Abstract
Object
Our objective was to use 7 T MRI to compare cartilage morphology (thickness) and collagen composition (T2 values) in cartilage repair patients and healthy controls.
Materials and methods
We scanned the knees of 11 cartilage repair patients and 11 controls on a 7 T MRI scanner using a high-resolution, gradient-echo sequence to measure cartilage thickness and a multi-echo spin-echo sequence to measure cartilage T2 values. We used two-tailed t tests to compare cartilage thickness and T2 values in: repair tissue (RT) versus adjacent cartilage (AC); RT versus healthy control cartilage (HC); AC versus HC.
Results
Mean thickness in RT, AC, HC were: 2.2 ± 1.4, 3.6 ± 1.1, 3.3 ± 0.7 mm. Differences in thickness between RT–AC (p = 0.01) and RT–HC (p = 0.02) were significant, but not AC–HC (p = 0.45). Mean T2 values in RT, AC, HC were: 51.6 ± 7.6, 40.0 ± 4.7, 45.9 ± 3.7 ms. Differences in T2 values between RT–AC (p = 0.0005), RT-HC (p = 0.04), and AC–HC (p = 0.004) were significant.
Conclusion
7 T MRI allows detection of differences in morphology and collagen architecture in: (1) cartilage repair tissue compared to adjacent cartilage and (2) cartilage repair tissue compared to cartilage from healthy controls. Although cartilage adjacent to repair tissue may be normal in thickness, it can demonstrate altered collagen composition.
Keywords: Cartilage repair, T2 mapping, Ultra high field, 7 T, MRI
Introduction
Over the last two decades, there has been remarkable progress in the field of cartilage restoration procedures. A wide variety of surgical techniques are now available to treat patients with symptomatic, focal cartilage defects including microfracture, osteochondral autografting, osteochondral allografting, matrix assisted autologous chondrocyte implantation, and juvenile cartilage cell implantation [1–5]. There has been a call for randomized, prospective studies comparing the effectiveness and outcomes of these different procedures so that evidence-based treatment decisions can be made [6]. Currently, the choice of treatment is guided by patient goals and expectations, the presence of concomitant injuries, history of prior treatment, and cartilage defect size, depth, and location [7].
There is a critical need to objectively assess cartilage repair tissue quality, which may serve as a marker for surgical and clinical outcomes in longitudinal patient studies [8]. Though not routinely performed for patient follow-up, the only true gold standard for assessment of repair tissue quality is arthroscopy with mechanical probing and biopsy for histologic analysis [8]. Magnetic resonance imaging (MRI) offers an attractive, noninvasive alternative to quantitatively assess the status of repair tissue, both in terms of its morphology and its biochemical composition.
The recent arrival of ultra high field, 7 T MRI canners offers promise for improved evaluation of cartilage structure and physiology [9–11]. Because image signal-to-noise ratio (SNR) scales approximately linearly with the magnitude of the main magnetic field, the “extra” SNR available at 7 T can be converted into increases in image spatial resolution or scanning speed [12]. This is beneficial for MRI of cartilage, since it is a tissue only 1–4 mm thick and long scan times are required in order to obtain images of cartilage with high resolution. In a recent article by Pepin et al. [13] published in the American Journal of Sports Medicine, there was a strong correlation between 7 T MRI measurements of cartilage thickness and histologic measurements of cartilage thickness in a canine model of joint degeneration. The authors concluded that 7 T MRI offers an alternative to histology for the detection of early cartilage degeneration and the evaluation of osteoarthritis treatments.
While many 3 T MRI studies of human cartilage repair patients exist, there are only a handful of studies of human cartilage repair patients using 7 T MRI. Trattnig et al. [14] used sodium MRI at 7 T to evaluate 12 patients after matrix-assisted autologous chondrocyte implantation and found that sodium signal intensity (and thus proteoglycan content) within repair tissue was lower than in native cartilage. Schmitt et al. [15] used both glycosaminoglycan chemical exchange saturation transfer and sodium MRI at 7 T and found evidence for lower cartilage proteoglycan content in patients after both microfracture and matrix-assisted autologous chondrocyte transplantation. And Chang et al. [16] used a sodium inversion recovery pulse sequence at 7 T to evaluate eleven patients after cartilage repair procedures and also found that sodium concentration in repair tissue was lower than sodium concentration within adjacent cartilage and within cartilage from the non-surgical knee compartment. While sodium MRI of cartilage repair tissue has been performed at 7 T, only one prior study has used 7 T MRI to quantify and compare cartilage thickness and T2 values (marker of collagen architecture) in the knees of four cartilage repair patients and 12 healthy controls in vivo. In this 7 T study Welsch et al. [17] did not find global differences in T2 values in repair tissue compared to healthy cartilage. However, this may have been due to the low number of cartilage repair patients in their study.
