. P < 0.05 were significant and are bold. P1 = comparison with Fancd2+/+ control unirradiated; P2 = comparison with Fancd2−/− unirradiated; and P3 = comparison with Fancd2 +/+ JP4-309 (preirradiation).
DISCUSSION
Deletion of the human FancD2 gene has been implicated in altered genome stability, apoptosis, cell cycle control, and sensitivity to DNA cross-linking agents (5, 6, 21–23). Recent evidence indicates a link between the FA pathway and that of the oxidative stress response suggesting a role for FA proteins in the cellular antioxidant defense system (5). Significant molecular evidence suggests that the function of the FA pathway is to serve as a platform for multiple elements of the DNA repair pathway. This subject has recently been reviewed in detail (1, 2).
The present studies demonstrate reduced duration of hematopoiesis in Fancd2−/− mouse LTBMCs consistent with reduced capacity of marrow to handle stress of long term culture (11, 12). An explanation for the greater levels of several parameters of hematopoiesis in Fancd2+/− marrow cultures is not yet known. Studies with freshly removed tissues from Fancd2−/− compared to Fancd2+/+ and Fancd2+/− mice are in progress. While Fancd2−/− mouse marrow stromal cell lines were radiosensitive, there was an unexpected radioresistance of IL-3-dependent hematopoietic progenitor cell lines and fresh marrow CFU-GM (granulocyte macrophage progenitor cells). Radioresistance of hematopoietic cells was confirmed with clonogenic radiation survival curves in each of eight Fancd2−/− hematopoietic cell clonal lines including two that were derived from 6 and 8 Gy irradiation surviving colonies in semi-solid medium plates (17).
Fancd2−/− radiosensitive stromal cells showed diminished recovery of comet tail intensity as a measure of DNA repair after 5 Gy irradiation and had lower antioxidant stores. In contrast, Fancd2−/− radioresistant IL-3-dependent hematopoietic progenitor cells showed rapid recovery of comet tail intensity as a measure of repaired DNA within 6 h after 5 Gy irradiation, and had higher baseline antioxidant stores and less antioxidant depletion after irradiation. The data indicate an intrinsic radioresistance of Fancd2−/− hematopoietic progenitor but not stromal cells and correlate with differences in clonogenic survival, comet tail intensity recovery as a measure of DNA repair capacity and oxidative stress response.
There was concordance between radiation-induced p21-CDKNIA transcripts and protein with Fancd2−/− stromal cells, but not with MnSOD or p53. The lack of concordance with MnSOD and p53 may be explained by timing of the sample collection for the proteins relative to the RNA.
We confirmed through genotype analysis of all our cell lines that the heterogeneity between bone marrow stromal and hematopoietic cells radiosensitivity was not attributable to revertant mosaicism, which has been reported in cells of some FA patients (24). In our current studies, Fancd2 gene copy number analysis showed that all cell lines studied maintained a biallelic loss of Fancd2 gene and the heterozygote Fancd2+/− cells had one Fancd2 allele, but gene mutations were not studied.
Cell cycle regulation and activation of cell cycle checkpoints is another pathway implicated in cellular responses to ionizing irradiation (23–25), and differed between radiosensitive Fancd2−/− stromal and radioresistant IL-3-dependent hematopoietic cells. There was no detectable cell cycle alteration in irradiated Fancd2−/− stromal cells; however, IL-3-dependent Fancd2−/− cells showed a significantly reduced G2/M pileup at 4 h after 5 Gy irradiation and at 4 and 24 h after 3 Gy irradiation compared to Fancd2+/+ IL-3 cells. The cell cycle alterations in irradiated IL-3-dependent Fancd2−/− cell lines were associated with low levels of Chk1 at baseline and after 5 Gy. Whether there was a survival advantage by down-regulating Chk1 during clonal cell line selection of Fancd2−/− IL-3-dependent cell lines is not known. Prior reports showed that Chk1 was overexpressed in FA hematopoietic stem cells, T-cells and erythroid progenitors (23, 26–27). In addition to the differential total antioxidant capacity, it is possible that difference in cell cycle population might have accounted for the distinctive radiosensitivity of Fancd2−/− mouse stromal cells compared to hematopoietic progenitor cells (25). Since human FancD2 function in DSB repair is tightly associated with homologous recombination in S/G2 phase and a higher G1 population was found in mouse Fancd2−/− hematopoietic progenitors, but not in mouse Fancd2−/− stromal cells, we plan to pursue this possible explanation in future studies.