With this as background, the goal of this study was to use 7 T MRI to perform both high resolution anatomic imaging and quantitative assessment of collagen architecture (T2 mapping) in cartilage repair patients and healthy controls in vivo. T2 mapping is an accepted method to quantitatively assess cartilage collagen architecture in vivo [18, 19]. Indeed, it is included in the MRI protocol for the Osteoarthritis Initiative, the longitudinal National Institutes of Health-funded, multicenter study of degenerative osteoarthritis [20]. We hypothesized that 7 T MRI would allow us to detect differences in the morphology (thickness) and collagen architecture (T2 values) of cartilage repair tissue compared to: (1) cartilage adjacent to the repair tissue and (2) cartilage in healthy controls.
Materials and methods
Subject recruitment
This study had institutional review board approval, and we obtained written informed consent from all subjects. We recruited 11 consecutive patients status post cartilage restoration procedure from an orthopaedic surgery practice (eight men, three women, mean age = 36.5 ± 14.9 years). The types of procedures included: displaced osteochondral fragment reattachment (n = 1), osteochondral allograft (n = 1), synthetic resorbable graft placement (OBI, n = 1), juvenile cartilage cell implantation (DeNovo, n = 2), microfracture (n = 3), matrix assisted autologous chondrocyte implantation (Carticel, n = 3). At the time of the MRI scan, the mean follow-up interval was 12.3 ± 10.9 months (range 3–30 months). We also recruited and one-to-one matched 11 healthy controls without history of knee trauma, surgery, inflammatory or metabolic disorder, or medication use (eight men, three women, mean age = 32.6 ± 5.9 years).
MRI scanning
We scanned the surgically-operated knee of patients and a randomly selected knee of healthy controls on a 7 T whole body MR scanner (Siemens, Erlangen, Germany) using a 28 channel receive array coil (Quality Electrodynamics, Mayfield Village, Ohio). For each patient, we manually calibrated the transmit power to account for differences in knee sizes and maintain equivalent excitation. For morphologic imaging, we used a three-dimensional fast low-angle shot sequence (3D-FLASH, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm, 80 sagittal or axial images (axial images were obtained for cartilage repair at the patella), parallel acceleration factor = 2, acquisition time = 7 min 9 s). This T1-weighted, fat suppressed, gradient-echo sequence is considered ideal for quantitative assessment of cartilage morphology [21]. For cartilage T2 mapping, we used a multi-echo spin-echo sequence (MESE, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm, 6 sagittal or axial slices, parallel acceleration factor = 1, acquisition time = 6 min 29 s) similar to the T2 mapping sequence employed in the Osteoarthritis Initiative MRI protocol [20].
Measurement of cartilage thickness and T2 values
A musculoskeletal radiologist identified the cartilage repair site for each patient on the MR images. The radiologist also confirmed the location of the repair site with arthroscopy reports from each patient’s procedure.
To measure cartilage thickness, we uploaded 3D-FLASH images to a computer workstation (Siemens, Erlangen, Germany). Since semiquantitative assessment of repair tissue thickness (complete, incomplete >50 %, incomplete <50 %, subchondral bone exposed) is a metric used by orthopaedic surgeons and radiologists to assess the outcome of a repair procedure [22], we chose to measure the smallest repair tissue thickness because we felt that this would most accurately reflect the outcome of the procedure. Under the guidance of a musculoskeletal radiologist, we used electronic calipers to measure the smallest thickness of cartilage repair tissue, cartilage adjacent to the repair tissue (on the same sagittal slice), and the cartilage of healthy controls (this was measured in the same compartment/anatomic location as the repair tissue). For healthy controls, we measured cartilage thickness in the same anatomic location as one of the repair sites.
To measure cartilage T2 values, we uploaded MESE images to MATLAB (Mathworks, Natick, MA). We obtained color cartilage T2 maps using the equation ln((S(TE)/S0) = (−TE/T2) + C), where S(TE) is the measured SI of the image at a given TE, S0 is the signal at the shortest TE, and C is the intercept. We used a weighted linear least squares fit for the monoexponential decay equation [23]. Under guidance of the musculoskeletal radiologist, we manually drew a region of interest around the cartilage repair tissue in a patient, and we drew identically-sized regions of interest around adjacent cartilage tissue in the patient, and cartilage from a healthy control in the same compartment/anatomic location as the repair site. We calculated the T2 value within each region of interest, and as in prior studies, we excluded the first echo from the multi-echo spin-echo sequence because this has been shown to minimize error in T2 values due to stimulated echoes [24, 25].
Statistical analysis
We used two-tailed t tests (SPSS 18.0, Somers, New York) to compare mean cartilage thickness and mean T2 values between: (1) repair cartilage and adjacent cartilage, (2) repair cartilage and cartilage from healthy controls, and (3) adjacent cartilage and cartilage from healthy controls. We considered a p value <0.05 to represent a statistically significant difference.
Results
Representative 7 T MR images of the repair procedures
Figure 1 shows a representative 7 T high resolution 3D-FLASH image and T2 cartilage map from a healthy control. Figures 2, 3, 4, 5, 6 and 7 show high resolution 3D-FLASH images and T2 maps from patients with the different types of cartilage repair procedures included in this study: displaced osteochondral fragment reattachment (Fig. 2), synthetic resorbable graft placement (Fig. 3), osteochondral allografting (Fig. 4), microfracture (Fig. 5), juvenile cartilage cell implantation (Fig. 6), and matrix-assisted autologous chondroycte implantation (Fig. 7).
Fig. 1.
Healthy volunteer. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate normal morphology and T2 values in healthy cartilage
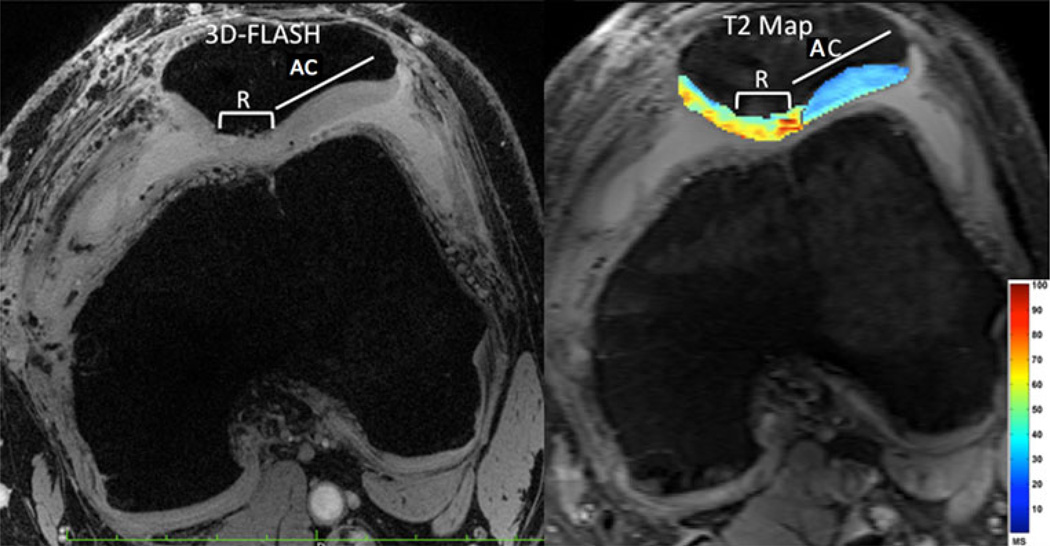
Fig. 2.
Displaced osteochondral fragment reattachment. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate slightly lower thickness and higher T2 values in the repair (R) cartilage compared to adjacent cartilage (AC)
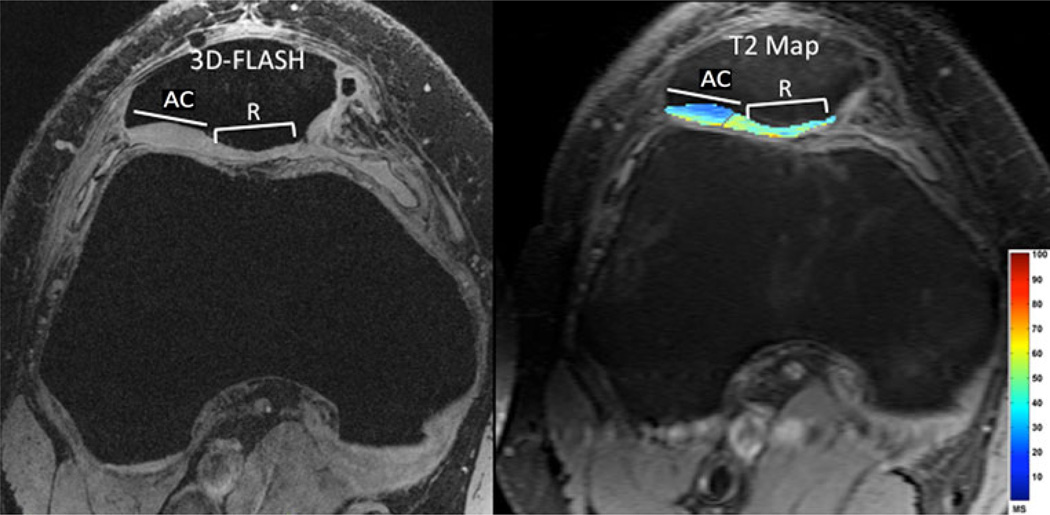
Fig. 3.