Because the FA pathway has been linked to that of the oxidative stress response (5, 6, 27–30), we tested the effect of the small molecule mitochondrial-targeted nitroxide JP4-039, which is a ROS scavenger and has been shown to be effective in the radioprotection of normal murine tissues in vivo (9, 19). Furthermore, JP4-039 ameliorates radiosensitivity in vitro of fibroblast cell lines derived from human Fanconi Anemia patient’s cell lines including FancD2−/− (4). The current studies showed radioprotection of mouse Fancd2−/− stromal, but not IL-3-dependent hematopoietic progenitor cells by JP4-039. Whether the lack of effect of JP4-039 on Fancd2−/− hematopoietic cell radioresistance reflects a saturated maximum antioxidant defense or a different radiation induced death pathway in Fancd2−/− cells will require further studies.
The reduced longevity of Fancd2−/− mouse long marrow cultures and radiosensitivity of marrow stromal cells, while hematopoietic cells were radioresistant suggests that the in vivo radiosensitivity of hematopoiesis in Fancd2−/− mice (10) may involve an indirect mechanism of radiation effect on stromal cells transmitted to hematopoietic cells. In vitro co-culture studies and selective marrow stromal cell transplantation studies may be required to test this hypothesis.
Supplementary Material
Acknowledgments
This manuscript is supported by NIH grant U19-A1068021 and the FA Research Foundation. This project used the UPCI animal facility that is supported in part by award P30CA047904.
References
- 1.Kee Y, D’Andrea A. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. 2012;122(11):3799–3806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins C, Kan J, Hoatlin ME. Targeting the Fanconi anemia pathway to identify tailored anticancer therapeutics. Anemia. 2012;2012:1–7. doi: 10.1155/2012/481583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter BP. Fanconi’s anemia and malignancies. Am J Hematol. 1996;53:99–110. doi: 10.1002/(SICI)1096-8652(199610)53:2<99::AID-AJH7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Xichen Z, et al. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603–12. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du W, Rani R, Sipple J, Schick J, Myers KC, Mehta P, et al. The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. 2012;119(18):4142–51. doi: 10.1182/blood-2011-09-381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Andrea AD, Grompe M. Molecular biology of Fanconi anemia: implications for diagnosis and therapy. Blood. 1997;90(5):1725–36. [PubMed] [Google Scholar]
- 7.Kalb R, Duerr M, Wagner M, Herterich S, Gross M, Digweed M, et al. Lack of sensitivity of primary fanconi’s anemia fibroblasts to UV and ionizing radiation. Radiat Res. 2004;161(3):318–25. doi: 10.1667/rr3138. [DOI] [PubMed] [Google Scholar]
- 8.Kuhnert VM, Kachnic LA, Li L, Purschke M, Gheorghiu L, Lee R, et al. FancD2-deficient human fibroblasts are hypersensitive to ionizing radiation at oxygen concentrations of 0% and 3% but not under normoxic conditions. Int J Radiat Biol. 2009;85(6):523–31. doi: 10.1080/09553000902883810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, et al. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants chemical. Pharmacology. 2007;74:801–09. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Parmar K, Kim J, Sykes SM, Shimamura A, Stuckert P, Zhu K, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp 1. Stem Cells. 2010;28:1186–95. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, et al. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS1−/−) mice. Radiat Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Sullivan R, Greenberger JS, Goff J, Shields D, Epperly M, Glowacki J. Cell logic parameters of accelerated osteoporosis in SAMP6 mice are demonstrated in long-term bone marrow culture senescence and in the logy of bone marrow stromal cell lines. Exp Hematol. 2012;40:499–509. [Google Scholar]