Synthetic resorbable graft placement. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate slightly lower thickness and higher T2 values in the repair (R) tissue compared to adjacent cartilage (AC)
Fig. 4.
Osteochondral allograft. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate lower thickness and higher T2 values in the repair (R) cartilage overlying the graft compared to adjacent cartilage (AC)
Fig. 5.
Microfracture. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate slightly lower thickness and higher T2 values in the repair (R) tissue compared to adjacent cartilage (AC)
Fig. 6.
Juvenile cartilage cell implantation. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate close to normal thickness, but higher T2 values in the repair (R) tissue compared to adjacent cartilage (AC)
Fig. 7.
Matrix-assisted autologous chondroycte implantation. High resolution 7 T 3D-FLASH image (left panel, TR/TE = 20 ms/5.1 ms, 0.234 mm × 0.234 mm × 1 mm) and T2 map (right panel, TR/TE = 3,000 ms/15, 30, 45, 60, 75, 90 ms, 0.586 mm × 0.586 mm × 2 mm) demonstrate lower thickness, but higher T2 values in the repair (R) tissue compared to adjacent cartilage (AC)
Thickness of repair tissue compared to adjacent cartilage and healthy control cartilage
The thicknesses of repair tissue, adjacent cartilage, and healthy control cartilage for each subject is listed in Table 1. Mean cartilage thickness in repair tissue, adjacent cartilage, and healthy control cartilage were: 2.2 ± 1.4, 3.6 ± 1.1, and 3.3 ± 0.7 mm, respectively. Differences in thickness between repair tissue and adjacent cartilage (p = 0.01) and between repair tissue and healthy control cartilage (p = 0.02) were statistically significant (Fig. 8a). Differences in thickness between adjacent cartilage and healthy control cartilage (p = 0.45) were not significant (Fig. 8a).
Table 1.
Tissue thickness and T2 values of cartilage repair tissue, adjacent cartilage adjacent to the repair tissue, and cartilage from healthy controls
| Repair type | Repair site | Follow-up time (months) |
Repair tissue thickness (mm) |
Repair tissue T2 value (ms) |
Adjacent cartilage thickness (mm) |
Adjacent cartilage T2 value (ms) |
Healthy cartilage thickness (mm) |
Healthy cartilage T2 value |
|---|---|---|---|---|---|---|---|---|
| Displaced osteochondral fragment reattachment | Patella | 3.5 | 4.1 | 54.6 | 4.7 | 34.5 | 4.4 | 53.4 |
| Osteochondral allograft | Medial femoral condyle | 32 | 1.0 | 45.6 | 3.7 | 42.9 | 3.0 | 42.3 |
| Synthetic resorbable graft placement | Lateral femoral condyle | 22 | 1.5 | 66.5 | 2.7 | 38.3 | 2.8 | 48.2 |
| Microfracture | Lateral femoral condyle | 30 | 1.0 | 51.2 | 2.4 | 39.0 | 3.3 | 46.7 |
| Microfracture | Lateral femoral trochlea | 15 | 1.0 | 49.8 | 2.8 | 41.2 | 2.4 | 40.9 |
| Microfracture | Patella | 8 | 4.0 | 47.6 | 5.1 | 48.9 | 3.9 | 42.6 |
| Matrix assisted autologous chondrocyte implantation | Lateral femoral condyle | 4 | 1.7 | 64.4 | 2.7 | 47.3 | 46.1 | |
| Juvenile cartilage cell implantation | Patella | 4 | 1.0 | 43.4 | 5.0 | 37.8 | 4.1 | 46.8 |
| Matrix assisted autologous chondrocyte implantation | Patella | 3 | 1.8 | 43.9 | 2.5 | 35.1 | 3.5 | 45.4 |
| Juvenile cartilage cell implantation | Patella | 13 | 4.6 | 47.8 | 4.5 | 35.8 | 4.1 | 49.6 |
| Matrix assisted autologous chondrocyte implantation | Lateral Femoral Condyle | 7 | 2.0 | 51.6 | 3.4 | 39.3 | 2.5 | 43.0 |
| Average | 12.3 ± 10.9 | 2.2 ± 1.4 mm | 51.6 ± 7.6 ms | 3.6 ± 1.1 mm | 40.0 ± 4.7 ms | 3.3 ± 0.7 mm | 45.9 ± 3.7 ms |
For healthy controls, cartilage thickness and T2 values were measured in the same anatomic location as one of the repair sites
Fig. 8.
Bar graphs demonstrating mean thickness (a) and T2 values (b) for repair tissue, cartilage adjacent to repair tissue, and cartilage from healthy controls. For cartilage thickness, there were significant differences in repair tissue compared to adjacent cartilage (p = 0.01) and in repair tissue compared to cartilage from healthy controls (p = 0.02), but not in adjacent cartilage compared to healthy control cartilage (0.45). For T2 values, there were significant differences in repair tissue compared to adjacent cartilage (p = 0.0005), in repair tissue compared to healthy control cartilage (p = 0.04), and in adjacent cartilage compared to healthy control cartilage (p = 0.004)
T2 values of repair tissue compared to adjacent cartilage and healthy control cartilage
The T2 values of repair tissue, adjacent cartilage, and healthy control cartilage are listed in Table 1. Mean T2 values in repair tissue, adjacent cartilage, and healthy control cartilage were: 51.6 ± 7.6, 40.0 ± 4.7, and 45.9 ± 3.7 ms, respectively. Differences in T2 values between repair tissue and adjacent cartilage (p = 0.0005), between repair tissue and healthy control cartilage (p = 0.04), and between adjacent cartilage and healthy control cartilage (p = 0.004) were all significant (Fig. 8b).
Discussion
We have used ultra high field, 7 T MRI to perform high resolution, morphologic imaging and T2 mapping of cartilage in cartilage repair patients and healthy controls. 7 T MRI detected significant differences in thickness and T2 values in repair tissue compared to adjacent cartilage and in repair tissue compared to cartilage from healthy controls. Although normal in thickness, cartilage adjacent to repair tissue demonstrated significantly different T2 values compared to cartilage from healthy controls. Longitudinal studies are needed to determine whether such altered collagen architecture/water content in cartilage adjacent to repair tissue represents a normal postoperative state or early degeneration.
Over the last few years, there have been an increasing number of publications describing the benefits of ultra high field 7 T MRI for imaging of the musculoskeletal system [9, 11, 26–31]. Because SNR scales approximately linearly with the magnitude of the main magnetic field, scanning at 7 T provides more than twofold and fourfold the SNR compared to scanning at 3 T and 1.5T (conventional field strength), respectively [11, 12]. The greater SNR of 7 T can be converted into increases in image spatial resolution, since image voxel size is directly proportional to SNR [12]. Alternatively, the greater SNR of 7 T can be used to decrease image acquisition times while maintaining the same image quality compared to 1.5 or 3 T either by decreasing the number of signal averages or by employing parallel imaging if a multichannel coil is used [9, 32, 33]. Although decreasing the number of signal averages or using parallel imaging incurs SNR losses, 7 T allows the use of these techniques because of the greater baseline intrinsic SNR available. This is all beneficial for cartilage imaging, since: (1) higher resolution images facilitates the detection of smaller morphologic cartilage defects and improves measurement precision [21] and (2) faster scanning will decrease the risk of motion artifact on images, which is critical when assessing a tissue that is only a few millimeters thick.
In this study, we used 7 T MRI to obtain high resolution morphologic images of cartilage (voxel size = 0.053 mm3) with total knee joint coverage in 7 min and 9 s. In comparison, previous morphologic cartilage imaging studies performed at 1.5 and 3 T have used 47–350 % larger voxel sizes of 0.078–0.72 mm3) with greater than 50 % longer scan times of approximately 12 min [21]. The higher SNR of 7 T allowed us to obtain these higher resolution images with a shorter scan time compared to 1.5 or 3 T. Because parallel imaging does incur an SNR penalty, which can render images lower in quality, we did not use a higher parallel acceleration factor greater than 2. It has been shown that at a parallel acceleration factor of 2 with a 28 channel knee coil at 7 T, the SNR is approximately equivalent to that obtained with a quadrature knee coil at 7 T [33].
Semiquantitative assessment of repair tissue thickness (complete, >50 % thickness, <50 % thickness, exposed subchondral bone) is a metric used by radiologists and orthopaedic surgeons to assess the outcome of a cartilage repair procedure [22]. The ideal outcome of a cartilage repair procedure is to obtain full-thickness repair tissue that completely fills a cartilage defect compared to the surrounding adjacent cartilage. We were able to quantify and detect decreases in the thickness of repair tissue compared to: (1) cartilage adjacent to the repair tissue and (2) normal cartilage from the same anatomic location in a healthy control. The ability to quantify and detect such differences using high-resolution 7 T MRI suggests that 7 T MRI can serve as a quantitative means to assess and monitor surgical outcome after cartilage repair procedures. We were not able to detect differences in the thickness of cartilage adjacent to repair tissue compared to normal cartilage in a healthy control. This suggests that the cartilage adjacent to the repair tissue is similar, at least on a macrostructural level, to cartilage from the healthy control. However, as discussed below, on the biochemical level, we found that the cartilage adjacent to repair tissue had different collagen and water content (based on different T2 values) compared to normal cartilage from healthy patients.