- 13.Mauch P, Greenberger JS, Botnick L, Hannon E, Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci U S A. 1980;77:2927–30. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakakeeny MA, Greenberger JS. Granulopoiesis longevity in continuous bone marrow cultures and factor-dependent cell line generation: significant variation among 28 inbred mouse strains and outbred stocks. J Natl Cancer Inst. 1982;68:305–17. [PubMed] [Google Scholar]
- 15.Greenberger JS, Sakakeeny MA, Humphries KC, Eaves CG, Eckner RJ. Demonstration of permanent factor-dependent multi-potential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci, USA. 1983;80:2931–35. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Geijn J. Time-dose response of human tumors and normal tissues during and after fractionated radiation treatment. A new model Radiother Oncol. 1988;2(1):57–78. doi: 10.1016/0167-8140(88)90193-4. [DOI] [PubMed] [Google Scholar]
- 17.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of superoxide dismutase is required for decreasing radiation induced cell damage. Radiat Res. 2003;160(5):568–78. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 18.Olive PL. Detection of hypoxia by measurement of DNA damage in individual cells from spheroids and murine tumours exposed to reductive drugs. I. Tirapzazmine. Br J Cancer. 1995;71:529–36. doi: 10.1038/bjc.1995.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol l Phys. 2011;80(3):860–68. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houghtaling S, Newell A, Akkari Y, Taniguchi T, Olson S, Grompe M. Fancd2 functions in a double strand break repair pathway that is distinct from non-homologous end joining. Hum Mol Genet. 2005;14(20):3027–33. doi: 10.1093/hmg/ddi334. [DOI] [PubMed] [Google Scholar]
- 21.Ceccaldi R, Parmar K, Mouly E, Delord M, Kim JM, Regairaz M, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11(1):36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Yang K, Dejsuphong D, D’Andrea AD. Regulation of Rev1 by the Fanconi anemia core complex. Nature Structural Molecular logy. 2012;19:164–70. doi: 10.1038/nsmb.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guervilly JH, Macé-Aimé G, Rosselli F. Loss of CHK1 function impedes DNA damage-induced FancD2 monoubiquitination but normalizes the abnormal G2 arrest in Fanconi anemia. Hum Mol Genet. 2008;17(5):679–89. doi: 10.1093/hmg/ddm340. [DOI] [PubMed] [Google Scholar]
- 24.Hoehn H, Kalb R, Neveling K, Friedl R, Bechtold A, Herterich S, et al. Monogr Hum Genet. Basel: Karger; 2007. Revertant Mosaicism in Fanconi Anemia: Natural Gene Therapy at Work; pp. 149–172. [Google Scholar]
- 25.Pawlik TM, Keyomarsi K. Role of Cell Cycle in Mediating Sensitivity to Radiotherapy. Int J Radiation Oncology l Phys. 2004;59(4):928–42. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Djuzenova CS, Flentje M. Characterization of Fanconi anemia fibroblasts in terms of clonogenic survival and DNA damage assessed by the Comet assay. Med Sci Monit. 2002;8(10):BR421–30. [PubMed] [Google Scholar]
- 27.Hamanoue S, Yagasaki H, Tsuruta T, Oda T, Yabe H, Yabe M, et al. Myeloid lineage-selective growth of revertant cells in Fanconi anemia. Br J Haematol. 2006;132(5):630–5. doi: 10.1111/j.1365-2141.2005.05916.x. [DOI] [PubMed] [Google Scholar]
- 28.Parmar K, D’Andrea AD. Stressed out: endogenous aldehydes damage hematopoietic stem cells. Cell Stem Cell. 2012;11:583–84. doi: 10.1016/j.stem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Kuhnert VM, Kachnic LA, Li L, Purschke M, Gheorghiu L, Lee R, et al. FANCD2-deficient human fibroblasts are hypersensitive to ionizing radiation at oxygen concentrations of 0% and 3% but not under normoxic conditions. Int J Radiat l. 2009;85(6):523–31. doi: 10.1080/09553000902883810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosselli F, Ridet A, Soussi T, Duchaud E, Alapetite C, Moustacchi E. p53-dependent pathway of radio-induced apoptosis is altered in Fanconi anemia. Oncogene. 1995;10:9–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.