As mentioned previously, only a handful of studies have used 7 T MRI to study cartilage repair patients in vivo. However, our finding that T2 values are higher in repair tissue compared to adjacent cartilage is in line with the results of previous studies of cartilage repair patients performed at 3 T in vivo. In 20 patients status post matrix-assisted autologous chondroycte implantation studied at 3T, Welsch et al. [14–17] found that T2 values of repair tissue were higher than those of native cartilage and that there were differences in T2 values depending on whether a collagen-based or hyaluronan-based scaffold was used [34]. Mamisch et al. [35] found that T2 values were higher in repair tissue compared to native cartilage when patients mechanically loaded their knees within a 3 T MRI scanner. Altogether, the higher T2 values in repair tissue compared to native cartilage likely reflect differences in collagen architecture, water content, and thus the biomechanical properties of repair tissue compared to native cartilage.
The finding that cartilage adjacent to repair tissue demonstrates different T2 values (and thus altered collagen architecture) compared to healthy cartilage is supportive of the results of prior synovial fluid and ex vivo histology studies, which have shown that the trauma to the articular surface results in: (1) release of collagen from the extracellular matrix into synovial fluid [36] and (2) a gradient of chondrocyte damage along the articular surface, with nonviable chondrocytes at the injury site and increasing numbers of viable chondrocytes further from the injury site [37]. Chondrocyte death would also contribute to decreased collagen content and abnormal extracellular matrix physiology in native cartilage surrounding the injury/repair site. This in turn would alter T2 values in the matrix of surrounding native cartilage. In this study, cartilage adjacent to repair tissue demonstrated lower T2 values compared to healthy cartilage from controls. This may reflect lower water content and greater compressibility of the cartilage adjacent to repair tissue [38–40].
This study has limitations. First, the number of subjects is low. However, as an initial feasibility study at 7 T, the number of subjects was sufficient to demonstrate significant differences between groups. Second, there was heterogeneity in the types of repair procedures performed, and thus the types of repair tissues being assessed. Therefore, the results of this study cannot be viewed as definitive because we cannot exclude the possibility that variation in the types of repair procedures accounts for the results. We chose, however, to image patients as they were consecutively referred to us by orthopaedic surgery in order to more accurately reflect clinical practice. In addition, the presence of heterogeneity in the patient population would likely only make it more difficult for 7 T MRI to detect differences between groups. Third, there was also heterogeneity in the follow-up intervals for the patients. This would also contribute to variation in the maturity and types of the repair tissues assessed. Because T2 values of repair tissue depend on the length of the follow-up interval, the heterogeneity in follow-up times in our study may have skewed our results. However, variation in follow-up interval would likely only make it more difficult for 7 T MRI to detect significant differences between groups. Fourth, it can be difficult to segment synovial fluid from cartilage tissue on the 3D FLASH images and T2 maps. We did try to mitigate the potential for this error by having a dedicated musculoskeletal radiologist oversee all of the segmentations. Fifth, we did not perform a zonal evaluation of cartilage T2 values in this study. We did initially desire to do this; however, because most of the repair tissue was partial thickness, we felt that our ability to accurately segment superficial and deep layers of repair tissue would be limited. Finally, 7 T MRI scanners are only available at handful of academic medical centers in the United States. However, there has been increasing annual demand for 7 T MRI scanners worldwide [12]. Furthermore, when 3 T MRI scanners were introduced approximately 15 years ago, they were also not widely available. 3 T MRI is now becoming the standard-of-care for musculoskeletal imaging as the diagnostic benefits become more evident [41, 42].
Conclusion
In conclusion, we have used 7 T MRI to quantify and compare cartilage thickness and T2 values in cartilage repair patients and healthy controls. 7 T MRI detected differences in tissue thickness and T2 values in repair tissue compared to native adjacent cartilage and in repair tissue compared to cartilage from healthy controls. In addition, although native cartilage adjacent to repair tissue was normal in thickness, it demonstrated lower T2 values compared to cartilage from healthy controls. This may reflect a normal postoperative due to decreased water content/greater compressibility, but the possibility of an early degenerative process cannot definitely be excluded. In the future, 7 T MRI can be used as a tool to objectively evaluate the structure and physiology of cartilage repair tissue and native adjacent cartilage. This will be valuable for future prospective clinical trials of cartilage repair procedures, in which quantitative metrics of joint health are needed in order to compare the outcomes and effectiveness of different surgical techniques.
Acknowledgments
The authors acknowledge Grant support from the Radiological Society of North America (RSNA RR0806, PI Chang) and the United States National Institutes of Health (NIH/NIAMS K23AR059748, PI Chang; R01AR056260, PI Regatte; R01AR053133, PI Regatte, R01AR060238, PI Regatte).
Contributor Information
Gregory Chang, Email: gregory.chang@nyumc.org, Department of Radiology, NYU Langone Medical Center, Center for Musculoskeletal Care, 333 East 38th Street, 6th Floor, Room 610, New York, NY 10016, USA.
Ding Xia, Quantitative Multinuclear Musculoskeletal Imaging Group, Department of Radiology, NYU Langone Medical Center, Center for Biomedical Imaging, 660 First Avenue, 4th Floor, New York, NY 10016, USA.
Orrin Sherman, Department of Orthopaedic Surgery, NYU Langone Medical Center, Center for Musculoskeletal Care, 333 East 38th Street, 4th Floor, New York, NY 10016, USA.
Eric Strauss, Department of Orthopaedic Surgery, NYU Langone Medical Center, Center for Musculoskeletal Care, 333 East 38th Street, 4th Floor, New York, NY 10016, USA.
Laith Jazrawi, Department of Orthopaedic Surgery, NYU Langone Medical Center, Center for Musculoskeletal Care, 333 East 38th Street, 4th Floor, New York, NY 10016, USA.
Michael P. Recht, Department of Radiology, NYU Langone Medical Center, Center for Musculoskeletal Care, 333 East 38th Street, 6th Floor, Room 610, New York, NY 10016, USA
Ravinder R. Regatte, Quantitative Multinuclear Musculoskeletal Imaging Group, Department of Radiology, NYU Langone Medical Center, Center for Biomedical Imaging, 660 First Avenue, 4th Floor, New York, NY 10016, USA
References
- 1.Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28:25–40. doi: 10.1016/j.csm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee–a review. Eur Radiol. 2009;19:1582–1594. doi: 10.1007/s00330-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 3.Gomoll AH, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470–2490. [PubMed] [Google Scholar]
- 4.Chang G, Sherman O, Madelin G, Recht M, Regatte R. MR imaging assessment of articular cartilage repair procedures. Magn Reson Imaging Clin N Am. 2011;19:323–337. doi: 10.1016/j.mric.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potter HG, Foo LF. Magnetic resonance imaging of articular cartilage: trauma, degeneration, and repair. Am J Sports Med. 2006;34:661–677. doi: 10.1177/0363546505281938. [DOI] [PubMed] [Google Scholar]
- 6.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469:2696–2705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(Suppl 1):148S–155S. doi: 10.1177/0363546509351143. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AF, Smith M. Progress in cartilage restoration. Am J Sports Med. 2009;37(Suppl 1):7S–9S. doi: 10.1177/0363546509354205. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Krug R, Carballido-Gamio J, Kelley DA, Xu D, Vigneron DB, Majumdar S. Rapid in vivo musculoskeletal MR with parallel imaging at 7 T. Magn Reson Med. 2008;59:655–660. doi: 10.1002/mrm.21455. [DOI] [PubMed] [Google Scholar]
- 10.Krug R, Carballido-Gamio J, Banerjee S, Stahl R, Carvajal L, Xu D, Vigneron D, Kelley DA, Link TM, Majumdar S. In vivo bone and cartilage MRI using fully-balanced steady-state free-precession at 7 tesla. Magn Reson Med. 2007;58:1294–1298. doi: 10.1002/mrm.21429. [DOI] [PubMed] [Google Scholar]
- 11.Regatte RR, Schweitzer ME. Ultra-high-field MRI of the musculoskeletal system at 7.0T. J Magn Reson Imaging. 2007;25:262–269. doi: 10.1002/jmri.20814. [DOI] [PubMed] [Google Scholar]
- 12.Robitaille P-M, Berliner LJ. Ultra high-field magnetic resonance imaging. New York, NY: Springer; 2006. [Google Scholar]
- 13.Pepin SR, Griffith CJ, Wijdicks CA, Goerke U, McNulty MA, Parker JB, Carlson CS, Ellermann J, LaPrade RF. A comparative analysis of 7.0-Tesla magnetic resonance imaging and histology measurements of knee articular cartilage in a canine posterolateral knee injury model: a preliminary analysis. Am J Sports Med. 2009;37(Suppl 1):119S–124S. doi: 10.1177/0363546509350439. [DOI] [PubMed] [Google Scholar]
- 14.Trattnig S, Welsch GH, Juras V, Szomolanyi P, Mayerhoefer ME, Stelzeneder D, Mamisch TC, Bieri O, Scheffler K, Zbyn S. 23Na MR imaging at 7 T after knee matrix-associated autologous chondrocyte transplantation preliminary results. Radiology. 2010;257:175–184. doi: 10.1148/radiol.10100279. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, Bachert P, Trattnig S. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 16.Chang G, Madelin G, Sherman OH, Strauss EJ, Xia D, Recht MP, Jerschow A, Regatte RR. Improved assessment of cartilage repair tissue using fluid-suppressed (2)(3)Na inversion recovery MRI at 7 Tesla: preliminary results. Eur Radiol. 2012;22:1341–1349. doi: 10.1007/s00330-012-2383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, Kraff O, Schweitzer ME, Szomolanyi P, Trattnig S. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43:619–626. doi: 10.1097/RLI.0b013e31817e9122. [DOI] [PubMed] [Google Scholar]
- 18.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 19.Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47:675–686. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 22.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Koff MF, Amrami KK, Felmlee JP, Kaufman KR. Bias of cartilage T2 values related to method of calculation. Magn Reson Imaging. 2008;26:1236–1243. doi: 10.1016/j.mri.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 25.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, Schmithorst VJ, Smith MB. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 26.Zuo J, Bolbos R, Hammond K, Li X, Majumdar S. Reproducibility of the quantitative assessment of cartilage morphology and trabecular bone structure with magnetic resonance imaging at 7 T. Magn Reson Imaging. 2008;26:560–566. doi: 10.1016/j.mri.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang G, Friedrich KM, Wang L, Vieira RL, Schweitzer ME, Recht MP, Wiggins GC, Regatte RR. MRI of the wrist at 7 Tesla using an eight-channel array coil combined with parallel imaging: preliminary results. J Magn Reson Imaging. 2010;31:740–746. doi: 10.1002/jmri.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser E, Stahlberg F, Ladd ME, Trattnig S. 7-T MR-from research to clinical applications? NMR Biomed. 2012;25:695–716. doi: 10.1002/nbm.1794. [DOI] [PubMed] [Google Scholar]
- 29.Juras V, Welsch G, Bar P, Kronnerwetter C, Fujita H, Trattnig S. Comparison of 3 T and 7 T MRI clinical sequences for ankle imaging. Eur J Radiol. 2012;81:1846–1850. doi: 10.1016/j.ejrad.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Krug R, Stehling C, Kelley DA, Majumdar S, Link TM. Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Invest Radiol. 2009;44:613–618. doi: 10.1097/RLI.0b013e3181b4c055. [DOI] [PubMed] [Google Scholar]
- 31.Krug R, Carballido-Gamio J, Banerjee S, Burghardt AJ, Link TM, Majumdar S. In vivo ultra-high-field magnetic resonance imaging of trabecular bone microarchitecture at 7 T. J Magn Reson Imaging. 2008;27:854–859. doi: 10.1002/jmri.21325. [DOI] [PubMed] [Google Scholar]
- 32.Pruessmann KP. Parallel imaging at high field strength: synergies and joint potential. Top Magn Reson Imaging. 2004;15:237–244. doi: 10.1097/01.rmr.0000139297.66742.4e. [DOI] [PubMed] [Google Scholar]
- 33.Chang G, Wiggins GC, Xia D, Lattanzi R, Madelin G, Raya JG, Finnerty M, Fujita H, Recht MP, Regatte RR. Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7 T. J Magn Reson Imaging. 2012;35:441–448. doi: 10.1002/jmri.23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, Trattnig S. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38:934–942. doi: 10.1177/0363546509354971. [DOI] [PubMed] [Google Scholar]
- 35.Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH. Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results. Radiology. 2010;254:818–826. doi: 10.1148/radiol.09090335. [DOI] [PubMed] [Google Scholar]
- 36.Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48:3130–3139. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
- 37.Tochigi Y, Buckwalter JA, Martin JA, Hillis SL, Zhang P, Vaseenon T, Lehman AD, Brown TD. Distribution and progression of chondrocyte damage in a whole-organ model of human ankle intra-articular fracture. J Bone Joint Surg Am. 2011;93:533–539. doi: 10.2106/JBJS.I.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthr Cartil. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, Smith MB. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 40.Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging. 2008;28:175–180. doi: 10.1002/jmri.21418. [DOI] [PubMed] [Google Scholar]
- 41.Gold GE, Suh B, Sawyer-Glover A, Beaulieu C. Musculoskeletal MRI at 3.0 T: initial clinical experience. AJR Am J Roentgenol. 2004;183:1479–1486. doi: 10.2214/ajr.183.5.1831479. [DOI] [PubMed] [Google Scholar]
- 42.Kuo R, Panchal M, Tanenbaum L, Crues JV., 3rd 3.0 Tesla imaging of the musculoskeletal system. J Magn Reson Imaging. 2007;25:245–261. doi: 10.1002/jmri.20815. [DOI] [PubMed] [Google Scholar]